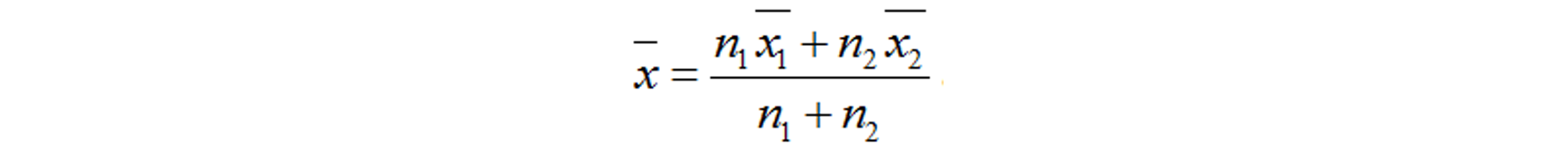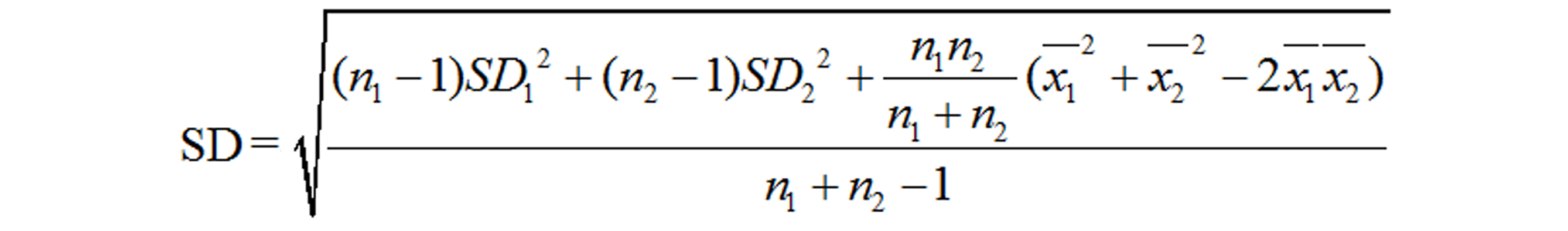Published online Mar 6, 2019. doi: 10.12998/wjcc.v7.i5.585
Peer-review started: December 20, 2018
First decision: January 12, 2019
Revised: February 2, 2019
Accepted: February 18, 2019
Article in press: February 18, 2019
Published online: March 6, 2019
Processing time: 79 Days and 1.6 Hours
Resistin is most likely involved in the pathogenesis of gestational diabetes mellitus (GDM), but the existing findings are inconsistent.
To review the literature investigating the associations of the risk of GDM with serum level of resistin.
A systematic literature search was performed using MEDLINE, EMBASE, and Web of Science (all databases). This meta-analysis included eligible studies that: (1) investigated the relationship between the risk of GDM and serum resistin; (2) included GDM cases and controls without GDM; (3) diagnosed GDM according to the oral glucose-tolerance test; (4) were performed in humans; (5) were published as full text articles in English; and (6) provided data with median and quartile range, median and minimum and maximum values, or mean and standard deviation. The pooled standardized mean difference (SMD) and 95% confidence interval (CI) were calculated to estimate the association between the risk of GDM and serum resistin. To analyze the potential influences of need for insulin in GDM patients and gestational age at blood sampling, we performed a subgroup analysis. Meta-regression with restricted maximum likelihood estimation was performed to assess the potentially important covariate exerting substantial impact on between-study heterogeneity.
