Published online Jan 6, 2020. doi: 10.12998/wjcc.v8.i1.88
Peer-review started: October 17, 2019
First decision: November 9, 2019
Revised: November 27, 2019
Accepted: December 22, 2019
Article in press: December 22, 2019
Published online: January 6, 2020
Processing time: 81 Days and 21.6 Hours
Other than surgery, endoscopic ultrasound-guided fine-needle aspiration (EUS-FNA) is the only procedure for histologically diagnosing autoimmune pancreatitis (AIP). However, adequate specimens are difficult to obtain. Recently, more adequate specimens were reported to be obtained with EUS-FNA with a wet suction technique (WEST) than with conventional EUS-FNA.
To histologically diagnose AIP by EUS-FNA with a WEST.
Eleven patients with possible type 1 AIP between February 2016 and August 2018 underwent EUS-FNA with a WEST (WEST group), with four punctures by 19 or 22 G needles. As a historical control, 23 type 1 AIP patients who underwent no fewer than four punctures with 19 or 22 G needles were enrolled (DRY group). Patient characteristics and histological findings were compared between the two groups.
Three histopathological factors according to the International Consensus Diagnostic Criteria were significantly greater in the WEST group than the DRY group [lymphoplasmacytic infiltrate without granulocytic infiltration: 9 (81.8%) vs 6 (26.1%), P = 0.003, storiform fibrosis: 5 (45.5%) vs 1 (4.3%), P = 0.008, abundant (> 10 cells/HPF) IgG4-positive cells: 7 (63.6%) vs 5 (21.7%), P = 0.026]. Level 1 or level 2 histopathological findings were observed more often in the WEST group than in the DRY group [8 (72.7%) vs 3 (13.0%), P = 0.001].
EUS-FNA with a WEST was more successful than standard EUS-FNA in histologically diagnosing AIP.
Core tip: Recently, more adequate specimens were reported to be obtained with endoscopic ultrasound-guided fine-needle aspiration (EUS-FNA) with a wet suction technique (WEST) than with the conventional method (DRY) of EUS-FNA. This study aimed to histologically diagnose autoimmune pancreatitis (AIP) by EUS-FNA with a WEST. Patient characteristics and histological findings were compared between the WEST group and the DRY group. Level 1 or level 2 histopathological findings were observed more often in the WEST group than in the DRY group. EUS-FNA with a WEST was more useful than standard EUS-FNA for histologically diagnosing AIP.
- Citation: Sugimoto M, Takagi T, Suzuki R, Konno N, Asama H, Sato Y, Irie H, Watanabe K, Nakamura J, Kikuchi H, Takasumi M, Hashimoto M, Kato T, Hikichi T, Notohara K, Ohira H. Can the wet suction technique change the efficacy of endoscopic ultrasound-guided fine-needle aspiration for diagnosing autoimmune pancreatitis type 1? A prospective single-arm study. World J Clin Cases 2020; 8(1): 88-96
- URL: https://www.wjgnet.com/2307-8960/full/v8/i1/88.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v8.i1.88
Autoimmune pancreatitis (AIP) was determined by Yoshida et al[1]. Some autoimmune pathogeneses caused pancreatitis with pancreatic enlargement, pancreatic duct narrowing, or invasion of lymphocytes with fibrosis. Hamano et al[2] described elevated serum IgG4 in AIP patients. The 2010 International Consensus Diagnostic Criteria (ICDC) for AIP defined pancreatitis with other organ involvement and elevated serum IgG4 as “type 1”; lymphoplasmacytic sclerosing pancreatitis (LPSP) was the most prominent histological characteristic[3]. Four items were mentioned as being significant to histologically diagnose LPSP. These items are periductal lymphoplasmacytic infiltrate without granulocytic infiltration, obliterative phlebitis, storiform fibrosis, and abundant (> 10 cells/HPF) IgG4-positive cells. If three of those four items are observed, that is defined as level 1 histological findings. If two items are observed, it is defined as level 2 histological findings.
AIP can be diagnosed by imaging characteristics and elevated serum IgG4 or by other criteria. Nevertheless, level 1 pancreatic histological findings of the ICDC are necessary to histologically diagnose AIP type 1. Other than surgical biopsy, endoscopic ultrasound-guided fine needle aspiration (EUS-FNA) is the only procedure that can histologically diagnose AIP. However, it is very hard to acquire a sufficient specimen via EUS-FNA[4-6]. The ICDC recommended using EUS-FNA for ruling out malignancy before a diagnostic steroid administration[3]. In 2017, Sugimoto et al[7] reported that the clinical characteristics of AIP differed from those of pancreatic cancer and that malignancy was ruled out by EUS-FNA in AIP patients.
