Published online Mar 6, 2019. doi: 10.12998/wjcc.v7.i5.656
Peer-review started: January 22, 2019
First decision: January 26, 2019
Revised: February 1, 2019
Accepted: February 18, 2019
Article in press: February 18, 2019
Published online: March 6, 2019
Processing time: 46 Days and 18.1 Hours
Paraganglioma/pheochromocytoma and medullary thyroid carcinoma can coexist and are often found in multiple endocrine neoplasia (MEN). However, very few cases highlight papillary thyroid carcinoma. We present herein a rare case of head and neck paraganglioma associated with papillary thyroid carcinoma.
A 51-year-old man presented to our department with right-sided neck swelling and hypertension. Physical examination showed neck masses with obvious pulsation. Concentrations of serum calcium, phosphorus, parathormone, thyroid stimulating hormone, free thyroxine, and calcitonin were within normal limits. Enhanced computed tomography revealed an irregular solid nodule, located in the carotid artery bifurcation. A low-density nodule of the thyroid isthmus with a spot-like dense shadow was also detected. The diagnosis of carotid body tumor was raised and an ultrasound-guided fine needle aspiration biopsy of the thyroid nodule revealed papillary thyroid carcinoma. The patient underwent surgery for lesion excision, total thyroidectomy, and neck dissection, and the pathology was reported as paraganglioma and papillary carcinoma. Genetic studies showed negative results for germline mutation of succinate dehydrogenase subunit D on 11q23. He was treated with 131I after surgery and remained disease-free so far.
The presence of concomitant paraganglioma and thyroid papillary carcinoma could be either coincidental or a result of an unknown mutation.
Core tip: The presence of concomitant paraganglioma and thyroid papillary carcinoma is extremely rare. We present a case of a head and neck paraganglioma associated with papillary thyroid carcinoma in a 51-year-old man. The major characteristics and imaging features of the lesion are discussed.
- Citation: Lin B, Yang HY, Yang HJ, Shen SY. Concomitant paraganglioma and thyroid carcinoma: A case report. World J Clin Cases 2019; 7(5): 656-662
- URL: https://www.wjgnet.com/2307-8960/full/v7/i5/656.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v7.i5.656
Carotid body tumors represent approximately 65% of head and neck paragangliomas, followed by glomus jugulare and glomus tympanicum tumors[1].
A paraganglioma can partly or wholly be associated with other tumors such as kidney cancer, parathyroid adenoma, thyroid carcinoma, gastrointestinal stromal tumors, and astrocytoma[2].
Coexistence of paraganglioma/pheochromocytoma (PHEO) and medullary thyroid carcinoma (MTC) is strongly suggestive of multiple endocrine neoplasia (MEN), in such cases, succinate dehydrogenase subunit B (SDHB) and subunit D (SDHD) mutation was frequently reported as positive[3,4].
Herein, we report a patient with a combination of paraganglioma and papillary thyroid carcinoma. The tumors were surgically removed with minimal blood loss and temporary neurological loss. An analysis of RET porto-oncogene mutation yielded negative results. To our best knowledge, this unusual association of the two tumors represents a novel entity.
In addition, we summarize the clinical manifestations and the imaging and pathological features of the tumors.
A 51-year-old man was admitted to our department with a year-long history of swelling on the right-sided neck.
He also had a history of hypertension for three years, but without any medical treatment, and his blood pressure and heart rate at presentation were 150/94 mmHg and 83 beats/min, respectively.
He was diagnosed with diabetes three years ago and took metformin to control the blood sugar levels. He did not describe other constitutional symptoms such as episodes of diaphoresis, weight loss, or palpitations.
The family history was unremarkable.
Obvious pulsation could be found on the right-sided neck masses. The masses were firm in texture and are not accompanied by pain. Cranial nerve examinations were intact, and the otolaryngology examination was negative.
