Published online May 26, 2020. doi: 10.12998/wjcc.v8.i10.1916
Peer-review started: February 10, 2020
First decision: March 27, 2020
Revised: April 18, 2020
Accepted: April 24, 2020
Article in press: April 24, 2020
Published online: May 26, 2020
Processing time: 105 Days and 0.7 Hours
The elevation of plasma von Willebrand factor (vWF) has been proposed to be a predictor of lung cancer. Type 2 diabetes mellitus (T2DM) causes endothelial activation, resulting in the secretion of vWF. However, the role of vWF in patients with T2DM complicated with lung cancer remains unclear.
To investigate the clinical value of serum vWF as a tumor marker in patients with T2DM combined with lung adenocarcinoma in situ (AIS).
This study enrolled 43 patients with T2DM combined with lung AIS (T2DM + AIS group), 43 patients with T2DM alone (T2DM group), 43 patients with lung AIS alone (AIS group), and 43 healthy volunteers (control group). The serum levels of vWF, insulin-like growth factor 1, and insulin-like growth factor binding protein 3 were determined. Multiple linear stepwise regression was performed to determine the correlations among variables.
Serum concentration of vWF in the T2DM + AIS group was significantly higher than those in the T2DM, AIS, and control groups (P < 0.05). Serum vWF levels in the T2DM and AIS groups were significantly higher than that in the control group (P < 0.05). There was no significant difference in serum vWF level between the T2DM and AIS groups. In the T2DM + AIS group, serum vWF was independently associated and positively correlated with serum levels of insulin-like growth factor 1 and insulin-like growth factor binding protein 3 (P < 0.05).
Serum vWF level may represent a novel biomarker for the early diagnosis of lung AIS.
Core tip: Previous evidence has indicated an increased cancer risk among patients with type 2 diabetes mellitus (T2DM). However, early diagnosis of lung adenocarcinoma in situ (AIS) in T2DM patients is difficult, resulting in a lost opportunity for curative-intent surgery in almost 70%-80% of lung cancer patients. This study revealed that serum levels of von Willebrand factor in patients with T2DM combined with lung AIS were significantly higher than those in patients with T2DM, and thus, can be used to screen lung AIS in T2DM patients. Thus, monitoring serum von Willebrand factor might promote the early diagnosis of lung AIS in T2DM patients.
- Citation: Zhou YY, Du X, Tang JL, Wang QP, Chen K, Shi BM. Serum von Willebrand factor for early diagnosis of lung adenocarcinoma in patients with type 2 diabetes mellitus. World J Clin Cases 2020; 8(10): 1916-1922
- URL: https://www.wjgnet.com/2307-8960/full/v8/i10/1916.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v8.i10.1916
Type 2 diabetes mellitus (T2DM) and lung cancer are the two major chronic non-communicable diseases worldwide. According to global survey data from the International Diabetes Federation in 2017, DM affects approximately 425 million people worldwide, and it is estimated that nearly 700 million will have this disease by 2045[1]. The prevalence of DM in China has substantially increased over the last 30 years, with an estimated prevalence of 11.6%, representing up to 113.9 million Chinese adults[2]. According to a report on the global burden of cancer in 2018, lung cancer is the most common malignancy, accounting for 11.6% of all cancer cases and 18.4% of all cancer-related deaths[3]. In addition, recent data released from the National Cancer Center of China showed that lung cancer ranks first in terms of both morbidity and mortality in China, with rates reaching 57.13 and 45.80 per 100000 people, respectively. Previous evidence has indicated an increased cancer risk among T2DM patients[4]. Lung cancer ranks top three in the incidence of malignant tumors in patients with T2DM[5]. However, early diagnosis of this disease in T2DM patients is difficult, resulting in a lost opportunity for curative-intent surgery in almost 70%-80% of lung cancer patients[6].
von Willebrand factor (vWF) is a multimeric glycoprotein that plays an essential role in hemostasis during vascular injury, via mediating platelet adhesion to the subendothelium and stabilizing coagulation factor VIII[7,8]. The elevation of plasma vWF in patients with advanced non-small-cell lung cancer has been shown[9], suggesting its potential role in the prediction of lung cancer. In addition, T2DM causes endothelial activation, resulting in the secretion of vWF[10]. However, the role of vWF in patients with T2DM complicated with lung cancer remains unclear.