However, recently, with a wet suction technique (WEST) of EUS-FNA, more adequate specimens were reported to be obtained than by the conventional method of EUS-FNA[8]. Therefore, we hypothesized that more AIP patients could be histopathologically diagnosed by WEST EUS-FNA. The aim of our study is to clarify the efficacy of EUS-FNA with a WEST for diagnosing AIP type 1.
This study was a single arm prospective study intended to clarify the efficacy of WEST EUS-FNA for diagnosing type 1 AIP. This study was performed at Fukushima Medical University. The Institutional Review Board of Fukushima Medical University approved this study. All patients agreed to participate in this study. This trial was registered in UMIN (ID: 000019768).
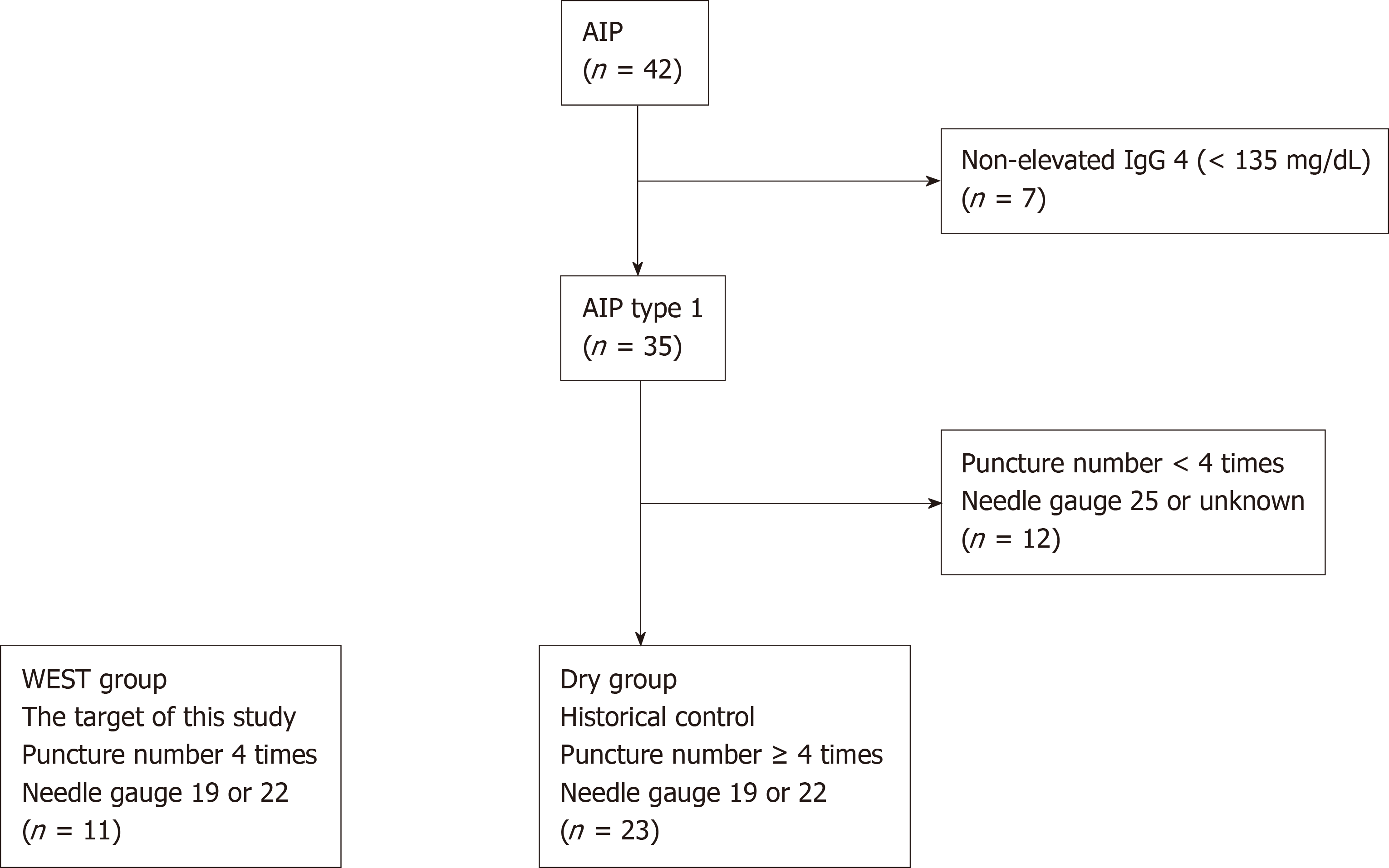
We recruited 11 patients who were suspected to have type 1 AIP at Fukushima Medical University between February 2016 and August 2018. They showed diffuse or focal pancreatic swelling on abdominal enhanced computed tomography with elevated serum IgG4 levels (> 135 mg/dL). To prove the efficacy of WEST EUS-FNA for diagnosing AIP, historical controls were used. The historical controls included 42 AIP patients who were diagnosed at Fukushima Medical University between July 2003 and July 2018 before the initiation of this study. Among these 42 patients, 35 had elevated IgG4 levels (≥ 135 mg/dL). In this study, WEST EUS-FNA was performed with four punctures using 19 or 22 G needles. The gauge of needle used during EUS-FNA was randomly chosen by each endoscopist. Therefore, 23 type 1 AIP patients who underwent procedures with no fewer than four punctures with 19 or 22 G needles were selected as the control group (Figure 1). The targets of this study were termed the WEST group. On the other hand, historical controls were termed the DRY group. The targets and historical controls were all diagnosed with type 1 AIP according to the ICDC.