The meta-analysis for the association between serum resistin level and GDM risk included 18 studies (22 comparisons) with 1041 cases and 1292 controls. The total results showed that the risk of GDM was associated with higher serum resistin level (SMD = 0.250, 95%CI: 0.116, 0.384). The “after 28 wk” subgroup, “no need for insulin” subgroup, and “need for insulin” subgroup indicated that higher serum resistin level was related to GDM risk (“after 28 wk” subgroup: SMD = 0.394, 95%CI: 0.108, 0.680; “no need for insulin” subgroup: SMD = 0.177, 95%CI: 0.018, 0.336; “need for insulin” subgroup: SMD = 0.403, 95%CI: 0.119, 0.687). The “before 14 wk” subgroup, “14-28 wk” subgroup, and “no information of need for insulin” subgroup showed a nonsignificant association between serum resistin level and GDM risk (“before 14 wk” subgroup: SMD = 0.087, 95%CI: -0.055, 0.230; “14-28 wk” subgroup: SMD = 0.217, 95%CI: -0.003, 0.436; “no information of need for insulin” subgroup: SMD = 0.356, 95%CI: -0.143, 0.855). The postpartum subgroup included only one study and showed that higher serum resistin level was related to GDM risk (SMD = 0.571, 95%CI: 0.054, 1.087) The meta-regression revealed that no need for insulin in GDM patients, age distribution similar between cases and controls, and ELISA all had a significant impact on between-study heterogeneity.
This meta-analysis supports that the maternal serum resistin level is associated with GDM risk.
Core tip: We conducted a meta-analysis of relevant high-quality studies, which revealed that the maternal serum resistin level is associated with gestational diabetes mellitus risk.
- Citation: Hu SM, Chen MS, Tan HZ. Maternal serum level of resistin is associated with risk for gestational diabetes mellitus: A meta-analysis. World J Clin Cases 2019; 7(5): 585-599
- URL: https://www.wjgnet.com/2307-8960/full/v7/i5/585.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v7.i5.585
Gestational diabetes mellitus (GDM) is defined by varying degrees of glucose intolerance that is first detected during pregnancy[1]. In recent decades, the prevalence of GDM has been increasing and fluctuates from 1.7% to 11.6%[2]. Poorly controlled GDM is associated with an increase in the incidence of gestational hypertension, preeclampsia, polyhydramnios, fetal macrosomia, birth trauma, operative delivery, and neonatal hypoglycemia[3-5]. During pregnancy, insulin resistance is enhanced physiologically, which parallels the growth of the fetoplacental unit and facilitates the diversion of glucose to the fetus. When the compensatory increase in insulin is not sufficient to maintain glycemic homeostasis, pregnant women develop GDM.
Resistin, which was named after its insulin resistance ability by Steppan et al[6] in 2001, is a hormone with a molecular weight of 12.5 kDa that consists of 108 amino acids. Steppan et al[6] found anti-diabetic drugs called thiazolidinediones, which markedly lowered serum resistin levels in mice during treatment. The immunoneutralization of endogenous resistin improved blood glucose and insulin action in this model of type 2 diabetes. The treatment of normal mice with recombinant resistin impaired glucose tolerance and insulin action. Insulin-stimulated glucose uptake by adipocytes was enhanced by neutralization of resistin and reduced by resistin treatment[6]. The association between elevated circulating resistin and insulin resistance in patients with type 2 diabetes has also been revealed[7-11].
The results of studies on the association between resistin and GDM risk vary greatly. Some studies have suggested that elevated circulating resistin is a risk factor for GDM[12-26], while some have suggested that elevated circulating resistin is a protective factor[27-30], and others have suggested that resistin circulating level is not associated with GDM risk[31-52]. Variations in gestational age at sample collection, assay methods, fasting state, sample size, ethnicity, diagnosis criteria, severity of GDM, and definitions of controls could account for the considerable differences of the results of these studies. In a study performed by Steppan et al[6], they found that resistin expression was adipocyte-specific. However, recent reports suggest that resistin is also expressed in multiple other tissues, such as pancreatic islets, skeletal muscles, mononuclear cells, placenta, and liver cells[51,53,54]. During pregnancy, the placenta synthesizes and secretes resistin into the maternal circulation[55,56]. One study found that resistin protein expression in placental tissue was much higher than that in subcutaneous adipose tissue in pregnant women’s abdomens, suggesting that the placenta is a major contributor of resistin in pregnancy[57]. Several studies have shown that the maternal circulating level of resistin gradually increases with gestational age and decreases significantly after delivery[17-19,23,37,40,42,44]. This process of change is consistent with the growth and delivery of the placenta.