In this study, we evaluated the clinical significance of serum vWF in patients with T2DM combined with lung adenocarcinoma in situ (AIS).
In this study, 43 patients with T2DM combined with lung AIS were enrolled at the Department of Thoracic Surgery (T2DM + AIS group) from April 2019 to June 2019. During the same period, 43 patients with T2DM alone admitted to the Endocrinology Department (T2DM group) and 43 patients with lung AIS alone admitted to the Department of Thoracic Surgery (AIS group) were enrolled. All patients with lung AIS were confirmed by postoperative pathology, according to the TNM staging criteria proposed by the International Association for the Study of Lung Cancer, American Thoracic Society, and European Respiratory Society in 2011[11]. Patients with T2DM were diagnosed using the 1999 World Health Organization criteria[12]. In addition, 43 healthy outpatients who underwent physical examination during the same period were selected as a normal control (control group). The exclusion criteria were as follows: Type 1 DM, gestational DM, cardiovascular and cerebrovascular diseases, hypertension, autoimmune diseases, acute and chronic infections, severe liver and kidney disorders, and other endocrine conditions. None of the patients received anticancer or anticoagulant drugs at enrollment. Patients with T2DM combined with lung AIS were diagnosed with T2DM before being diagnosed with lung AIS. Informed consent was obtained from all patients and volunteers.
Demographic characteristic data including age, gender, and medical history were collected from the medical chart. Physical examination including anthropometric measurements (height, body weight, and hip and waist circumferences) and blood pressure measurement was conducted by specifically trained staff. Body mass index (BMI) was calculated as weight in kilograms divided by the square of height in meters (kg/m2).
Venous blood samples (20 mL) were drawn after overnight fasting, of which 5 mL was centrifuged for 10 min at 3000 r/min. Then, the serum was separated and stored in a refrigerator at -70 °C for further testing of vWF concentration by an enzyme-linked immunoassay (Elabscience Biotechnology Co. Ltd, Wuhan, China). The remaining 15 mL blood samples were used for determination of biochemical indicators including insulin-like growth factor 1 (IGF-1), insulin-like growth factor binding protein 3 (IGFBP3), triglyceride (TG), total cholesterol (TC), low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), glycosylated hemoglobin (HbAlc), fasting plasma glucose (FPG), and fasting insulin (FINS). The concentrations of TC, TG, HDL-C, LDL-C, and hsC-RP were measured with a Hitachi 7600 automatic biochemical analyzer (Tokyo, Japan). HbA1C was assayed by high-performance liquid chromatography (TosohG8; Tosoh Bioscience, Tokyo, Japan). Insulin resistance was assessed according to homeostatic model assessment-estimated insulin resistance (HOMA-IR) = FINS (μIU/mL) × FPG (mmol)/22.5.
All statistical analyses were performed using GraphPad Prism 7. Quantitative data are expressed as the means ± SD. The t-test and one-way analysis of variance were used to test differences between two groups and among the four groups, respectively. Correlations between serum vWF concentrations and clinical characteristics were analyzed using multiple linear stepwise regression analysis. P < 0.05 was considered statistically significant.