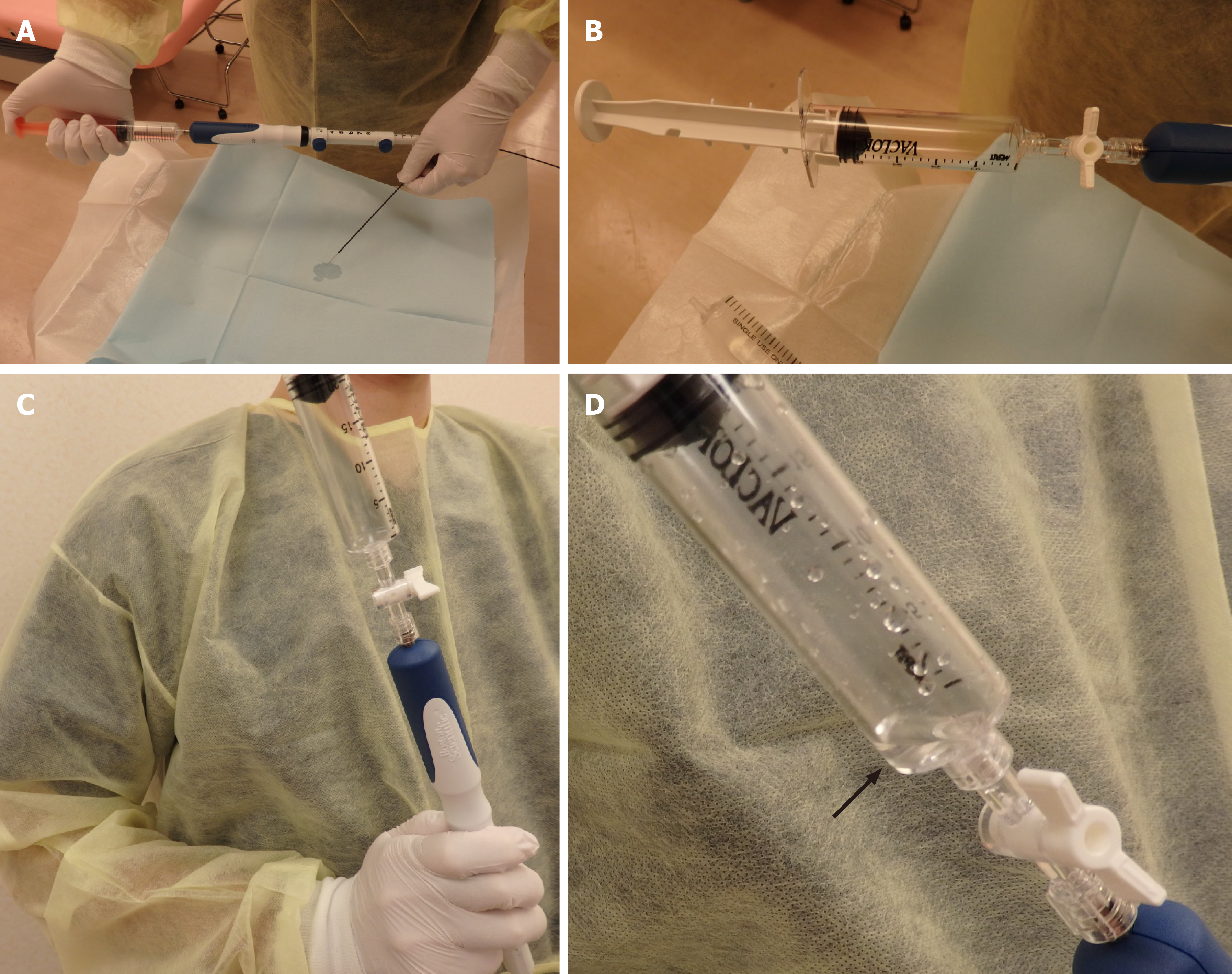
All procedures were performed under the guidance of a professional endoscopist who was well versed in EUS-FNA (TT) and who had performed no less than 1500 EUS-FNA procedures. After a patient was well sedated with intravenous midazolam, an echoendoscope was inserted. The swollen pancreas was viewed on the monitor, and the vessels along the puncture line were confirmed by color doppler echo imaging. Then, the puncture needle was prepared for WEST. The EUS-FNA with a WEST was performed according to the methods described in a previous report by Attam et al[8]. First, the stylet was removed from the needle, and saline solution was injected until the needle was filled (Figure 2A). After a suction syringe was loaded to 20 mL of suction in a locked position, the syringe was set at the edge of the needle without an extension tube (Figure 2B). The needle was inserted into the target lesion, and the lock of the suction syringe was opened. Saline solution flowed into the suction syringe because of the negative pressure (Figures 2C and D), and the needle was moved back and forth 20 times per puncture. The punctures were performed 4 times according to the instructions in the past report by Suzuki et al[9]. In patients with focal pancreatic swelling, samples were obtained from the swollen tissue. In patients with diffuse pancreatic swelling, samples were obtained at the farthest point from the puncture. If the target was the pancreatic head, a transduodenal puncture was performed. On the other hand, if the target was a pancreatic body or tail, the transgastric approach was performed.
In the WEST group, the echoendoscope used was GF-UCT260 or GF-UC240AL-5 (Olympus Medical Systems, Tokyo, Japan). The ultrasonography apparatus was EU-ME2 (Olympus Medical Systems). The biopsy needles were Expect 22 or 19 G (Boston Scientific, MA, United States). The biopsy needles were randomly selected by the endoscopists.
