Published online May 26, 2020. doi: 10.12998/wjcc.v8.i10.1887
Peer-review started: December 30, 2019
First decision: April 1, 2020
Revised: April 7, 2020
Accepted: April 22, 2020
Article in press: April 22, 2020
Published online: May 26, 2020
Processing time: 146 Days and 20.4 Hours
Leiomyosarcoma is a subtype of soft tissue sarcoma with adverse outcomes. Leiomyosarcoma accounts for nearly 70% of all uterine sarcomas and is responsible for a considerable proportion of deaths because of uterine cancer. Clinical characteristics and relevant diagnosis of pelvic leiomyosarcoma should be further explored.
To identify the outcome and relevant perioperative evaluation of patients with pelvic leiomyosarcoma.
The Kaplan-Meier method was used to determine progression-free survival and overall survival rates. Factors predictive of outcomes were identified using univariate and multivariate Cox proportional hazards models.
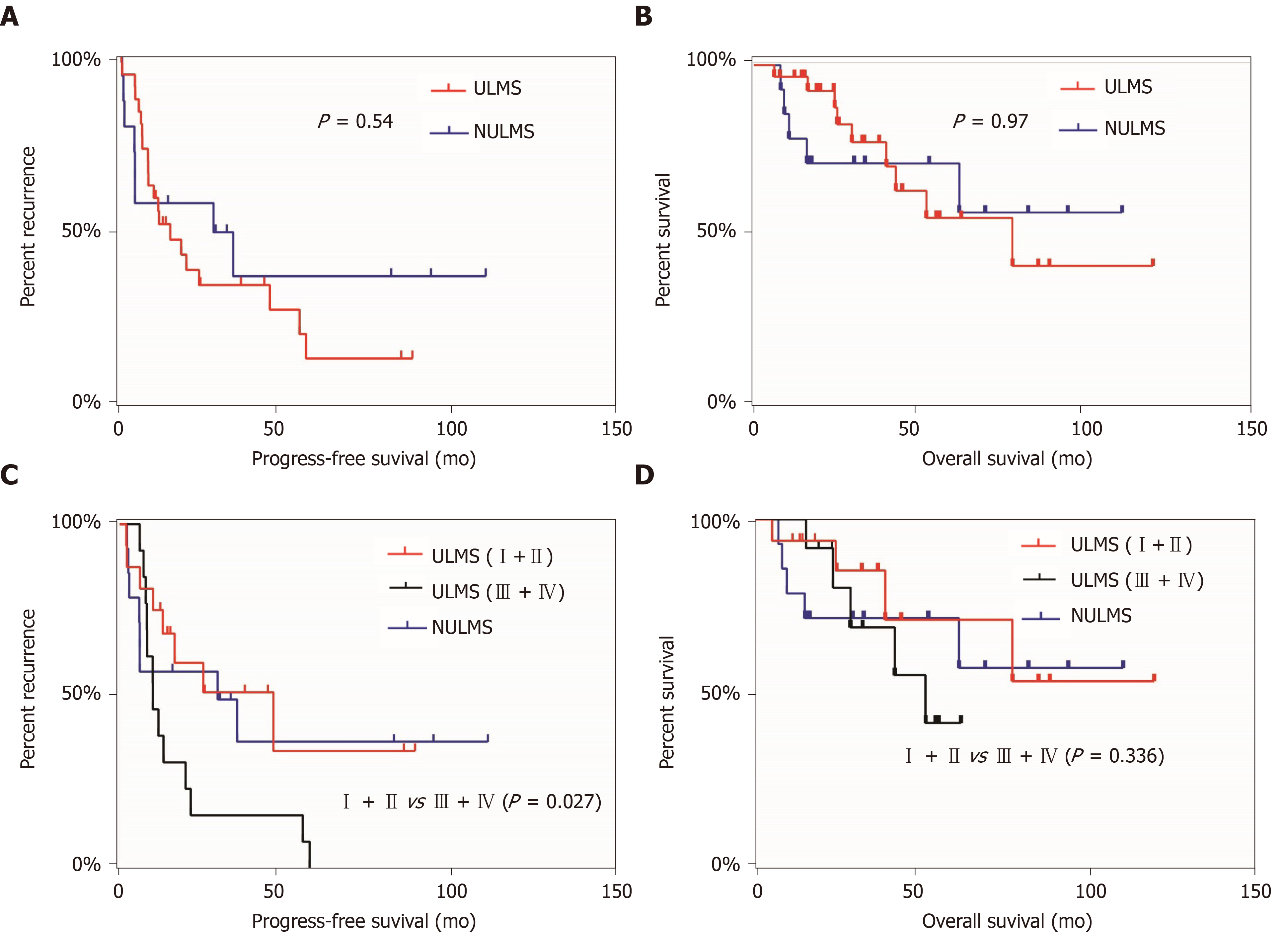
Fifty-one patients with pelvic leiomyosarcoma were enrolled and divided into two groups including uterine leiomyosarcoma and non-uterine leiomyosarcoma. Overall, 28.6% and 45.5% of uterine leiomyosarcoma and non-uterine leiomyosarcoma patients, respectively, had elevated carbohydrate antigen 125 levels, whereas 45.7% and 68.8%, respectively, underwent ultrasonography. Although 68.8% of uterine leiomyosarcoma patients were initially diagnosed with hysteromyoma, 72.7% of non-uterine leiomyosarcoma patients had pelvic and abdominal masses. Moreover, 93.3% of the recurrent lesions were detected using ultrasonography. Patients with International Federation of Gynaecology and Obstetrics (FIGO) stages III–IV disease had poorer progression-free survival values than those with FIGO stages I–II (P = 0.027) disease. FIGO stage was significantly associated with poor progression-free survival in the univariate (hazard ratio = 2.64, P = 0.03) and multivariate (hazard ratio = 2.49, P = 0.048) analyses.