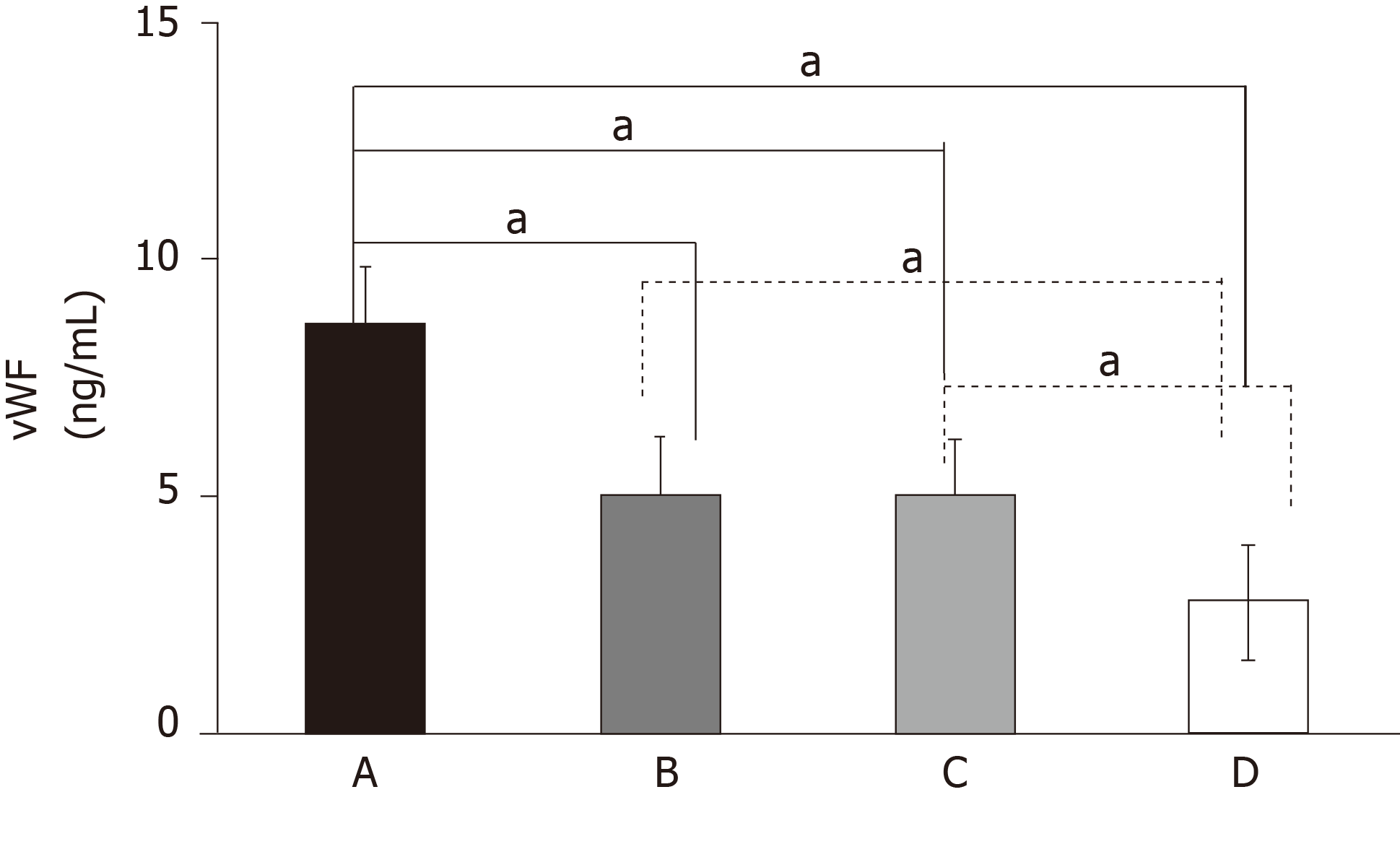
There were no differences in age, gender distribution, BMI value, or serum concentrations of TG and LDL-C among the four groups of patients (P > 0.05). Patients in the T2DM + AIS, T2DM, and AIS groups had higher concentrations of serum vWF, IGF-1, and IGFBP3 than the healthy volunteers. Moreover, patients in the T2DM + AIS group had the highest values of serum vWF and IGF-1, which were higher than those in the T2DM and AIS groups (P < 0.05 or P < 0.01; Figure 1). However, there was no statistical difference in serum vWF or IGF-1 concentration between the T2DM and AIS groups. In addition, no difference was found in serum IGFBP3 concentration among the T2DM + AIS, T2DM, and AIS groups. The demographic and biochemical characteristics of subjects are shown in Table 1.