Notably, a previous meta-analysis published in 2013 that included 10 papers showed no association between circulating resistin levels and GDM[58]. However, the authors suggested that these results should be interpreted with caution owing to the large heterogeneity among the studies. Since 2013, there have been many high-quality articles on serum resistin levels and GDM. Therefore, we think it is necessary to conduct another meta-analysis, and since there is a sufficient number of articles, we can perform a meta-regression analysis and subgroup analysis to explore possible influencing factors. The purpose of this study was to review the literature on the association of resistin and GDM risk, and attempt to find potential influence factors to interpret the considerable differences of the results of these studies.
The databases MEDLINE, EMBASE, and Web of Science (all databases, including Web of Science Core Collection, BIOSIS Citation Index, etc.) were searched up to October 11, 2018 to find articles focused on the relationship between the risk of GDM and serum resistin level. The following keywords were used in PubMed and Web of Science: (“Diabetes, Gestational” [Mesh] OR “GDM” OR “gestational diabetes” OR “gestational diabetic” OR “diabetic pregnancy”) AND (“resistin” [MeSH] OR resistin OR RETN OR ADSF OR RSTN OR XCP1 OR FIZZ3 OR RETN1). The following keywords were used in EMBASE: (“pregnancy diabetes mellitus”/exp OR “gestational diabetes” OR “gestational diabeti” OR “diabetic pregnancy” OR “gdm”) AND (“resistin”/exp OR “resistin” OR “RETN” OR “ADSF” OR ”RSTN” OR ”XCP1” OR ”FIZZ3” OR ”RETN1”). We manually searched all the reference lists of the included studies and relevant reviews to find additional eligible studies.
This meta-analysis included eligible studies that: (1) investigated the relationship between the risk of GDM and serum resistin; (2) included GDM cases and controls without GDM; (3) diagnosed GDM according to the oral glucose-tolerance test; (4) were performed in humans; (5) were published as full text articles in English; and (6) provided data with median and quartile range, median and minimum and maximum values, or mean and standard deviation (SD). We excluded the studies with overlapping data. Information of the first author, study location (country), study design, year of publication, diagnostic criteria, number of patients and controls, gestational age at the time of blood sampling, assay methods, need for insulin in GDM patients, and the mean and SD of serum resistin levels was extracted. The quality of each included study was evaluated based on the Newcastle-Ottawa Scale (NOS) recommended by the Agency for Healthcare Research and Quality of the US[59]. Comparability of cases and controls on the basis of the design or analysis was evaluated based on whether the gestational age and body mass index (BMI) at blood sampling matched. The NOS total score of the literature included had to be greater than or equal to 5 points. Disputes were resolved by discussion with a third author during data extraction and quality evaluation. The equation “SD = SEM × (n)1/2” was used to calculate SD from the standard error of the mean (SEM). If we need to merge the data of subgroups, we used the following equations: Equation 1 and Equation 2[60]. The equation “SD = interquartile range/1.35” was used to calculate SD from interquartile ranges. And we treated the medians as means[61]. However, if we were provided with the minimum and maximum values, we calculated means and SDs according to the equations in the study of Hozo et al[62]. When serum resistin levels were measured in both nonfasting and fasting blood samples at the same gestational age, we chose the fasting result here.
The pooled standardized mean difference (SMD) and 95% confidence interval (CI) were used to estimate the relationship between the risk of GDM and serum resistin. Subgroup analyses were performed to detect whether “need for insulin” or “gestational age at blood sampling” influenced the relationship between the risk of GDM and serum resistin. We divided the subjects into four subgroups according to the gestational age at blood sampling (“before 14 wk”, “14-28 wk”, “after 28 wk”, and “postpartum”). If the gestational age of the included study did not completely overlap with the gestational age of the subgroup, we would assign the study into the subgroup with the most overlap with the gestational age of the study. The study of Kuzmicki et al[14] was assigned to the “14-28 wk” subgroup. The studies of Kralisch et al[39], Siddiqui et al[25], and Palik et al[17] were assigned to the subgroup of “after 28 wk”. Additionally, based on the “need for insulin in GDM patients”, the subjects were divided into three subgroups: (1) need for insulin; (2) no need for insulin; and (3) no information. The Z test was used to determine the significance of the pooled SMD, with α set at 0.05.