Serum tumour biomarkers cannot be used for pelvic leiomyosarcoma diagnosis. FIGO stage is critical to predict the outcome of uterine leiomyosarcoma. Ultrasonography is more reliable for postoperative follow-up than preoperative diagnosis.
Core tip: Owing to a lack of long-term leiomyosarcoma-focused studies, the clinical features of pelvic leiomyosarcoma among women remain unclear. Our study showed that serum tumour biomarkers cannot be used for pelvic leiomyosarcoma diagnosis and ultrasonography is more reliable for postoperative follow-up than preoperative diagnosis.
- Citation: Sun Q, Yang X, Zeng Z, Wei X, Li KZ, Xu XY. Outcomes of patients with pelvic leiomyosarcoma treated by surgery and relevant auxiliary diagnosis. World J Clin Cases 2020; 8(10): 1887-1896
- URL: https://www.wjgnet.com/2307-8960/full/v8/i10/1887.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v8.i10.1887
Leiomyosarcoma is a subtype of soft tissue sarcoma that is derived from smooth muscles located in the uterus and soft tissue. The most frequently involved organs are the retroperitoneal space, extremities, and uterus. Despite its low annual incidence (approximately 0.36–0.64 per 100000 women), leiomyosarcoma accounts for nearly 70% of all uterine sarcomas and is responsible for a considerable proportion of deaths because of uterine cancer[1-3]. Leiomyosarcoma can occur at any site, but it occurs most commonly in the retroperitoneum and proximal extremities[4,5]. However, there is a lack of convincing data on pelvic non-uterine leiomyosarcoma[6], and the biological behaviour of this tumour type, including the associated metastases and prognoses, requires further exploration.
Although various diagnostic tools have been used to preoperatively evaluate uterine leiomyosarcoma, they have a low sensitivity for differentiating uterine leiomyosarcoma from fibroids; a previous study revealed that only approximately 54% of uterine leiomyosarcoma lesions were unidentified before surgery[7]. Moreover, unlike in the case of ovarian cancer, regular serum tumour markers have limited value in diagnosing uterine leiomyosarcoma[8-10]. Of all auxiliary examinations, only medical imaging can identify the existence and position of lesions. Computed tomography, magnetic resonance imaging, positron emission tomography, and ultrasonography can be used in the examination and follow-up of leiomyosarcoma patients[11-13].
The treatment methods and prognostic factors related to leiomyosarcoma require extensive research because of the disease’s high malignant potential. Previous studies have confirmed that the standard surgical resection of uterine leiomyosarcoma is associated with improved prognoses[12,14]. Lymphadenectomy is recommended following the intraoperative discovery of palpable lymph nodes in women with extrauterine disease[15,16]. Recent studies have implied that tumour stage, size, and relapse status, and mitotic index are associated with overall survival and progression-free survival in uterine leiomyosarcoma[17-19]. Furthermore, the disease stage at diagnosis is an important predictor of survival[20,21]. However, there is a lack of standardised treatment guidelines and specialised investigations focusing on non-uterine leiomyosarcoma of the pelvic cavity. Studies that compared the clinical differences have not shown consistent results[4,22]. Thus, there is an urgent need to identify clinical characteristics of non-uterine leiomyosarcoma to provide early diagnosis and proper treatment.
Owing to a lack of long-term leiomyosarcoma-focused studies, the clinical and ultrasonographic features of uterine leiomyosarcoma and non-uterine leiomyosarcoma among women remain unclear. Therefore, we retrospectively analysed patients with uterine leiomyosarcoma and non-uterine leiomyosarcoma to identify their clinical characteristics in detail.
Fifty-one patients treated between 2000 and 2018 were retrospectively examined in our study; the patients included 35 (68.6%) with uterine leiomyosarcoma and 16 (31.4%) with non-uterine leiomyosarcoma. The study was approved by the Ethical Committee of the Medical Faculty of Tongji Medical College, and verbal informed consent was obtained from the participants or their relatives. The tumour stages were evaluated based on International Federation of Gynaecology and Obstetrics (FIGO) criteria[23]. The outcomes included progression-free survival and overall survival; progression-free survival was calculated from the surgery date when the diagnosis was first confirmed to the date of detection of recurrence via imaging examination. Overall survival was calculated from the date of surgery that first confirmed the diagnosis to the date of death or that of the last follow-up for survivors.