In the DRY group, the echoendoscope used was GF-UCT260, GF-UCT240AL-5, or GF-UC240AL-5 (Olympus Medical Systems, Tokyo, Japan). SSD5000 (ALOKA, Tokyo, Japan), EU-ME2, or EU-ME1 (Olympus Medical Systems) was used as the ultrasonography apparatus. The biopsy needles were Expect 22 or 19 G (Boston Scientific, MA, United States); EZ Shot 22 G or NA11J-KB (Olympus Medical System); or EchoTip 19, 22, EchoTip ProCore 19 G, or Quick-Core 19 G (Cook Medical Inc., NC, United States).
The primary endpoint was the level 1 or level 2 histopathological finding according to the ICDC[3]. Biopsy of the pancreatic duct by EUS-FNA is difficult; therefore, an item on the ICDC such as “periductal lymphoplasmacytic infiltrate without granulocytic infiltration” was replaced with “Lymphoplasmacytic infiltrate without granulocytic infiltration” according to the Clinical Diagnostic Criteria for AIP 2011 of Japan[10]. The secondary outcomes were each of the four items involved in the ICDC histopathological findings, namely, lymphoplasmacytic infiltrate without granulocytic infiltration, obliterative phlebitis, storiform fibrosis, and abundant (> 10 cells/HPF) IgG4-positive cells). All pathological diagnoses were performed by more than two pathologists.
Patient characteristics (age, sex, type of pancreatic swelling, serum level of IgG4), items related to EUS-FNA (19 or 22 G needle, number of needle passes, histology, or adverse events), primary outcome and secondary outcomes were compared between the WEST group and the DRY group.
The results of conventional EUS-FNA for AIP type 1 patients in our hospital indicated that level 1 or level 2 histopathological findings were observed in 13% (3/23) of patients. The results of WEST EUS-FNA were expected to be 60%, which was better than a previous multicenter study[11]. Eleven patients were needed in this study for an α error of 0.05 and statistical power of 0.8.
Age was compared by Student’s t test. Serum IgG4 level and the number of needle passes were compared using the Mann-Whitney U test. Nominal variables were compared by Fisher’s exact test. A P value < 0.05 was defined as statistically significant. All statistical analyses were performed using EZR (Saitama Medical Centre, Jichi Medical University, Saitama, Japan), which is a graphical user interface for R (The R Foundation for Statistical Computing, Vienna, Austria)[12].