| T2DM + AIS (n = 43) | T2DM (n = 43) | AIS (n = 43) | Control (n = 43) | |
| Male/Female | 21/22 | 21/22 | 21/22 | 21/22 |
| Age, yr | 58 ± 8 | 57 ± 10 | 59 ± 11 | 58 ± 10 |
| BMI, kg/m2 | 23.32 ± 3.59 | 24.10 ± 3.74 | 23.43 ± 3.74 | 23.20 ± 3.18 |
| IGF-1, ng/mL | 131.4 ± 50.45 | 111.0 ± 30.21a | 106.6 ± 35.80b | 56.93 ± 24.65bde |
| IGFBP3, μg/mL | 3.48 ± 0.78 | 3.53 ± 1.09 | 3.13 ± 1.33 | 1.71 ± 0.79bde |
| vWF, ng/mL | 9.18 ± 0.53 | 5.33 ± 0.24b | 4.86 ± 0.15b | 2.69 ± 0.44bde |
| HbA1c, % | 7.65 ± 1.79 | 9.75 ± 2.51b | 5.05 ± 0.52bd | 5.29 ± 0.52bd |
| TC, mmol/L | 4.33 ± 0.99 | 4.72 ± 1.38 | 4.75 ± 0.85 | 4.91 ± 0.87b |
| TG, mmol/L | 1.48 ± 0.73 | 1.55 ± 0.81 | 1.38 ± 0.54 | 1.48 ± 0.81 |
| HDL-C, mmol/L | 1.14 ± 0.38 | 1.19 ± 0.33 | 1.32 ± 0.27 | 1.38 ± 0.32ac |
| LDL-C, mmol/L | 2.48 ± 0.81 | 2.65 ± 1.17 | 2.74 ± 0.64 | 2.81 ± 0.75 |
| FBG, mmol/ L | 7.36 ± 2.66 | 7.14 ± 4.09 | 5.13 ± 0.52bd | 5.17 ± 1.19bd |
| FINS, mU/L | 9.94 ± 8.69 | 14.43 ± 7.60b | 15.27 ± 2.29b | 15.22 ± 2.09b |
| HOMA-IR | 2.97 ± 2.44 | 4.74 ± 3.82b | 3.46 ± 0.62 | 3.52 ± 1.29 |
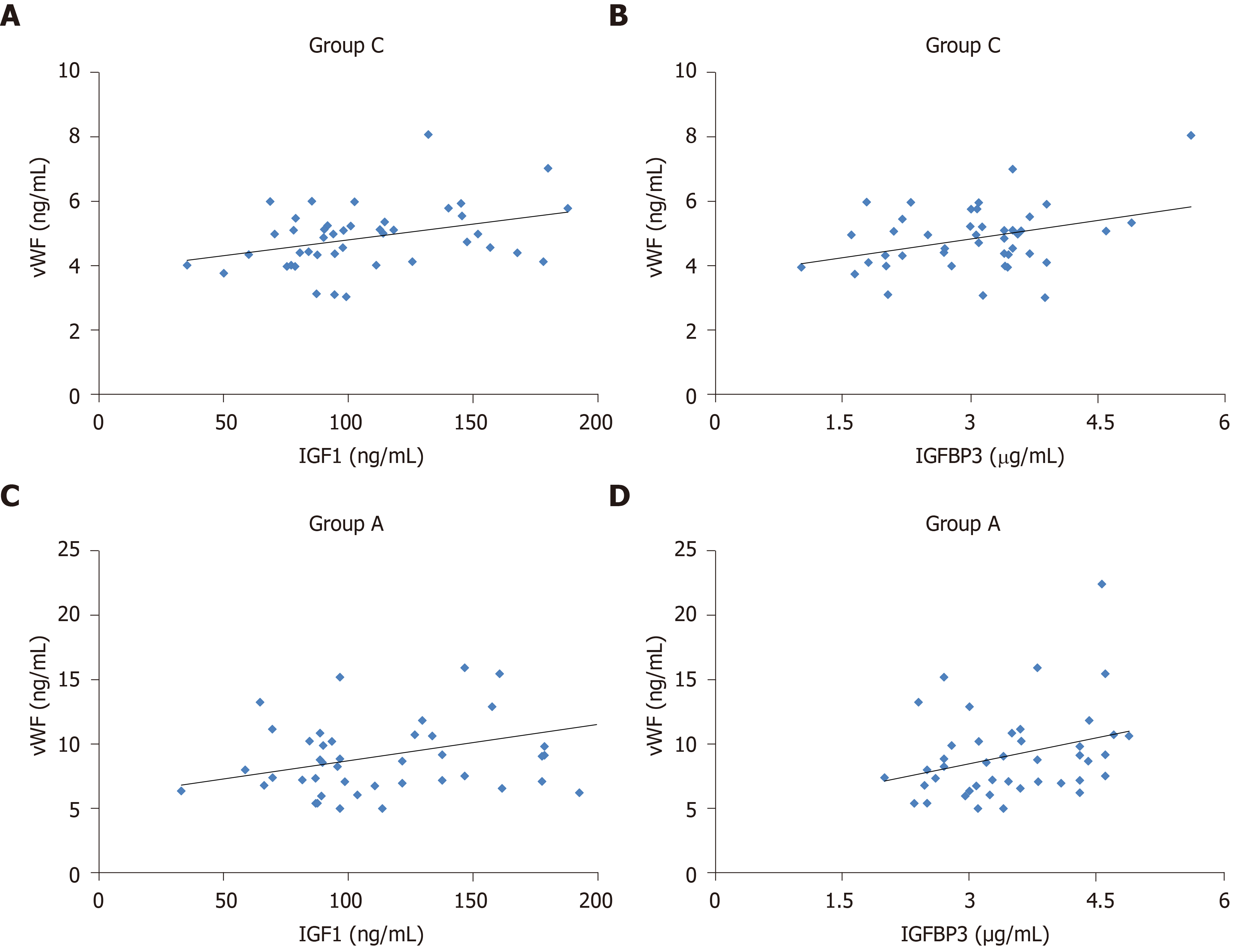
We performed multiple linear stepwise regression analysis to assess the independent predictors using gender, age, vWF, BMI, FPG, HbA1c, HOMA-IR, TC, TG, LDL-C, HDL-C, FINS, and HOMA-IR as independent variables. For patients with lung AIS (AIS group), serum vWF was an independent factor influencing serum levels of IGF-1 (F = 5.989, R2 = 0.128, P < 0.05; Figure 2A) and IGFBP3 (F = 6.117, R2 = 0.129, P < 0.05; Figure 2B). Similarly, for patients with T2DM combined with lung AIS (T2DM + AIS group), serum vWF was independently associated and positively