The overall survival and progression-free survival of patients were estimated using Kaplan-Meier curves, and differences between uterine leiomyosarcoma and non-uterine leiomyosarcoma patients were compared using a log-rank test. Pearson’s χ2 test or Fisher’s exact test was used to assess the association among clinicopathologic features. Statistical significance and differences in survival were assessed using the Cox proportional hazards regression model. Factors with P values < 0.05 in the univariate analysis were included in the multivariate Cox regression analysis. For all analyses, the level of statistical significance was set at P < 0.05. Data analyses were performed using the Statistical Product and Service Solutions software (SPSS, version 22.0; IBM Corp., Armonk, NY, United States).
Fifty-one women with leiomyosarcoma were enrolled and were divided into the uterine leiomyosarcoma and non-uterine leiomyosarcoma groups based on tumour origin. Thirty-five (68.6%) patients were classified as having uterine leiomyosarcoma, whereas 16 (31.4%) had non-uterine leiomyosarcoma. We followed 43 women till May 2019. The patients’ characteristics are shown in Table 1. The mean ages of the patients in the uterine leiomyosarcoma and non-uterine leiomyosarcoma groups were 49.6 (range, 32-71) years and 46 (range, 29-66) years, respectively. The majority of the patients in both the groups initially presented with menopause (60% in the uterine leiomyosarcoma group and 56.3% in the non-uterine leiomyosarcoma group). Other medical conditions such as hypertension and diabetes mellitus were rarely observed, with < 20% of the patients presenting with these two disorders. Initial symptoms were particularly important for patient diagnoses. A plurality of patients with uterine leiomyosarcoma (45.7%) did not state obvious symptoms, and their lesions were diagnosed during medical examination. Furthermore, 25.7% of the patients with uterine leiomyosarcoma sought medical advice for menorrhagia and menstrual disorders, which were the most frequently reported complaints. Additionally, 17.1% of the patients with uterine leiomyosarcoma complained of abdominal pain and distention, and 5.7% had a palpable mass and postmenopausal bleeding. In contrast, 50% of patients with non-uterine leiomyosarcoma presented with abdominal pain and abdominal distention, whereas other complaints were rarely reported.
| Characteristic | Uterine leiomyosarcoma (n = 35) | Non-uterine leiomyosarcoma (n = 16) |
| Age [mean (range)], yr | 49.6 (32-71) | 46.0 (29-66) |
| Menopause, n (%) | ||
| No | 21 (60) | 9 (56.3) |
| Yes | 14 (40) | 7 (43.7) |
| Medical disease, n (%) | ||
| Hypertension | 6 (17.1) | 2 (12.5) |
| Diabetes mellitus | 1 (2.9) | 2 (12.5) |
| History of operation, n (%) | ||
| Hysteromyoma surgery | 20 (57.1) | 6 (37.5) |
| Cesarean section | 5 (14.3) | 3 (18.8) |
| Other abdominal surgeries1 | 8 (22.9) | 6 (37.5) |
| Clinical symptoms, n (%) | ||
| Menorrhagia and menstrual disorder | 9 (25.7) | 0 (0) |
| Abdominal pain and abdominal distention | 6 (17.1) | 8 (50) |
| Palpable mass | 2 (5.7) | 1 (6.25) |
| Limb pain | 0 (0) | 1 (6.25) |
| Postmenopausal bleeding | 2 (5.7) | 1 (6.25) |
| No obvious discomfort and examination revealed abnormalities | 16 (45.7) | 4 (25) |
| Ascites, n (%) | ||
| Increased | 5 (14.3) | 2 (12.5) |
| Normal | 30 (85.7) | 14 (87.5) |
| Number of pregnancies [mean (range)], n (%) | 2.86 (0-6) | 3.31 (2-6) |
| FIGO stage, n (%) | ||
| I | 16 (45.7) | — |
| II | 4 (11.4) | — |
| III | 9 (25.7) | — |
The levels of serum tumour markers, i.e., carbohydrate antigen (CA)125, alpha-fetoprotein, carcinoembryonic antigen (CEA), CA199, CA153, and CA724, were assessed for their potential association with leiomyosarcoma considering their roles in other malignant gynaecological diseases[8,24,25]. Because of our study’s long time span, only a proportion of our patients underwent serum tumour marker screening (Table 2). Most patients were screened for CA125 levels (60% in the uterine leiomyosarcoma group and 68.8% in the non-uterine leiomyosarcoma group); however, the CA125 positive rates were not high in either group. Only 28.6% and 45.5% of patients in the uterine leiomyosarcoma and non-uterine leiomyosarcoma groups, respectively, had elevated CA125 levels. Of the 42.9% and 63.6% of uterine leiomyosarcoma and non-uterine leiomyosarcoma patients, respectively, whose CEA levels were assessed, only one uterine leiomyosarcoma patient had elevated levels (with no non-uterine leiomyosarcoma patient having such elevations). Moreover, the alpha-fetoprotein and CA199 levels were normal in both groups. Only a minority of patients with uterine leiomyosarcoma underwent CA153 and CA724 screening; none of the patients had elevated CA153 levels and two had elevated CA724 levels, accounting for 40% of all the examined patients. These observations demonstrate that serum tumour markers have limited ability in leiomyosarcoma diagnosis; therefore, we next examined the medical imaging findings.