Concentrations of serum calcium, phosphorus, and parathormone were normal. Besides, radiotracer-labeled metaiodobenzyl-guanidine scintigraphy and serum and urine catecholamine and metanephrine levels were negative. Laboratory tests combined with abdominal computed tomography (CT) excluded the diagnosis of a PHEO. Serum thyroid stimulating hormone and free thyroxine, calcitonin, and carcinoembryonic antigen were within normal limits.
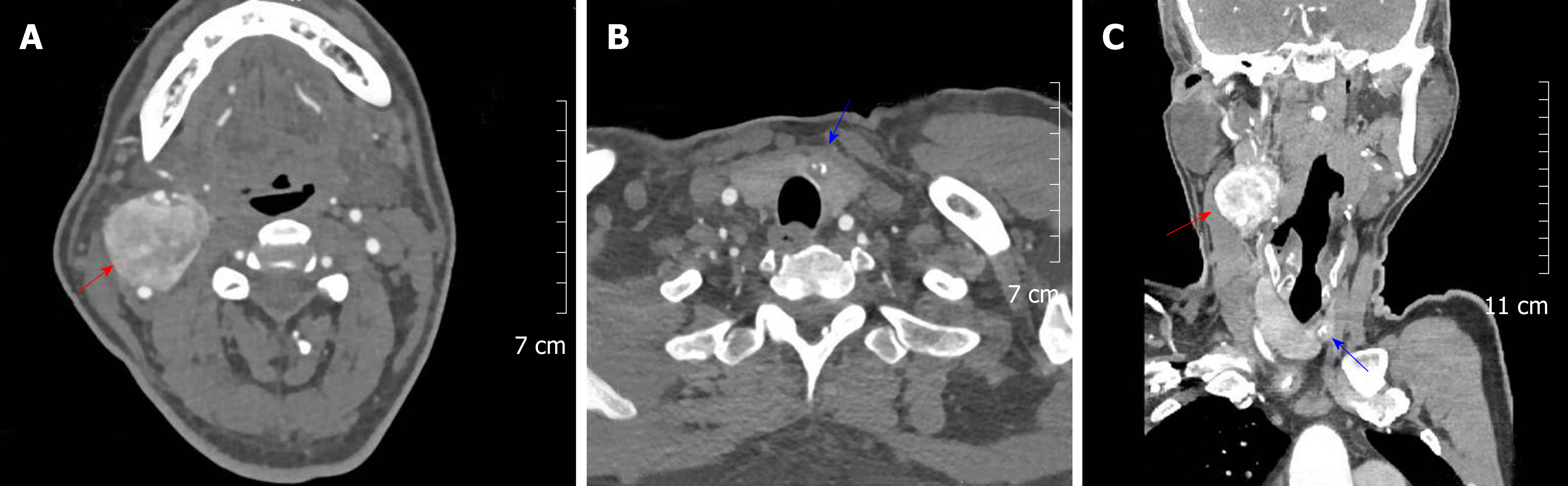
Enhanced CT revealed two irregular solid nodules, consisting of 3.5 × 3.6 × 4.0 cm soft tissue density located in the right carotid artery bifurcation with heterogeneous reinforcement. The mass surrounded both the internal and external carotid arteries; however, a clear boundary between the tumor and the artery could be found. At the same time, a low-density nodule of the thyroid isthmus measuring about 11 mm in diameter with a spot-like dense shadow could be seen. Carotid angiography demonstrated a blood-rich tumor at the carotid bifurcation that surrounded the internal and external carotid arteries. Figure 1 shows the paraganglioma and thyroid cancer, respectively (Figure 1 A and B), which were displayed simultaneously in the same section (Figure 1C).
Ultrasound showed a hypoechoic mass near the isthmus measuring about 18 × 15 × 12 mm in size. The boundary of the mass was unclear and calcification could be seen in the internal echo. An ultrasound-guided fine needle aspiration biopsy (FNAB) of the thyroid nodule revealed papillary thyroid carcinoma.
Based on the clinical characteristics and radiographic results, a combination of carotid body tumor and thyroid papillary carcinoma was raised.