No significant differences were observed in patient characteristics between the WEST and DRY groups except for sex (Table 1). Females were significantly more common in the WEST group than in the DRY group (male/female, WEST group 6/5, DRY group 21/2, P = 0.024).
| WEST group (n = 11) | DRY group (n = 23) | P value | |
| Age (yr, means ± SD) | 62.9 ± 12.4 | 61.0 ± 9.6 | 0.626 |
| Sex, male/female | 6/5 | 21/2 | 0.024 |
| Pancreatic swelling type, diffuse/focal | 6/5 | 11/12 | 1.0 |
| Serum IgG4 level [mg/dL, median (range)] | 568 (177-2100) | 447 (149-1480) | 0.663 |
Regarding the comparison of procedures and results related to EUS-FNA, several items were significantly different between the two groups (Table 2). The number of needle passes was significantly lower in the WEST group than in the DRY group [median (range), 4 (4-4) vs 5 (4-9), P < 0.001]. In terms of the histopathological findings according to the ICDC, three items were observed to be significantly greater in the WEST group than in the DRY group [lymphoplasmacytic infiltrate without granulocytic infiltration: 9 (81.8%) vs 6 (26.1%), P = 0.003, storiform fibrosis: 5 (45.5%) vs 1 (4.3%), P = 0.008, abundant (> 10 cells/HPF) IgG4-positive cells: 7 (63.6%) vs 5 (21.7%), P = 0.026]. Level 1 histopathological findings were observed more in the WEST group than in the DRY group [4 (36.4%) vs 1 (4.3%), P = 0.029]. Level 1 or level 2 histopathological findings were observed more often in the WEST group than in the DRY group [8 (72.7%) vs 3 (13.0%), P = 0.001]. Adverse events were not observed in either group.
| WEST group (n = 11) | DRY group (n = 23) | P value | |
| EUS-FNA needle (19 G/22 G) | 1/10 | 8/15 | 0.214 |
| Number of needle passes [median (range)] | 4 (4-4) | 5 (4-9) | < 0.001 |
| Histopathological findings | |||
| Lymphoplasmacytic infiltrate without granulocytic infiltration | 9 (81.8) | 6 (26.1) | 0.003 |
| Obliterative phlebitis | 2 (18.2) | 0 (0) | 0.098 |
| Storiform fibrosis | 5 (45.5) | 1 (4.3) | 0.008 |
| Abundant (> 10 cells/HPF) IgG4 positive cells | 7 (63.6) | 5 (21.7) | 0.026 |
| Level 1 histopathological findings | 4 (36.4) | 1 (4.3) | 0.029 |
| Level 1 or level 2 histopathological findings | 8 (72.7) | 3 (13.0) | 0.001 |
| Adverse events | 0 (0) | 0 (0) | |
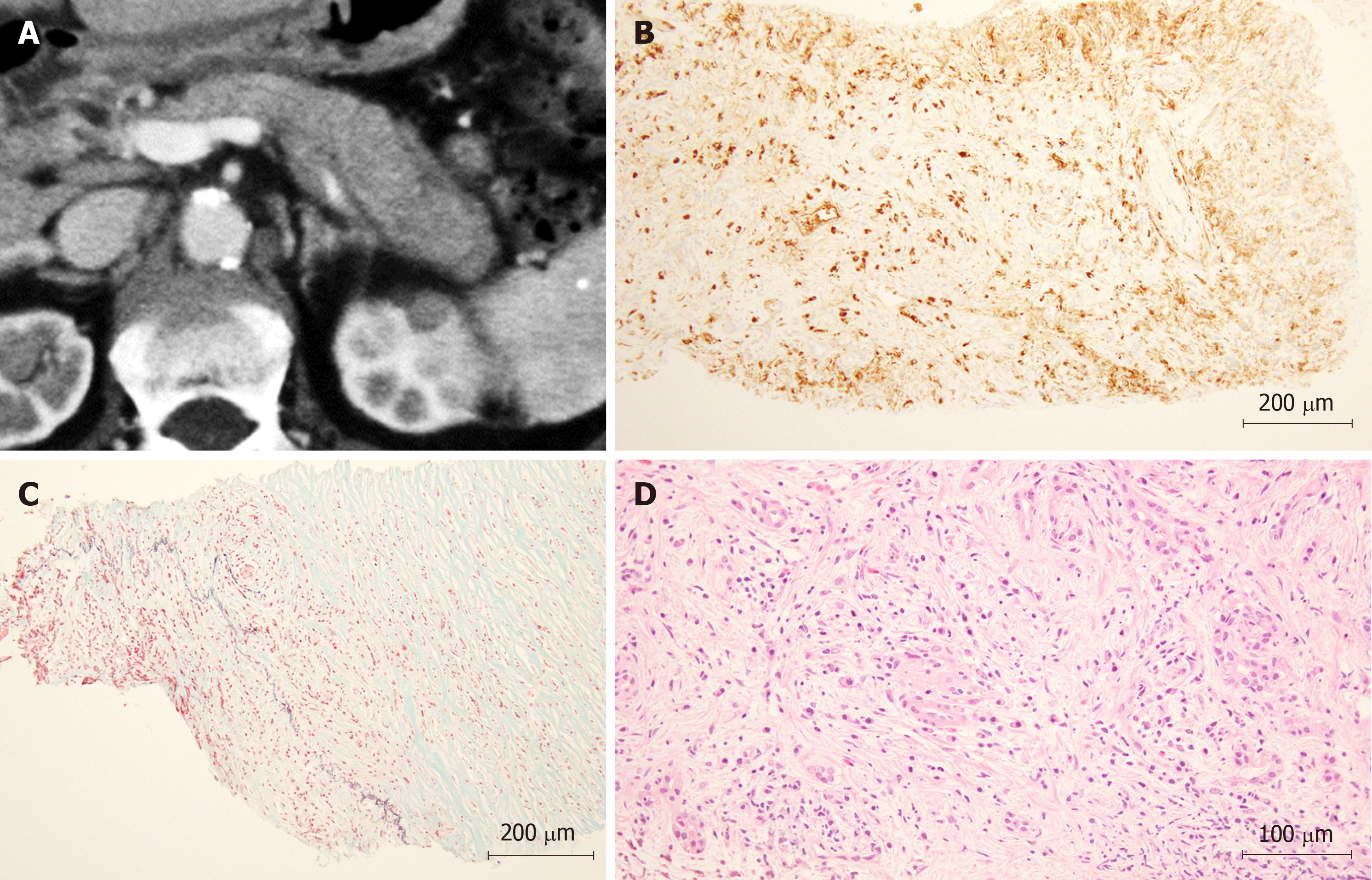
In Figure 3, a representative case of a patient diagnosed with AIP by WEST EUS-FNA is shown.
In this study, we investigated the efficacy of WEST EUS-FNA for diagnosing AIP. Three histopathological items according to the ICDC were observed more frequently in the WEST group than in the DRY group. Level 1 histopathological findings and level 2 histopathological findings were observed more in the WEST group than in the DRY group.