| Characteristic | Uterine leiomyosarcoma (n = 35) | Non-uterine leiomyosarcoma (n = 16) |
| Serum tumor markers, n (%) | ||
| CA125 | 21 (60) | 11 (68.8) |
| Elevated | 6 (28.6) | 5 (45.5) |
| Not elevated | 15 (71.4) | 6 (54.5) |
| CEA | 15 (42.9) | 7 (63.6) |
| Elevated | 1 (6.7) | 0 (0) |
| Not elevated | 14 (93.3) | 7 (100) |
| AFP | 14 (40) | 7 (63.6) |
| Elevated | 0 (0) | 0 (0) |
| Not elevated | 14 (100) | 7 (100) |
| CA199 | 12 (34.3) | 7 (63.6) |
| Elevated | 0 (0) | 0 (0) |
| Not elevated | 12 (100) | 7 (100) |
| CA153 | 5 (14.3) | 0 (0) |
| Elevated | 0 (0) | — |
| Not elevated | 5 (100) | — |
| CA724 | 5 (14.3) | 0 (0) |
| Elevated | 2 (40) | — |
| Not elevated | 3 (60) | — |
| Medical imaging examination, n (%) | ||
| Ultrasonography | 16 (45.7) | 11 (68.8) |
| Hysteromyoma | 11 (68.8) | 1 (9.1) |
| Pelvic and abdominal mass | 4 (25) | 8 (72.7) |
| Malignant occupancy of cervix | 1 (6.2) | 2 (18.2) |
| CT | 2 (5.7) | 1 (6.3) |
| MRI | 1 (2.9) | 0 (0) |
Ultrasonography was the most widely performed procedure and was performed in 45.7% and 68.8% of patients in the uterine leiomyosarcoma and non-uterine leiomyosarcoma groups, respectively. However, the majority of the patients with uterine leiomyosarcoma (68.8%) were diagnosed with hysteromyoma, whereas the majority of patients with non-uterine leiomyosarcoma (72.7%) were diagnosed with pelvic and abdominal masses. These data imply that ultrasonography has limited ability in distinguishing between benign and malignant foci.
We followed 43 patients, including 29 with uterine leiomyosarcoma (67.4%) and 14 with non-uterine leiomyosarcoma (32.6%). In the Kaplan-Meier analysis, no significant differences were found in the progression-free survival (P = 0.54) and overall survival values (P = 0.97) between patients in the uterine leiomyosarcoma and non-uterine leiomyosarcoma groups (Figure 1A and 1B). Because FIGO stage is a known prognostic indicator[26], we divided uterine leiomyosarcoma patients into the early and late-stage groups and compared their survival curves with those of non-uterine leiomyosarcoma patients. We found significant differences in progression-free survival values (P = 0.027) between the early- and late-stage groups (Figure 1C); however, this did not apply to overall survival (Figure 1D).
We incorporated various clinical characteristics to identify factors that affected the prognosis of patients with leiomyosarcoma. In the univariate Cox regression analysis, FIGO stage (uterine leiomyosarcoma I–II vs III–IV: Hazard ratio = 2.64, P = 0.03), chemotherapy (hazard ratio = 2.95, P = 0.03), and neoplasm metastasis (hazard ratio = 3.31, P = 0.006) were independent factors that were significantly related to poor progression-free survival. Factors with P values < 0.05 in the univariate analysis were included in the multivariate Cox regression analysis. Because collinearity existed between FIGO stage and tumour metastasis, we only included FIGO stage and chemotherapy in the multivariate analysis (Table 3). FIGO stage was a significant predictor of progression-free survival (hazard ratio = 2.49, P = 0.048), whereas chemotherapy was not (hazard ratio = 1.47, P = 0.47). Univariate Cox regression analysis was also performed for overall survival; however, there was no significant relationship between any of the clinical variables investigated and overall survival.