The patient underwent thyroidectomy, neck dissection, and surgery for removal of the right-sided lesion.
During surgery, the dissection of the encapsulated mass from the carotid bifurcation was performed. The internal carotid artery and external carotid artery remained intact. Total thyroidectomy removed a nodular left lobe and normal-appearing right lobe, isthmus, and pyramidal lobe, with right-side selective neck dissection (levels II-V).
Total thyroidectomy removed a nodular left lobe and normal-appearing right lobe, isthmus, and pyramidal lobe, with right-side selective neck dissection (levels II-V).
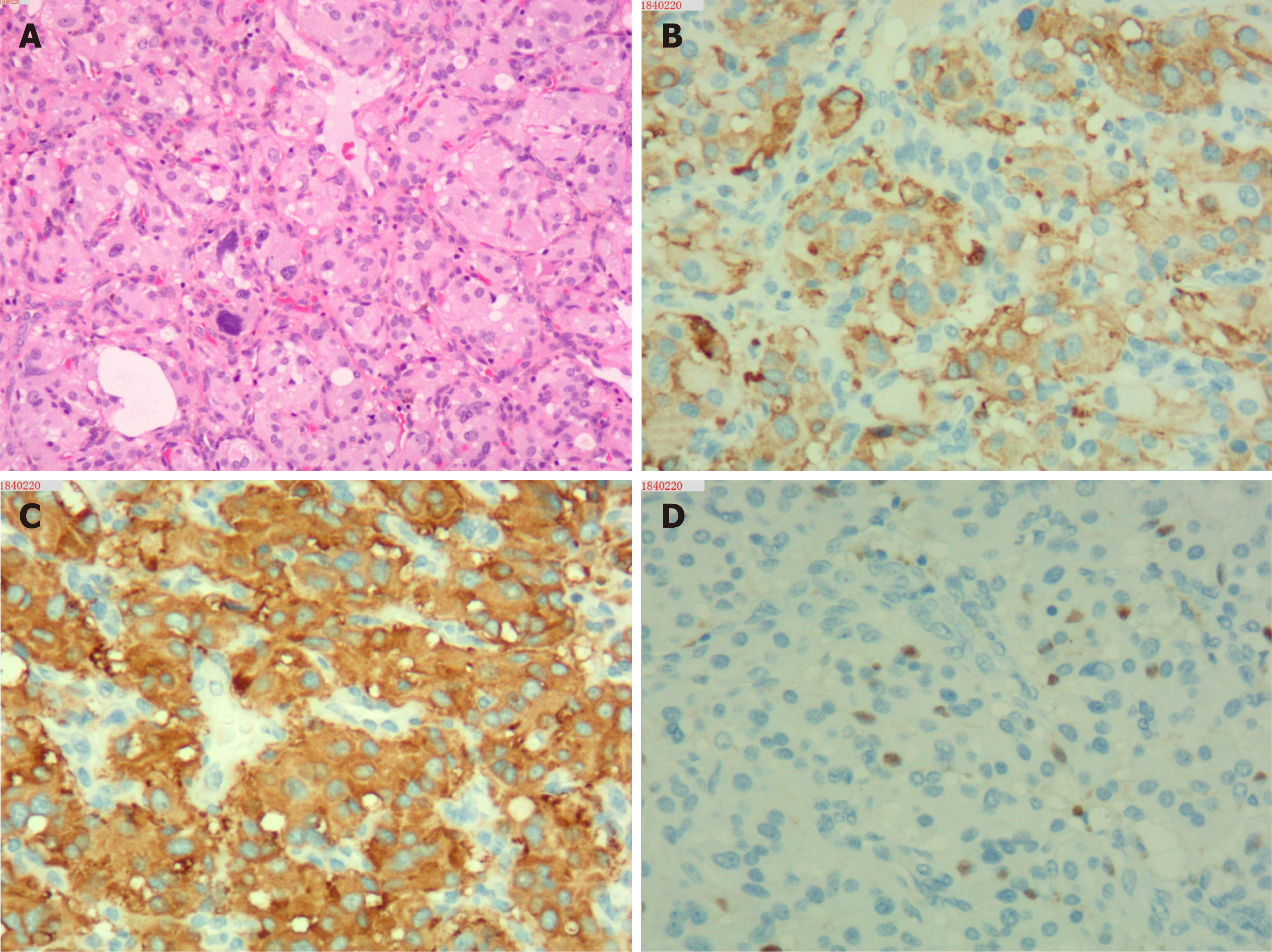