correlated with serum levels of IGF-1 (F = 4.996, R2 = 0.109, P < 0.05; Figure 2C) and IGFBP3 (F = 4.146, R2 = 0.092, P < 0.05; Figure 2D). Moreover, TC (F = 4.245, R2 = 0.094, P < 0.05) and LDL-C (F = 7.79, R2 = 0.159, P < 0.01) were independent factors for serum IGF-1.
The vWF is an adhesive multimeric glycoprotein with the gene located on the short arm of the chromosome[13]. It is mainly synthesized, stored, and released from endothelial cells and megakaryocytes. Under stimulation, vWF is released from these cells and tethers to sites of vascular damage, where it binds to receptors on the platelet plasma membrane, mediating platelet adhesion to the subendothelial matrix and increasing the possibility of microthrombosis[14]. On the other hand, vWF, which bridges between the platelets constituents of the vascular subendothelium and platelet membrane receptors, may be involved in the development and progression of tumors[14,15].
In this study, patients with T2DM combined with lung AIS had higher serum vWF levels than healthy controls, suggesting that there may be a close relationship between the occurrence of lung AIS and the hypercoagulable state of the body. A malignant tumor can directly invade blood vessels, leading to intimal lesions and endothelial injury. During this process, activated endothelial cells, partially via release of vWF to mediate platelet interaction with the endothelial surface, recruit platelets to the site of injury, further forming an obvious hypercoagulable state. Thus, elevated vWF level is a marker of vascular endothelial injury, and detection of vWF may help determine the hypercoagulability state in patients with lung AIS[16].