| Factor | Univariate analysis | Multivariate analysis | ||||
| Hazard ratio | 95%CI | P value | Hazard ratio | 95%CI | P value | |
| Fage | 0.98 | 0.94-1.01 | 0.25 | |||
| Primary site (uterine/non-uterine) | 0.77 | 0.34-1.76 | 0.54 | |||
| FIGO stage | ||||||
| III-IV/I-II | 2.64 | 1.08-6.44 | 0.03 | 2.49 | 1.01-6.16 | 0.048 |
| Non-uterine /I-II | 1.20 | 0.45-3.23 | 0.71 | |||
| Non-uterine /III-IV | 0.51 | 0.20-1.26 | 0.14 | |||
| Diameter of tumor (> 8 cm/≤ 8 cm) | 2.65 | 0.81-8.68 | 0.11 | |||
| Myoma surgical history (yes/no) | 1.60 | 0.73-3.53 | 0.24 | |||
| Surgery (laparotomy/laparoscopy) | 1.11 | 0.90-1.33 | 0.87 | |||
| Ascites (increased/normal) | 2.17 | 0.92-5.13 | 0.08 | |||
| Menopause (yes/no) | 0.81 | 0.38-1.73 | 0.59 | |||
| Chemotherapy (yes/no) | 2.95 | 1.13-7.70 | 0.03 | 1.47 | 0.52-4.22 | 0.47 |
| CA125 (elevated/normal) | 0.99 | 0.39-2.58 | 0.99 | |||
| Lymphadenectomy (yes/no) | 1.36 | 0.63 -2.93 | 0.43 | |||
| Neoplasm metastasis (yes/no) | 3.31 | 1.4 -7.82 | 0.006 | |||
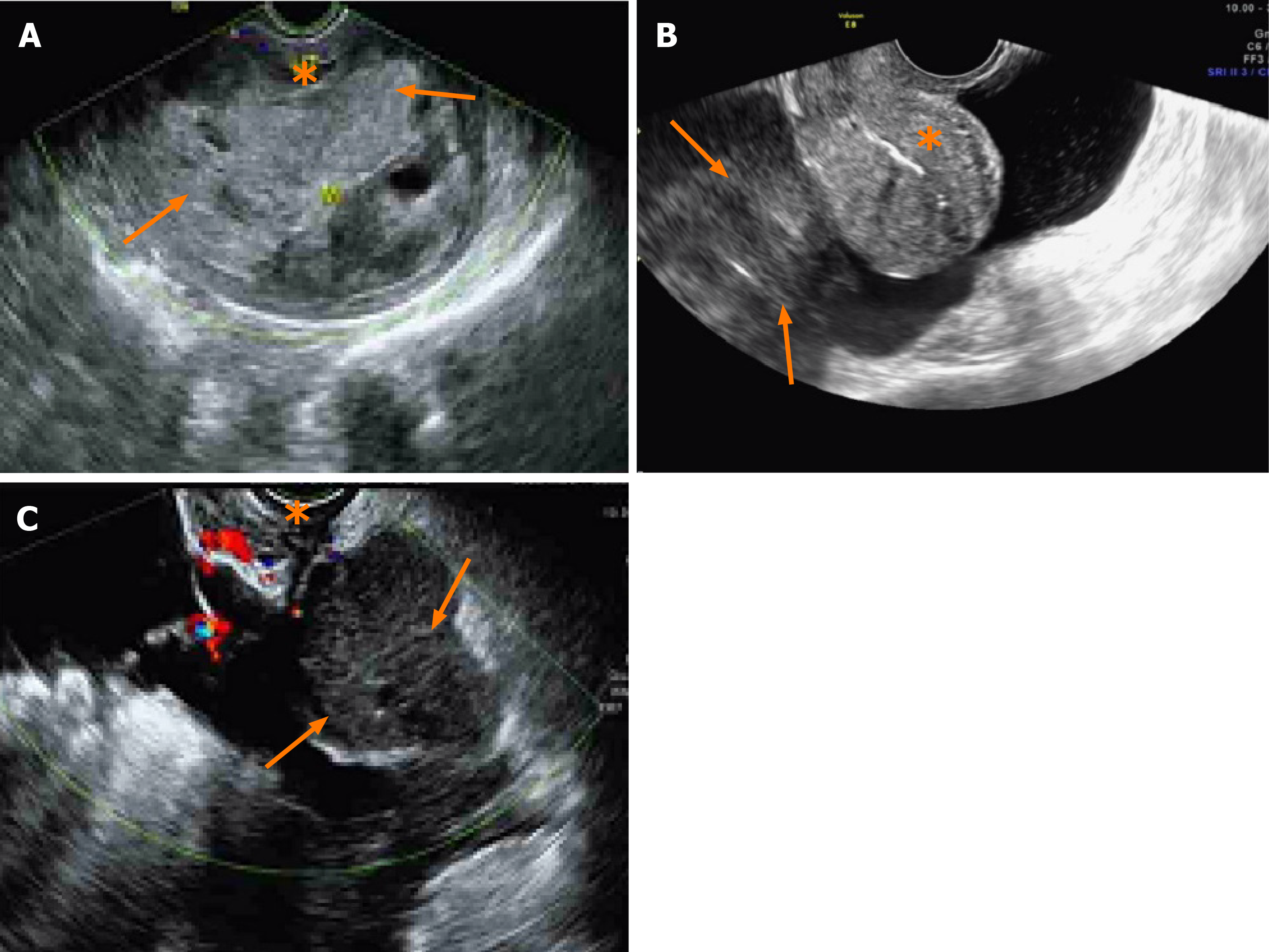
Ultrasonography is the most widely used imaging examination for the screening and postoperative follow-up of patients with leiomyosarcoma. We found that ultrasonography could indicate the location of lesions before operation (Figure 2A and 2B). Additionally, 93.3% of the recurrences (14/15) were detected by ultrasonography, and only one patient experienced pulmonary relapse. Ultrasonography could clearly identify substantive masses in the pelvic cavity (Figures 2C). These observations imply that ultrasonography may serve as a comprehensive examination technique for recurrent pelvic leiomyosarcoma lesions.