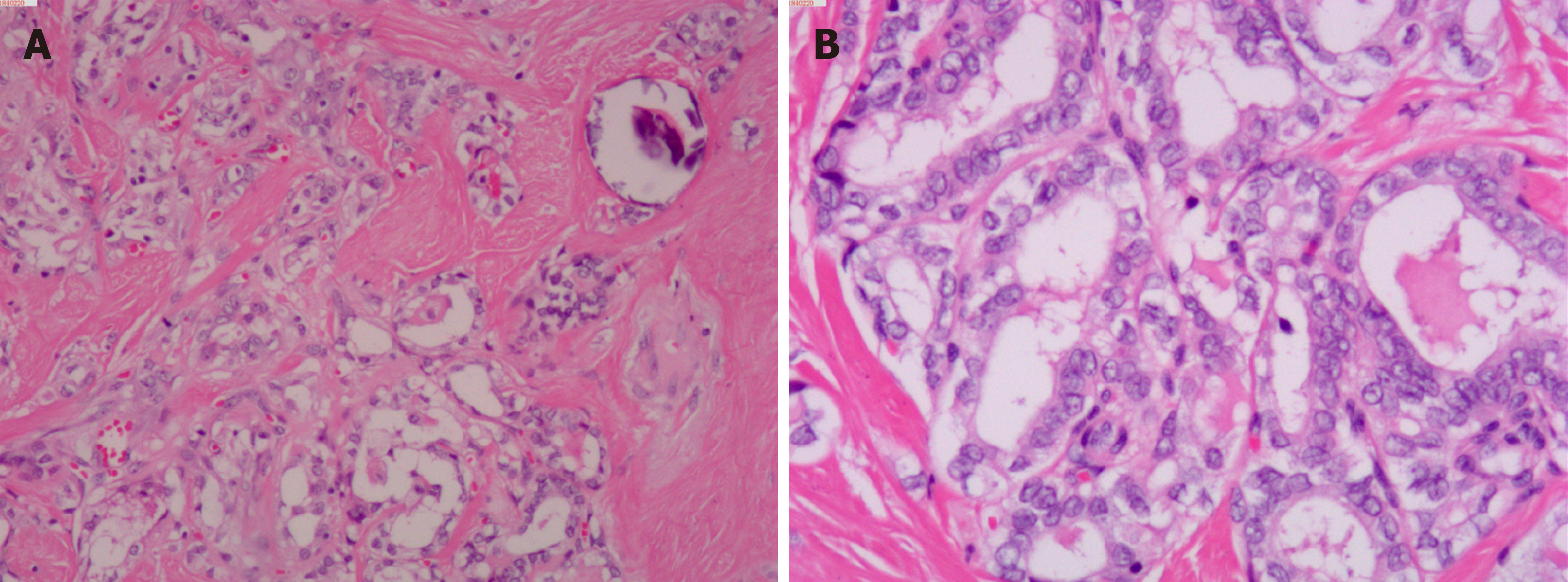
Upon microscopic analysis, the tumor at the carotid artery bifurcation appeared to have rich blood supply, formed by epithelial cells lying in a trabecular pattern and arranged in a “Zellballen” structure (Figure 2). In the thyroid tumor, the cells lining the papillary structures showed nuclear grooves and nuclear clearing, which are characteristic nuclear features of papillary thyroid carcinoma (Figure 3). The final histopathologic diagnosis was paraganglioma and thyroid papillary carcinoma. Immunohistochemical staining revealed positive staining for chromogranin and synaptophysin; the sustentacular cells stained positively for S100 protein.