The Q test and the I2 statistic were used to estimate the heterogeneity across studies[63,64]. If P < 0.1 in the Q test, and I2 > 50%, we used the random effects model to pool the data; and meta-regression with restricted maximum likelihood estimation (REML) was performed to assess the potentially important covariate exerting substantial impact on between-study heterogeneity. The following covariates were included in the meta-regression analysis: need for insulin (no need for insulin, need for insulin, OR no information; dummy variable), assay method (ELISA OR others), and maternal age distribution (similar between cases and controls OR different between cases and controls) in each study. Begg’s funnel plot and Egger’s test and sensitivity analysis were used to assess the publication bias and the stability of the results. STATA 12.0 software (Stata Corporation, College Station, TX) was the only analysis software in this meta-analysis. The statistical methods of this study were reviewed by Jun-Xia Yan from Department of Epidemiology and Health Statistics, Central South University, China.
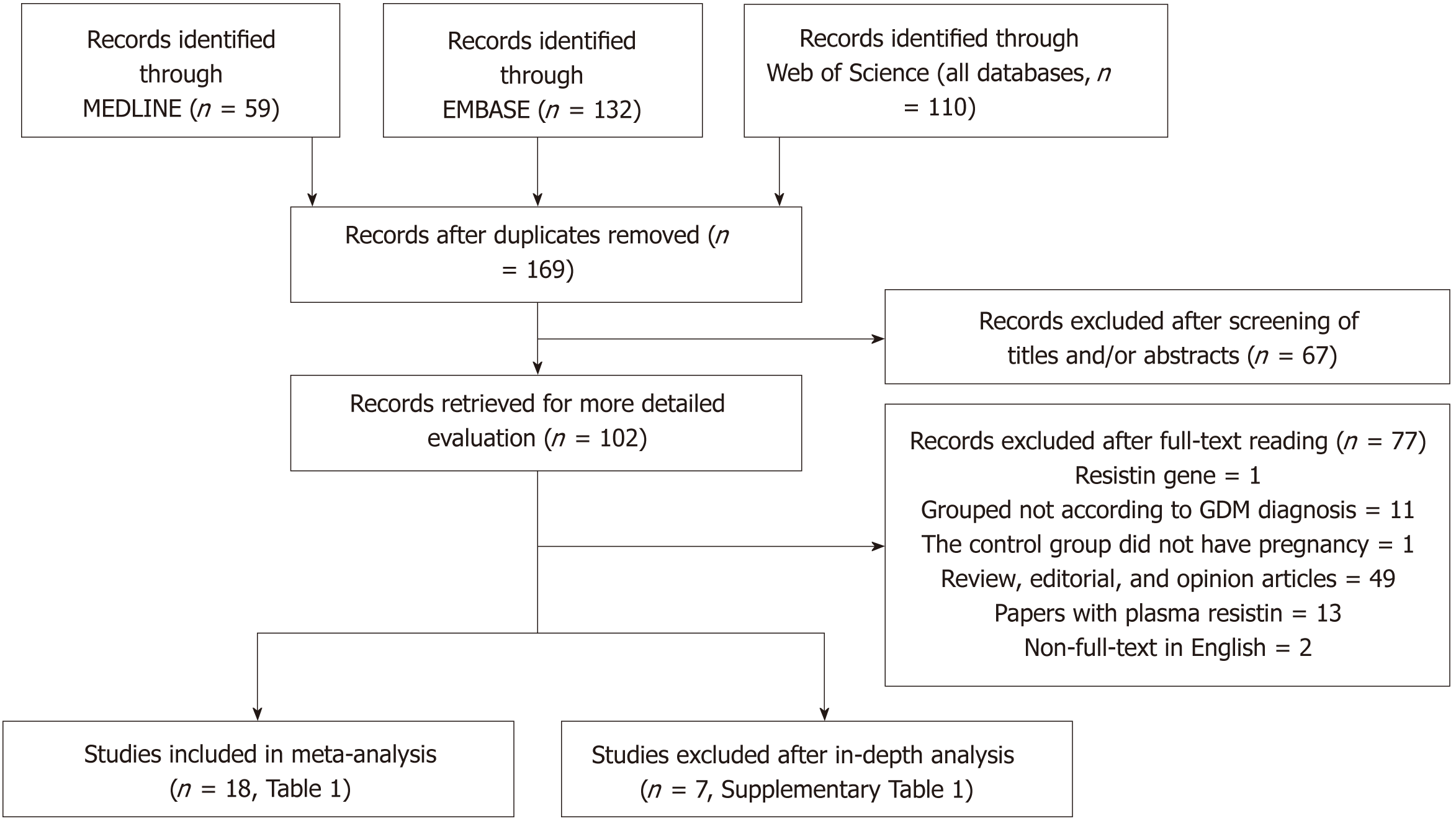
The process of study selection is shown in Figure 1. Three hundred and one records of potentially relevant studies were identified. Of these, 199 records were excluded based on their title and/or abstract (“repetitive publications”: n = 132; “conference abstracts”: n = 18; “reports of animal studies”: n = 6; “reports of studies that investigated outcomes irrelevant to this meta-analysis”: n = 31; “adipokines other than resistin”: n = 1; “studies of which the biological material was not maternal blood”: n = 11). A further 77 full-text articles were excluded because these were: (1) review, editorial, and opinion articles (n = 49); (2) studies in which interviewees were not grouped according to whether they had GDM (n = 11); (3) a study in which the controls were not pregnant women and had never been pregnant (n = 1); (4) papers with plasma resistin concentrations reported (n = 13); (5) non-full-text articles in English (n = 2); and (6) RETN gene study (n = 1). After in-depth analysis, seven more studies were excluded. The specific reasons for the exclusion and the detailed information of these seven papers are shown in Supplementary Table 1. The 18 studies (22 comparisons) that were ultimately selected for our meta-analysis included 1041 cases and 1292 controls (Tables 1 and 2).