Although uterine leiomyosarcoma and non-uterine leiomyosarcoma are ostensibly the same disease, we gathered ample evidence implying the presence of significant differences in terms of clinical features, prognoses, and pathological characteristics. In our retrospective study, we assessed clinical aspects and prognoses of 35 and 16 patients with uterine leiomyosarcoma and non-uterine leiomyosarcoma, respectively, and summarised the ultrasonographic features of primary and recurrent tumour sites. We confirmed that patients with leiomyosarcoma do not necessarily exhibit elevated serum levels of known tumour biomarkers and that medical imaging is useful for identifying the presence of lesions but not their identities. Unlike in other gynaecological cancers[27], pelvic lymph node metastases were rarely observed in our leiomyosarcoma patients.
In terms of treatment, platinum-based chemotherapy accounted for the largest proportion of postoperative adjuvant therapies in both patients with uterine leiomyosarcoma and those with non-uterine leiomyosarcoma. The prognoses of our patients were poor, with no significant differences in progression-free survival and overall survival rates between the uterine leiomyosarcoma and non-uterine leiomyosarcoma groups. However, the univariate and multivariate analyses revealed that FIGO stage was significantly associated with progression-free survival. Because serum tumour biomarkers were found to be unreliable diagnostic features, imaging has a particularly important role for the diagnosis of leiomyosarcoma[28]. Ultrasonography was the most widely used modality, although its accuracy in diagnosing uterine leiomyosarcoma was unsatisfactory because most of such tumours were initially considered leiomyomas. In contrast to uterine leiomyosarcoma, most non-uterine leiomyosarcoma lesions were detected as pelvic masses by ultrasonography. In both uterine leiomyosarcoma and non-uterine leiomyosarcoma patients, however, ultrasonography accurately diagnosed relapse, demonstrating this technique’s potential in monitoring leiomyosarcoma recurrence.
While both uterine leiomyosarcoma and non-uterine leiomyosarcoma are sarcomas, they have varied anatomical locations, clinical characteristics, and gene expression patterns[29]. Our study also found that patients with these two types of leiomyosarcoma had different clinical symptoms because most patients with uterine leiomyosarcoma experienced menorrhagia and menstrual disorder, whereas those with non-uterine leiomyosarcoma more commonly presented with abdominal pain and distention. We found no significant differences in the prognoses of patients with uterine leiomyosarcoma and non-uterine leiomyosarcoma in terms of both progression-free survival and overall survival, consistent with the findings of Farid et al[29] but not with those of Lamm et al[4]. Such discrepancies can be attributed to the rare occurrence of leiomyosarcoma. Consistent with previous studies, we demonstrated that FIGO stage was the most significant factor associated with patient prognoses[12]. Chemotherapy may affect the prognosis of patients[30,31]. Moreover, we found that chemotherapy use was independently associated with poor progression-free survival in the univariate (but not multivariate) analysis.
The differential diagnosis of uterine leiomyoma and leiomyosarcoma remains a challenge in the medical imaging field. Ultrasonography is a reliable tool used to examine the uterus and accurately identify uterine leiomyomas[32]. Exacoustos et al[13] reported that uterine leiomyosarcoma lesions are significantly larger than uterine leiomyomas and have different grayscale sonographic characteristics. Furthermore, colour Doppler and power Doppler vascular patterns could be used as ancillary diagnostic methods to differentiate uterine sarcomas from other tumour types[32]. Our study has some limitations pertaining to its retrospective nature; therefore, prospective studies focusing on the medical imaging-based diagnosis of leiomyosarcoma are required, particularly those investigating the utility of power Doppler and contrast-enhanced ultrasonography.
In conclusion, our study suggests that serum tumour markers are of little value in leiomyosarcoma diagnosis, and ultrasonography cannot accurately differentiate leiomyosarcoma from uterine leiomyoma, although it remains valuable in monitoring recurrence. Our study also showed that FIGO stage is significantly associated with patient outcomes.
Leimyosarcoma of the pelvic cavity heavily affects the health and life of women, but clinical characteristics and relevant diagnosis of the disease are still unclear. It is necessary to carry out clinical research related to pelvic leiomyosarcoma.
Preoperative diagnosis of pelvic leiomyosarcoma is very difficult, and only the pathological examination after operation can provide an accurate diagnosis for this disease. We would like to study the causes affecting diagnosis and factors affecting outcomes of those patients.
This study aimed to identify the outcomes and relevant perioperative evaluation of patients with pelvic leiomyosarcoma.
We used Kaplan-Meier method to determine progression-free survival and overall survival rates. Univariate and multivariate Cox proportional hazards models were used to comprehensively analyse the prognostic factors
The results indicated that serum biomarkers had limited ability in leiomyosarcoma diagnosis and ultrasonography could not accurately differentiate leiomyosarcoma from uterine leiomyoma. However, most of the recurrent lesions could be detected using ultrasonography. FIGO stage was significantly associated with poor progression-free survival in the univariate and multivariate analyses.
Serum tumour markers and ultrasonography cannot accurately diagnose pelvic leiomyosarcoma. Ultrasonography remains valuable in monitoring recurrence. FIGO stage is significantly associated with patient outcomes.