Analysis of the RET proto-oncogene mutation, von Hippel Lindau (VHL) mutation, SDHB mutation, and SDHD mutation showed negative results.
Nifedipine was administered after the operation as the patient continued to be hypertensive. The patient experienced two weeks of hoarseness after operation without other neurological symptoms. He was treated with 131I after surgery and so far disease-free. The patient is still being followed.
Paragangliomas are rare neuroendocrine neoplasms that originate from chromaffin cells of the adrenal medulla. CBT is a form of head and neck paraganglioma arising at the carotid bifurcation.
MEN is characterized by thyroid, adrenal medulla, and parathyroid neuro-endocrine cell proliferation or tumor, and the clinical manifestations are MTC, PHEO, and primary parathyroid primary hyperparathyroidism[5,6]. A few cases were reported to exhibit combinations of PHEO, abdominal paraganglioma, and papillary thyroid carcinoma[7,8]. However, the coexistence of head and neck paraganglioma and papillary thyroid carcinoma was extremely rare and only reported in three cases (Table 1).
| Author (yr) | Topography | Diagnosis | Genetic testing |
| Rasquin et al[11], 2018 | Adrenal | PTC and PHEO | EGLN1, FH, KIF1B, MEN1, NF1, RET, SDHAF2, SDHC, SDHD, TMEM127, VHL, and SDHA: (-) |
| Bugalho, et al[7], 2015 | Carotid body | PTC and PGL/PHEO | VHL, SDHB, SDHC, SDHD, SDHAF2, TMEM127, MAX, PTEN: (-) |
| Adrenal | PTC and PGL/PHEO | SDHB (+) | |
| Adrenal | PTC and PGL/PHEO | VHL, SDHB, SDHC, SDHD, SDHAF2, TMEM127, MAX, PTEN: (-) | |
| Adrenal | PTC and PGL/PHEO | SDHB (+) | |
| Huguet et al[12], 2013 | Adrenal | PTC and PGLs | SDHD (+) |
| Papathomas et al[13], 2013 | Carotid body | PTC and PGL and PHEO | SDHD (+) |
| Sisson et al[8], 2012 | Adrenal | PTC and PHEO and PA | Not performed |
| Nasser et al[14], 2009 | Adrenal | PTC and PHEO | RET: (-) |
| Zbuk et al[15], 2007 | Carotid body | PTC and PGLs | PTEN (+), SDHC (+) |
| Yang et al[16], 2007 | Adrenal | PTC and PHEO and PGL | RET, VHL, SDHB, SDHD: (-) |
| Hashiba et al[17], 2006 | Adrenal | PTC and PHEO | Not performed |
| Neumann et al[18], 2004 | Adrenal | PTC and PGL | SDHB (+) |
| Adrenal | PTC and PGL | SDHD (+) |
Clinically, thyroid masses, as well as symptoms of increased secretion of catecholamines such as paroxysmal hypertension, headache, palpitations, and sweating are most common. The high blood pressure in our patient raised the suspicion of MEN, but the normal serum and urine catecholamine and metanephrine levels and abdominal ultrasound eliminate PHEO.
When an endocrine gland tumor is discovered, the possibility of MEN should be considered and screened for. Serum calcitonin is a special indicator for the diagnosis of MTC, which can be more than 1000 pg/mL. CT, digital subtraction angiography (DSA), and MRI are helpful to locate the paraganglioma, while ultrasonography and FNAB are more reliable for detection of thyroid tumors.
CT angiography (CTA) is required for preoperative diagnosis and treatment strategies. It can significantly improve the recognition of tumors and identify the anatomical relationship between the tumor and important blood vessels. Correct diagnosis of CBT by careful clinical physical examination is not difficult, but the advantage of CTA is that it can help identify the “feeding artery” of the tumor and provide critical information for surgery.
Moreover, it was through CT that an asymptomatic thyroid tumor was found, which could further confirm the diagnosis.