Regarding EUS-FNA for AIP, the results of procedures performed with standard needles and more complex needles were reported. Regarding previous reports using standard needles, Iwashita et al[13] reported that 17 of 44 AIP patients could be diagnosed by EUS-FNA with a 19 G needle. The reports using a 22 G needle are described below. Ishikawa et al[4] diagnosed LPSP in 9 of 47 AIP patients. Imai et al[5] could not histopathologically diagnose AIP. Interestingly, a multicenter study found that the diagnosability of AIP by EUS-FNA was poor[6], and another multicenter study reported that 57.7% of patients were diagnosed with level 2 or higher based on the histopathological findings[14].
Second, some previous reports involved more complex needles. Mizuno et al[15] reported that EUS Tru-Cut biopsy (EUS-TCB) resulted in a diagnosis of LPSP in 45.5% of cases. In a report by Kanno et al[11], EUS-FNA using 22 G automated spring-loaded PowerShot needles resulted in a diagnoses of ICDC level 1 and 2 histological findings in 56% and 24% of AIP cases. The histopathological diagnosability of EUS-FNA for AIP was improved by needle choice; however, it was difficult to statistically compare the sensitivity and accuracy between the standard needles and more complex needles.
On the other hand, the histopathological accuracy by WEST EUS-FNA was statistically superior to that by the standard EUS-FNA method or EUS-TCB in this study (seven historical control cases underwent EUS-TCB). Moreover, the positive result of this study was achieved with standard needles. Though the reasons underlying the increased size of the specimens collected by WEST are not clear, it is thought that the saline solution coating the lining of the needle leads to better transmission of the applied suction or that the saline solution acts as a stylet, reducing the contamination from GI tissue[8].
In recent reports, EUS-FNA using a 22 G SharkCore needle was effective for histologically diagnosing AIP[16,17]. By the development of puncture methods such as WEST or special puncture needles, the diagnosability of AIP by EUS-FNA will be improved.
Some limitations exist in this study. First, a small number of patients at a single institution were involved in this study. Second, historical controls were used as a control group. In the future, a multicenter randomized controlled trial (RCT) with more cases is needed. However, AIP patients are rare, rending an RCT difficult to conduct. Third, we used EUS-TCB needles such as Quick-Core 19 G or ProCore 19 G (Cook Medical Inc., NC, United States) in the historical control patients. In past reports, these needles were useful for obtaining more specimens[18,19]. However, these needles were not used in the WEST group. Therefore, they were not a factor in the superiority of the WEST technique.
In conclusion, WEST EUS-FNA was more useful for histologically diagnosing AIP than was standard EUS-FNA.