Moreover, we found that serum vWF in patients with T2DM combined with lung AIS was significantly higher compared to those with T2DM or lung AIS alone. In patients with T2DM combined with lung AIS, endothelial dysfunction and platelet activation usually occur in the early stage of the disease, probably due to hyperglucemia-induced vascular endothelial injury, leading to elevation of circulating vWF and increased vascular permeability, which further promote the development of lung AIS. However, the results of this study do not explain whether elevation of vWF is a simple superposition of the effects caused by T2DM and lung AIS, or whether it is the result of the interaction between T2DM and lung AIS, which needs further investigation.
Multiple linear stepwise regression analysis showed that vWF was an independent factor influencing serum IGF-1 and IGFPB3 levels in patients with lung AIS, suggesting that it might be a potential risk factor for lung AIS. In addition, vWF was also an independent factor influencing serum levels of IGF-1 and IGFPB3 in patients with T2DM and lung AIS, indicating that elevated serum vWF might also be an independent risk factor for lung AIS in patients with T2DM. However, there was no significant difference in serum vWF concentration between patients with T2DM and lung AIS alone. The above-mentioned data indicate that serum vWF may be helpful for the early screening of lung AIS in T2DM patients.
To the best of our knowledge, this is the first study to investigate the role of vWF in patients with T2DM complicated with lung cancer. Several limitations merit consideration. First, this was a case-control study; therefore, the temporal relationship between vWF and lung AIS needs to be elucidated in future prospective studies. Second, the current study measured vWF one time, which may not represent the long-term levels of vWF. In addition, some random measurement errors were inevitable. Future studies with multiple measurements are warranted to validate our findings.
This study revealed that serum levels of vWF in patients with T2DM combined with lung AIS were significantly higher than those in patients with T2DM, and thus, can be used to screen lung AIS in T2DM patients. Thus, monitoring serum vWF might promote the early diagnosis of lung AIS in T2DM patients.
Type 2 diabetes mellitus (T2DM) and lung cancer are the two major chronic non-communicable diseases worldwide. Previous evidence has indicated an increased cancer risk among patients with T2DM. However, early diagnosis of lung adenocarcinoma in situ (AIS) in T2DM patients is difficult, resulting in a lost opportunity for curative-intent surgery in almost 70%‒80% of lung cancer patients. T2DM causes endothelial activation, resulting in the secretion of plasma von Willebrand factor (vWF). The elevation of plasma vWF has been proposed to be a predictor of lung cancer. However, the role of vWF in patients with T2DM complicated with lung cancer remains unclear.
Since the early diagnosis of AIS in T2DM patients is difficult, which currently results in a lost opportunity for surgery in 70%-80% of patients with lung cancer, we were motivated to examine whether the plasma vWF could be a useful biomarker to diagnose lung AIS among patients with T2DM. If vWF could serve as a useful biomarker, then it could save additional 70%-80% of T2DM patients with lung cancer for the opportunity in treatment and therapy.
We aimed to examine whether vWF could serve as a useful biomarker to diagnose patients with T2DM combined with AIS. Specifically, we aimed to assess whether vWF levels were elevated among patients with T2D combined with AIS compared to patients with T2DM only, patients with AIS only, and patients without T2DM and AIS.
We conducted a cross-sectional case-control study. The study enrolled 43 patients with T2DM combined with lung AIS (T2DM + AIS group), 43 patients with T2DM alone (T2DM group), 43 patients with lung AIS alone (AIS group), and 43 healthy volunteers (control group). The serum levels of vWF were measured using an enzyme-linked immunoassay (Elabscience Biotechnology Co. Ltd, Wuhan, China), and compared among the four groups.
Serum concentrations of vWF in the T2DM + AIS group were significantly higher than those in the T2DM, AIS, and control groups (P < 0.05). Serum vWF levels in the T2DM and AIS groups were significantly higher than those in the control group (both P < 0.05). There was no significant difference in serum vWF level between the T2DM and AIS groups.
For the first time, we have found that serum levels of vWF in patients with T2DM combined with lung AIS were significantly higher than those in patients with T2DM only, patients with AIS only, and healthy individuals. Therefore, serum vWF level may serve as a novel biomarker for the early diagnosis of lung AIS among T2DM patients. This provides an important implication for the clinical practice and public health intervention that vWF could be introduced as a new test to diagnose or screen T2DM patients with lung cancer.