| Ref. | Location | Study design | Number GDM/C | GDM diagnosis | Time for sampling | Assay method | Need for insulin n (%) | Resistin level, ng/mL mean ± SD | ||
| GDM | Control | P | ||||||||
| Tsiotra et al[49], 2018 | Greece | CC | 15/23 | 75 g ADA | 39 wk | LUMINEX xMAP | 4 (26.7) | 26.147 ± 7.337 | 23.440 ± 1.567 | NS |
| Lobo et al[42], 2018 | Brazil | CC | 15/30 | 75 g IADPSG | < 14 wk | ELISA | 0 | 14.047 ± 5.334 | 12.357 ± 4.747 | NS |
| 18/28 | 14-20 wk | 0 | 14.420 ± 6.157 | 14.219 ± 5.721 | NS | |||||
| Siddiqui et al[25], 2018 | Saudi Arabia | CC | 14/21 | 100 g ADA | 24-32 wk | Randox evidence biochip analyzer | NI | 9.1 ± 4.2 | 6.1 ± 1.6 | 0.02 |
| Bagci et al[21], 2018 | Turkey | CC | 40/40 | 100 g NDDG | 24-28 wk | ELISA | NI | 16.78 ± 6.83 | 12.79 ± 5.02 | 0.004 |
| Khanam et al[37], 2017 | Australia | Cohort | 52/71 | 75 g ADIPS | 14 wk | Multiplex assay kits | 0 | 0.667 ± 0.515 | 0.697 ± 0.249 | NS |
| 52/71 | 18 wk | 0 | 0.896 ± 0.547 | 0.806 ± 0.416 | NS | |||||
| Ravnsborg et al[45], 2016 | Denmark | CC | 199/208 | 75 g 2 h ≥ 9.0 mmol/L | 8+1-13+6 wk | ELISA | 78 (39.2) | 14.06 ± 6.43 | 13.22 ± 4.87 | NS |
| Huang et al[13], 2016 | China | CC | 43/24 | 75 g IADPSG | 37-40 wk | ELISA | 17 (39.5) | 19240 ± 5860 | 14570 ± 4890 | < 0.05 |
| Takhshid and Zare[48], 2015 | Iran | CC | 75/70 | 100 g Carpenter and Coustan | 30 wk | ELISA | NI | 13.0 ± 6.6 | 11.4 ± 6.9 | NS |
| Boyadzhieva et al[32], 2013 | Bulgaria | CC | 127/109 | 75 g IADPSG | 24-28 wk | BioVendor® kits | 0 | 10.11 ± 2.2 | 9.09 ± 3.2 | NS |
| Nanda et al[43], 2012 | UK | CC | 60/240 | 75 g fasting ≥ 6.0 mmol/L or 2 h ≥ 7.8 mmol/L | 11-13 wk | ELISA | 0 | 8.32 ± 4.58 | 8.28 ± 3.38 | NS |
| Skvarca et al[47], 2012 | Slovenia | CS | 30/25 | 100 g Carpenter and Coustan | Approximately 27 wk | ELISA | 0 | 2.00 ± 0.68 | 2.40 ± 1.26 | NS |
| Akdeniz et al[31], 2011 | Turkey | CC | 20/22 | ADA | Before delivery | ELISA | 20 (100) | 8.7 ± 2.1 | 8.1 ± 2.5 | NS |
| Vitoratos et al[18], 2011 | Greece | Cohort | 30/30 | 100 g Carpenter and Coustan | 26-28 wk | Sandwich immunoassay kit | 0 | 0.21 ± 0.08 | 0.19 ± 0.12 | NS |