Apart from surgical biopsy, endoscopic ultrasound-guided fine needle aspiration (EUS-FNA) is the only method used to histologically diagnose autoimmune pancreatitis (AIP) type 1. Nevertheless, it is very difficult to obtain an adequate specimen by EUS-FNA for a histological diagnosis of AIP type 1. However, recently, with EUS-FNA with a wet suction technique (WEST), adequate specimens were reported to be obtained more often than those obtained by conventional EUS-FNA.
EUS-FNA with a WEST could overcome the difficulty of histologically diagnosing AIP type 1.
To investigate the efficacy of EUS-FNA with a WEST for diagnosing AIP type 1.
Eleven AIP type 1 patients were recruited for this study and underwent EUS-FNA with a WEST for histological diagnosis. The histological diagnosability was compared between the WEST group and a historical control group that included AIP type 1 patients who underwent conventional EUS-FNA before this study (DRY group).
Three histopathological features [lymphoplasmacytic infiltrate without granulocytic infiltration, storiform fibrosis, and abundant (>10 cells/HPF) IgG4-positive cells] according to the International Consensus Diagnostic Criteria were significantly greater in the WEST group than in the DRY group. Level 1 or level 2 histopathological findings were observed more often in the WEST group than in the DRY group.
EUS-FNA with a WEST was more useful than standard EUS-FNA in the histological diagnosis of AIP.
The histological diagnosability of EUS-FNA for AIP will be developed by the improvement of puncture needles and puncture methods, such as WEST.
We thank all the staff at the Department of Gastroenterology of Fukushima Medical University, the Department of Endoscopy of Fukushima Medical University Hospital, and the gastroenterology ward of Fukushima Medical University Hospital.
| 1. | Yoshida K, Toki F, Takeuchi T, Watanabe S, Shiratori K, Hayashi N. Chronic pancreatitis caused by an autoimmune abnormality. Proposal of the concept of autoimmune pancreatitis. Dig Dis Sci. 1995;40:1561-1568. [PubMed] |
| 2. | Hamano H, Kawa S, Horiuchi A, Unno H, Furuya N, Akamatsu T, Fukushima M, Nikaido T, Nakayama K, Usuda N, Kiyosawa K. High serum IgG4 concentrations in patients with sclerosing pancreatitis. N Engl J Med. 2001;344:732-738. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2026] [Cited by in RCA: 1897] [Article Influence: 75.9] [Reference Citation Analysis (0)] |
| 3. | Shimosegawa T, Chari ST, Frulloni L, Kamisawa T, Kawa S, Mino-Kenudson M, Kim MH, Klöppel G, Lerch MM, Löhr M, Notohara K, Okazaki K, Schneider A, Zhang L; International Association of Pancreatology. International consensus diagnostic criteria for autoimmune pancreatitis: guidelines of the International Association of Pancreatology. Pancreas. 2011;40:352-358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1050] [Cited by in RCA: 1085] [Article Influence: 72.3] [Reference Citation Analysis (0)] |
| 4. | Ishikawa T, Itoh A, Kawashima H, Ohno E, Matsubara H, Itoh Y, Nakamura Y, Hiramatsu T, Nakamura M, Miyahara R, Ohmiya N, Goto H, Hirooka Y. Endoscopic ultrasound-guided fine needle aspiration in the differentiation of type 1 and type 2 autoimmune pancreatitis. World J Gastroenterol. 2012;18:3883-3888. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 66] [Cited by in RCA: 52] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 5. | Imai K, Matsubayashi H, Fukutomi A, Uesaka K, Sasaki K, Ono H. Endoscopic ultrasonography-guided fine needle aspiration biopsy using 22-gauge needle in diagnosis of autoimmune pancreatitis. Dig Liver Dis. 2011;43:869-874. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 61] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 6. | Morishima T, Kawashima H, Ohno E, Yamamura T, Funasaka K, Nakamura M, Miyahara R, Watanabe O, Ishigami M, Shimoyama Y, Nakamura S, Hashimoto S, Goto H, Hirooka Y. Prospective multicenter study on the usefulness of EUS-guided FNA biopsy for the diagnosis of autoimmune pancreatitis. Gastrointest Endosc. 2016;84:241-248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 75] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 7. | Sugimoto M, Takagi T, Suzuki R, Konno N, Asama H, Watanabe K, Nakamura J, Kikuchi H, Waragai Y, Takasumi M, Sato Y, Hikichi T, Ohira H. Endoscopic Ultrasonography-Guided Fine Needle Aspiration Can Be Used to Rule Out Malignancy in Autoimmune Pancreatitis Patients. J Ultrasound Med. 2017;36:2237-2244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 8. | Attam R, Arain MA, Bloechl SJ, Trikudanathan G, Munigala S, Bakman Y, Singh M, Wallace T, Henderson JB, Catalano MF, Guda NM. "Wet suction technique (WEST)": a novel way to enhance the quality of EUS-FNA aspirate. Results of a prospective, single-blind, randomized, controlled trial using a 22-gauge needle for EUS-FNA of solid lesions. Gastrointest Endosc. 