Since the current study is the first to examine the clinical utility of vWF for the diagnosis of lung cancer among patients with T2DM patients, the temporal relationship cannot be determined. Future studies with larger sample sizes and prospective study design are warranted to validate our findings, and further examine if vWF could predict the risk of lung cancer among T2DM patients. Studies regarding the cost-effectiveness of measuring this biomarker in clinical practice or in large-scale public health screening programs are also needed.
| 1. | Jafar N, Hippalgaonkar N, Parikh NI. Preeclampsia and Hypertension in Pregnancy. In: Reference Module in Biomedical Sciences, 2017. [DOI] [Full Text] |
| 2. | Xu Y, Wang L, He J, Bi Y, Li M, Wang T, Wang L, Jiang Y, Dai M, Lu J, Xu M, Li Y, Hu N, Li J, Mi S, Chen CS, Li G, Mu Y, Zhao J, Kong L, Chen J, Lai S, Wang W, Zhao W, Ning G. 2010 China Noncommunicable Disease Surveillance Group. Prevalence and control of diabetes in Chinese adults. JAMA. 2013;310:948-959. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1961] [Cited by in RCA: 2200] [Article Influence: 169.2] [Reference Citation Analysis (0)] |
| 3. | Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53206] [Cited by in RCA: 56675] [Article Influence: 7084.4] [Reference Citation Analysis (135)] |
| 4. | Xu Z, Wang X, Wu P, Pang X, Luo C, Zhang P, Zeng H, Peng W. Surgical treatment for mono-segmental lumbar tuberculosis by single-stage posterior debridement, compact bone grafting and posterior single-segment fixation. Injury. 2015;46:1311-1316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 5. | Lee JY, Jeon I, Lee JM, Yoon JM, Park SM. Diabetes mellitus as an independent risk factor for lung cancer: a meta-analysis of observational studies. Eur J Cancer. 2013;49:2411-2423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 113] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 6. | Hall GC, Roberts CM, Boulis M, Mo J, MacRae KD. Diabetes and the risk of lung cancer. Diabetes Care. 2005;28:590-594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 75] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 7. | Randi AM, Smith KE, Castaman G. von Willebrand factor regulation of blood vessel formation. Blood. 2018;132:132-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 166] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 8. | Denis CV, Lenting PJ. von Willebrand factor: at the crossroads of bleeding and thrombosis. Int J Hematol. 2012;95:353-361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 41] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 9. | Guo R, Yang J, Liu X, Wu J, Chen Y. Increased von Willebrand factor over decreased ADAMTS-13 activity is associated with poor prognosis in patients with advanced non-small-cell lung cancer. J Clin Lab Anal. 2018;32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 40] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 10. | Chen SF, Xia ZL, Han JJ, Wang YT, Wang JY, Pan SD, Wu YP, Zhang B, Li GY, Du JW, Gao HQ, de Groot PG, de Laat B, Hollestelle MJ. Increased active von Willebrand factor during disease development in the aging diabetic patient population. Age (Dordr). 2013;35:171-177. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 11. | Ried M, Eicher MM, Neu R, Sziklavari Z, Hofmann HS. Evaluation of the new TNM-staging system for thymic malignancies: impact on indication and survival. World J Surg Oncol. 2017;15:214. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 33] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 12. | American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2008;31 Suppl 1:S55-S60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 692] [Cited by in RCA: 724] [Article Influence: 40.2] [Reference Citation Analysis (0)] |
| 13. | James PD, Lillicrap D. The molecular characterization of von Willebrand disease: good in parts. Br J Haematol. 2013;161:166-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 14. | Luo GP, Ni B, Yang X, Wu YZ. von Willebrand factor: more than a regulator of hemostasis and thrombosis. Acta Haematol. 2012;128:158-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 68] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 15. | Terraube V, Marx I, Denis CV. Role of von Willebrand factor in tumor metastasis. Thromb Res. 2007;120 Suppl 2:S64-S70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 72] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 16. | Randi AM. Angiogenesis and the ADAMTS13-VWF balance. Blood. 2017;130:1-2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See:
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Berezin AE S-Editor: Zhang L L-Editor: Wang TQ E-Editor: Liu MY