RET was identified as a MEN-2 susceptibility gene in 1993, and the gene carrier’s penetrance rate is almost 100%[9]. In head and neck paraganglioma, a mutation of the D subunit of the SDH gene is identified in 50%-94% of cases, while a mutation of the B subunit is identified in 10%-20% of cases[10]. Neumann et al[18] suggested that whether thyroid malignancies are also components of SDHB or SDHD related disease awaits further confirmation. The genetic testing in the reported cases is reviewed in Table 1. The results of these studies indicate that the PTC-PGL/PHEO seems to have a heterogeneous genetic background. However, the genetic testing of our cases is not the same as previous studies. Whether this association is coincidental or has a genetic underlying relationship remains identification.
Onset involves multiple organs, and the treatment emphasizes multidisciplinary cooperation. Different lesions are mainly treated by related specialists, but it is necessary to avoid isolated treatment of a single subject. Removal of the paraganglioma and papillary cancer was the optimal treatment in this case, but it was necessary to exclude PHEO, as otherwise, other procedures could have induced hypertensive crisis. However, paraganglioma resection at the carotid bifurcation remains a challenge for surgeons because of its rich blood supply.
To our best understanding and knowledge, no known syndrome or conceivable interrelationships among the tumors explained this combination presentation. This case highlights that the presence of concomitant paraganglioma and thyroid papillary carcinoma could be either coincidental or a result of an underlying unknown mutation.
The authors thank Ms. YJ Huang for her support of the study.
| 1. | Lee JH, Barich F, Karnell LH, Robinson RA, Zhen WK, Gantz BJ, Hoffman HT; American College of Surgeons Commission on Cancer; American Cancer Society. National Cancer Data Base report on malignant paragangliomas of the head and neck. Cancer. 2002;94:730-737. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 278] [Cited by in RCA: 271] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 2. | Brandi ML, Gagel RF, Angeli A, Bilezikian JP, Beck-Peccoz P, Bordi C, Conte-Devolx B, Falchetti A, Gheri RG, Libroia A, Lips CJ, Lombardi G, Mannelli M, Pacini F, Ponder BA, Raue F, Skogseid B, Tamburrano G, Thakker RV, Thompson NW, Tomassetti P, Tonelli F, Wells SA, Marx SJ. Guidelines for diagnosis and therapy of MEN type 1 and type 2. J Clin Endocrinol Metab. 2001;86:5658-5671. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1115] [Cited by in RCA: 920] [Article Influence: 36.8] [Reference Citation Analysis (0)] |
| 3. | Chandrasekharappa SC, Guru SC, Manickam P, Olufemi SE, Collins FS, Emmert-Buck MR, Debelenko LV, Zhuang Z, Lubensky IA, Liotta LA, Crabtree JS, Wang Y, Roe BA, Weisemann J, Boguski MS, Agarwal SK, Kester MB, Kim YS, Heppner C, Dong Q, Spiegel AM, Burns AL, Marx SJ. Positional cloning of the gene for multiple endocrine neoplasia-type 1. Science. 1997;276:404-407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1389] [Cited by in RCA: 1275] [Article Influence: 44.0] [Reference Citation Analysis (5)] |
| 4. | Decker RA, Peacock ML, Watson P. Hirschsprung disease in MEN 2A: increased spectrum of RET exon 10 genotypes and strong genotype-phenotype correlation. Hum Mol Genet. 1998;7:129-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 68] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 5. | Skogseid B, Rastad J, Gobl A, Larsson C, Backlin K, Juhlin C, Akerström G, Oberg K. Adrenal lesion in multiple endocrine neoplasia type 1. Surgery. 1995;118:1077-1082. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 63] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 6. | Shepherd JJ. The natural history of multiple endocrine neoplasia type 1. Highly uncommon or highly unrecognized? Arch Surg. 1991;126:935-952. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 108] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 7. | Bugalho MJ, Silva AL, Domingues R. Coexistence of paraganglioma/pheochromocytoma and papillary thyroid carcinoma: a four-case series analysis. Fam Cancer. 2015;14:603-607. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 8. | Sisson JC, Giordano TJ, Avram AM. Three endocrine neoplasms: an unusual combination of pheochromocytoma, pituitary adenoma, and papillary thyroid carcinoma. Thyroid. 2012;22:430-436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 9. | Machens A. Early malignant progression of hereditary medullary thyroid cancer. N Engl J Med. 2004;350:943. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 11] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 10. | Astuti D, Hart-Holden N, Latif F, Lalloo F, Black GC, Lim C, Moran A, Grossman AB, Hodgson SV, Freemont A, Ramsden R, Eng C, Evans DG, Maher ER. Genetic analysis of mitochondrial complex II subunits SDHD, SDHB and SDHC in paraganglioma and phaeochromocytoma susceptibility. Clin Endocrinol (Oxf). 2003;59:728-733. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 78] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 11. | Rasquin L, Prater J, Mayrin J, Minimo C. Simultaneous Pheochromocytoma, Paraganglioma, and Papillary Thyroid Carcinoma without Known Mutation. Case Rep Endocrinol. 2018;2018:6358485. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 12. | Huguet I, Walker L, Karavitaki N, Byrne J, Grossman AB. Dandy-Walker malformation, papillary thyroid carcinoma, and SDHD-associated paraganglioma syndrome. J Clin Endocrinol Metab. 2013;98:4595-4596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 13. | Papathomas TG, Gaal J, Corssmit EP, Oudijk L, Korpershoek E, Heimdal K, Bayley JP, Morreau H, van Dooren M, Papaspyrou K, Schreiner T, Hansen T, Andresen PA, Restuccia DF, van Kessel I, van Leenders GJ, Kros JM, Looijenga LH, Hofland LJ, Mann W, van Nederveen FH, Mete O, Asa SL, de Krijger RR, Dinjens WN. Non-pheochromocytoma (PCC)/paraganglioma (PGL) tumors in patients with succinate dehydrogenase-related PCC-PGL syndromes: a clinicopathological and molecular analysis. Eur J Endocrinol. 2013;170:1-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 93] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 14. | Nasser T, Qari F. Pheochromocytoma, papillary thyroid carcinoma. Saudi Med J. 2009;30:1087-1090. [PubMed] |
| 15. | Zbuk KM, Patocs A, Shealy A, Sylvester H, Miesfeldt S, Eng C. Germline mutations in PTEN and SDHC in a woman with epithelial thyroid cancer and carotid paraganglioma. Nat Clin Pract Oncol. 2007;4:608-612. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 25] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 16. | Yang JH, Bae SJ, Park S, Park HK, Jung HS, Chung JH, Min YK, Lee MS, Kim KW, Lee MK. Bilateral pheochromocytoma associated with paraganglioma and papillary thyroid carcinoma: report of an unusual case. Endocr J. 2007;54:227-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 17. | Hashiba T, Maruno M, Fujimoto Y, Suzuki T, Wada K, Isaka T, Izumoto S, Yoshimine T. Skull metastasis from papillary thyroid carcinoma accompanied by neurofibromatosis type 1 and pheochromocytoma: report of a case. Brain Tumor Pathol. 2006;23:97-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 18. | Neumann HP, Pawlu C, Peczkowska M, Bausch B, McWhinney SR, Muresan M, Buchta M, Franke G, Klisch J, Bley TA, Hoegerle S, Boedeker CC, Opocher G, Schipper J, Januszewicz A, Eng C; European-American Paraganglioma Study Group. Distinct clinical features of paraganglioma syndromes associated with SDHB and SDHD gene mutations. JAMA. 2004;292:943-951. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 676] [Cited by in RCA: 686] [Article Influence: 31.2] [Reference Citation Analysis (0)] |
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, Research and Experimental
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See:
P- Reviewer: Mogulkoc R, Coskun A S- Editor: Dou Y L- Editor: Wang TQ E- Editor: Bian YN