2015;81:1401-1407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 96] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 9. | Suzuki R, Irisawa A, Bhutani MS, Hikichi T, Takagi T, Sato A, Sato M, Ikeda T, Watanabe K, Nakamura J, Tasaki K, Obara K, Ohira H. Prospective evaluation of the optimal number of 25-gauge needle passes for endoscopic ultrasound-guided fine-needle aspiration biopsy of solid pancreatic lesions in the absence of an onsite cytopathologist. Dig Endosc. 2012;24:452-456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 43] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 10. | Naitoh I, Nakazawa T, Hayashi K, Miyabe K, Shimizu S, Kondo H, Yoshida M, Yamashita H, Umemura S, Hori Y, Ohara H, Joh T. Clinical evaluation of international consensus diagnostic criteria for type 1 autoimmune pancreatitis in comparison with Japanese diagnostic criteria 2011. Pancreas. 2013;42:1238-1244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 11. | Kanno A, Masamune A, Fujishima F, Iwashita T, Kodama Y, Katanuma A, Ohara H, Kitano M, Inoue H, Itoi T, Mizuno N, Miyakawa H, Mikata R, Irisawa A, Sato S, Notohara K, Shimosegawa T. Diagnosis of autoimmune pancreatitis by EUS-guided FNA using a 22-gauge needle: a prospective multicenter study. Gastrointest Endosc. 2016;84:797-804.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 95] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 12. | Kanda Y. Investigation of the freely available easy-to-use software 'EZR' for medical statistics. Bone Marrow Transplant. 2013;48:452-458. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9275] [Cited by in RCA: 14533] [Article Influence: 1117.9] [Reference Citation Analysis (0)] |
| 13. | Iwashita T, Yasuda I, Doi S, Ando N, Nakashima M, Adachi S, Hirose Y, Mukai T, Iwata K, Tomita E, Itoi T, Moriwaki H. Use of samples from endoscopic ultrasound-guided 19-gauge fine-needle aspiration in diagnosis of autoimmune pancreatitis. Clin Gastroenterol Hepatol. 2012;10:316-322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 120] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 14. | Kanno A, Ishida K, Hamada S, Fujishima F, Unno J, Kume K, Kikuta K, Hirota M, Masamune A, Satoh K, Notohara K, Shimosegawa T. Diagnosis of autoimmune pancreatitis by EUS-FNA by using a 22-gauge needle based on the International Consensus Diagnostic Criteria. Gastrointest Endosc. 2012;76:594-602. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 99] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 15. | Mizuno N, Bhatia V, Hosoda W, Sawaki A, Hoki N, Hara K, Takagi T, Ko SB, Yatabe Y, Goto H, Yamao K. Histological diagnosis of autoimmune pancreatitis using EUS-guided trucut biopsy: a comparison study with EUS-FNA. J Gastroenterol. 2009;44:742-750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 132] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 16. | Runge TM, Hart PA, Sasatomi E, Baron TH. Diagnosis of autoimmune pancreatitis using new, flexible EUS core biopsy needles: report of 2 cases. Gastrointest Endosc. 2017;85:1311-1312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 7] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 17. | Bhattacharya A, Cruise M, Chahal P. Endoscopic ultrasound guided 22 gauge core needle biopsy for the diagnosis of Autoimmune pancreatitis. Pancreatology. 2018;18:168-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 18. | Thomas T, Kaye PV, Ragunath K, Aithal G. Efficacy, safety, and predictive factors for a positive yield of EUS-guided Trucut biopsy: a large tertiary referral center experience. Am J Gastroenterol. 2009;104:584-591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 80] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 19. | Mukai S, Itoi T, Yamaguchi H, Sofuni A, Tsuchiya T, Tanaka R, Tonozuka R, Honjo M, Fujita M, Yamamoto K, Matsunami Y, Asai Y, Kurosawa T, Nagakawa Y. A retrospective histological comparison of EUS-guided fine-needle biopsy using a novel franseen needle and a conventional end-cut type needle. Endosc Ultrasound. 2019;8:50-57. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 67] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See:
Manuscript source: Invited manuscript
Specialty type: Medicine, Research and Experimental
Country of origin: Japan
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Aseni P, Löhr JM, Vitali F S-Editor: Dou Y L-Editor: A E-Editor: Liu MY