Although accurate preoperative diagnosis of pelvic leiomyosarcoma was very difficult, our results showed that FIGO stage was significantly related to prognosis. Therefore, improving the early diagnosis rate is a promising field in the future.
| 1. | Toro JR, Travis LB, Wu HJ, Zhu K, Fletcher CD, Devesa SS. Incidence patterns of soft tissue sarcomas, regardless of primary site, in the surveillance, epidemiology and end results program, 1978-2001: An analysis of 26,758 cases. Int J Cancer. 2006;119:2922-2930. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 396] [Cited by in RCA: 473] [Article Influence: 23.7] [Reference Citation Analysis (0)] |
| 2. | Ricci S, Stone RL, Fader AN. Uterine leiomyosarcoma: Epidemiology, contemporary treatment strategies and the impact of uterine morcellation. Gynecol Oncol. 2017;145:208-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 140] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 3. | Zivanovic O, Jacks LM, Iasonos A, Leitao MM, Soslow RA, Veras E, Chi DS, Abu-Rustum NR, Barakat RR, Brennan MF, Hensley ML. A nomogram to predict postresection 5-year overall survival for patients with uterine leiomyosarcoma. Cancer. 2012;118:660-669. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 112] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 4. | Lamm W, Natter C, Schur S, Köstler WJ, Reinthaller A, Krainer M, Grimm C, Horvath R, Amann G, Funovics P, Brodowicz T, Polterauer S. Distinctive outcome in patients with non-uterine and uterine leiomyosarcoma. BMC Cancer. 2014;14:981. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 28] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 5. | Miettinen M. Smooth muscle tumors of soft tissue and non-uterine viscera: biology and prognosis. Mod Pathol. 2014;27 Suppl 1:S17-S29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 72] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 6. | Shoushtari AN, Landa J, Kuk D, Sanchez A, Lala B, Schmidt N, Okoli C, Chi P, Dickson MA, Gounder MM, Keohan ML, Crago AM, Tap WD, D'Angelo SP. Overall Survival and Response to Systemic Therapy in Metastatic Extrauterine Leiomyosarcoma. Sarcoma. 2016;2016:3547497. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 7. | Skorstad M, Kent A, Lieng M. Preoperative evaluation in women with uterine leiomyosarcoma. A nationwide cohort study. Acta Obstet Gynecol Scand. 2016;95:1228-1234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 38] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 8. | Karakaya BK, Başer E, Bildacı B, Cömert EÇ, Bayraktar N, Dursun P, Kuşçu E, Ayhan A. Alternative tumor markers in the diagnosis of ovarian cancer. Ginekol Pol. 2016;87:565-769. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 9. | Sölétormos G, Duffy MJ, Othman Abu Hassan S, Verheijen RH, Tholander B, Bast RC, Gaarenstroom KN, Sturgeon CM, Bonfrer JM, Petersen PH, Troonen H, CarloTorre G, Kanty Kulpa J, Tuxen MK, Molina R. Clinical Use of Cancer Biomarkers in Epithelial Ovarian Cancer: Updated Guidelines From the European Group on Tumor Markers. Int J Gynecol Cancer. 2016;26:43-51. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 200] [Cited by in RCA: 177] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 10. | Patsner B, Yim GW. Predictive value of preoperative serum CA-125 levels in patients with uterine cancer: The Asian experience 2000 to 2012. Obstet Gynecol Sci. 2013;56:281-288. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 21] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 11. | Gaetke-Udager K, McLean K, Sciallis AP, Alves T, Maturen KE, Mervak BM, Moore AG, Wasnik AP, Erba J, Davenport MS. Diagnostic Accuracy of Ultrasound, Contrast-enhanced CT, and Conventional MRI for Differentiating Leiomyoma From Leiomyosarcoma. Acad Radiol. 2016;23:1290-1297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 30] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 12. | Park JY, Kim DY, Suh DS, Kim JH, Kim YM, Kim YT, Nam JH. Prognostic factors and treatment outcomes of patients with uterine sarcoma: analysis of 127 patients at a single institution, 1989-2007. J Cancer Res Clin Oncol. 2008;134:1277-1287. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 73] [Cited by in RCA: 60] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 13. | Exacoustos C, Romanini ME, Amadio A, Amoroso C, Szabolcs B, Zupi E, Arduini D. Can gray-scale and color Doppler sonography differentiate between uterine leiomyosarcoma and leiomyoma? J Clin Ultrasound. 2007;35:449-457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 88] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 14. | Sagae S, Yamashita K, Ishioka S, Nishioka Y, Terasawa K, Mori M, Yamashiro K, Kanemoto T, Kudo R. Preoperative diagnosis and treatment results in 106 patients with uterine sarcoma in Hokkaido, Japan. Oncology. 2004;67:33-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 85] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 15. | Tasci T, Karalok A, Taskin S, Ureyen I, Kımyon G, Tulek F, Ozfuttu A, Turan T, Tulunay G, Kose MF, Ortac F. Does Lymphadenectomy Improve Survival in Uterine Leiomyosarcoma? Int J Gynecol Cancer. 2015;25:1031-1036. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 16. | Zhou J, Shan G, Chen Y. The effect of lymphadenectomy on survival and recurrence in patients with ovarian cancer: a systematic review and meta-analysis. Jpn J Clin Oncol. 2016;46:718-726. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 26] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 17. | Garcia C, Kubat JS, Fulton RS, Anthony AT, Combs M, Powell CB, Littell RD. Clinical outcomes and prognostic markers in uterine leiomyosarcoma: a population-based cohort. Int J Gynecol Cancer. 2015;25:622-628. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 25] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 18. | Rauh-Hain JA, Hinchcliff EM, Oduyebo T, Worley MJ, Andrade CA, Schorge JO, George S, Muto MG, del Carmen MG. Clinical outcomes of women with recurrent or persistent uterine leiomyosarcoma. Int J Gynecol Cancer. 2014;24:1434-1440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 19. | Lusby K, Savannah KB, Demicco EG, Zhang Y, Ghadimi MP, Young ED, Colombo C, Lam R, Dogan TE, Hornick JL, Lazar AJ, Hunt KK, Anderson ML, Creighton CJ, Lev D, Pollock RE. Uterine leiomyosarcoma management, outcome, and associated molecular biomarkers: a single institution's experience. Ann Surg Oncol. 2013;20:2364-2372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 59] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 20. | Foley OW, Rauh-Hain JA, Clemmer J, Clark RM, Hall T, Diver EJ, Schorge JO, del Carmen MG. Trends in the treatment of uterine leiomyosarcoma in the Medicare population. Int J Gynecol Cancer. 2015;25:453-458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 21. | Abeler VM, Røyne O, Thoresen S, Danielsen HE, Nesland JM, Kristensen GB. Uterine sarcomas in Norway. A histopathological and prognostic survey of a total population from 1970 to 2000 including 419 patients. Histopathology. 2009;54:355-364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 282] [Cited by in RCA: 296] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 22. | Oosten AW, Seynaeve C, Schmitz PI, den Bakker MA, Verweij J, Sleijfer S. Outcomes of first-line chemotherapy in patients with advanced or metastatic leiomyosarcoma of uterine and non-uterine origin. Sarcoma. 2009;2009:348910. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 32] [Article Influence: 1.9] [Reference Citation Analysis (1)] |
| 23. | Prat J. FIGO staging for uterine sarcomas. Int J Gynaecol Obstet. 2009;104:177-178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 245] [Cited by in RCA: 247] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 24. | Dolscheid-Pommerich RC, Keyver-Paik M, Hecking T, Kuhn W, Hartmann G, Stoffel-Wagner B, Holdenrieder S. Clinical performance of LOCI™-based tumor marker assays for tumor markers CA 15-3, CA 125, CEA, CA 19-9 and AFP in gynecological cancers. Tumour Biol. 2017;39:1010428317730246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 19] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 25. | Bian J, Sun X, Li B, Ming L. Clinical Significance of Serum HE4, CA125, CA724, and CA19-9 in Patients with Endometrial Cancer. Technol Cancer Res Treat. 2017;16:435-439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 48] [Article Influence: 4.8] [Reference Citation Analysis (1)] |
| 26. | Terek MC, Akman L, Hursitoglu BS, Sanli UA, Ozsaran Z, Tekindal MA, Dikmen Y, Zekioglu O, Ozsaran AA. The retrospective analysis of patients with uterine sarcomas: A single-center experience. J Cancer Res Ther. 2016;12:309-313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 27. | Matsuo K, Grubbs BH, Mikami M. Quality and quantity metrics of pelvic lymph node metastasis and risk of para-aortic lymph node metastasis in stage IB-IIB cervical cancer. J Gynecol Oncol. 2018;29:e10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 28. | Thomakos N, Rodolakis A, Zagouri F, Zacharakis D, Sotiropoulou M, Akrivos N, Haidopoulos D, Papadimitriou CA, Dimopoulos MA, Antsaklis A. Serum CA 125, CA 15-3, CEA, and CA 19-9: a prognostic factor for uterine carcinosarcomas? Arch Gynecol Obstet. 2013;287:97-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 29. | Farid M, Ong WS, Tan MH, Foo LS, Lim YK, Chia WK, Soh LT, Poon D, Lee MJ, Ho ZC, Jeevan R, Chin F, Teo M, Quek R. The influence of primary site on outcomes in leiomyosarcoma: a review of clinicopathologic differences between uterine and extrauterine disease. Am J Clin Oncol. 2013;36:368-374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 31] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 30. | Littell RD, Tucker LY, Raine-Bennett T, Palen TE, Zaritsky E, Neugebauer R, Embry-Schubert J, Lentz SE. Adjuvant gemcitabine-docetaxel chemotherapy for stage I uterine leiomyosarcoma: Trends and survival outcomes. Gynecol Oncol. 2017;147:11-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 35] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 31. | Bogani G, Fucà G, Maltese G, Ditto A, Martinelli F, Signorelli M, Chiappa V, Scaffa C, Sabatucci I, Lecce F, Raspagliesi F, Lorusso D. Efficacy of adjuvant chemotherapy in early stage uterine leiomyosarcoma: A systematic review and meta-analysis. Gynecol Oncol. 2016;143:443-447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 45] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 32. | Fascilla FD, Cramarossa P, Cannone R, Olivieri C, Vimercati A, Exacoustos C. Ultrasound diagnosis of uterine myomas. Minerva Ginecol. 2016;68:297-312. [PubMed] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See:
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ueda H S-Editor: Zhang L L-Editor: Wang TQ E-Editor: Liu MY