| 30/30 | 38 wk | 0 | 0.28 ± 0.2 | 0.21 ± 0.13 | 0.02 | |||||
| 30/30 | The third postpartum day | 0 | 0.25 ± 0.11 | 0.19 ± 0.10 | 0.03 | |||||
| Kleiblova et al[38], 2010 | Czech Republic | CC | 10/13 | 75 g Czech Diabetes Association | At delivery | ELISA | 10 (100) | 12.6 ± 4.2 | 10.9 ± 2.1 | NS |
| Vitoratos et al[50], 2010 | Greece | CC | 30/30 | 100 g Carpenter and Coustan | 24-26 wk | Sandwich immunoassay kit | 0 | 0.1913 ± 0.1096 | 0.21 ± 0.0822 | NS |
| Kralisch et al[39], 2009 | Germany | CC | 40/80 | 75 g ADA | G: 29 ± 3 wk; C: 28 ± 4 wk | ELISA | NI | 6.5 ± 2.7 | 7.4 ± 3.3 | NS |
| Kuzmicki et al[14], 2009 | Poland | CC | 81/82 | 75 g WHO | 24-31 wk | Quantikine immunoassay kit | 0 | 21.9 ± 5.9 | 19.0 ± 5.9 | < 0.001 |
| Palik et al[17], 2007 | Hungary | CS | 30/15 | 75 g WHO | 26-30 wk | ELISA | 30 (100) | 15.76 ± 3.52 | 13.0 ± 3.60 | < 0.001 |
| Ref. | Case definition adequacy | Representativeness of cases | Selection of controls | Definition of controls | Comparability of cases and controls | Ascertainment of exposure | Same method of ascertainment for cases and controls | Non-response rate | Total quality score |
| Tsiotra et al[49], 2018 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | NI | 5 |
| Lobo et al[42], 2018 | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 9 |
| Siddiqui et al[25], 2018 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | NI | 5 |
| Bagci et al[21], 2018 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | NI | 5 |
| Ravnsborg et al[45], 2016 | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 9 |
| Huang et al[13], 2016 | 1 | 0 | 0 | 1 | 2 | 1 | 1 | NI | 6 |
| Takhshid and Zare[48], 2015 | 1 | 0 | 0 | 1 | 2 | 1 | 1 | NI | 6 |
| Boyadzhieva et al[32], 2013 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | NI | 7 |
| Nanda et al[43], 2012 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | NI | 6 |
| Skvarca et al[47], 2012 | 1 | 0 | 0 | 1 | 2 | 1 | 1 | NI | 6 |
| Akdeniz et al[31], 2011 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | NI | 5 |
| Kleiblova et al[38], 2010 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | NI | 5 |
| Vitoratos et al[50], 2010 | 1 | 0 | 0 | 1 | 2 | 1 | 1 | NI | 6 |
| Kralisch et al[39], 2009 | 1 | 0 | 0 | 1 | 2 | 1 | 1 | NI | 6 |
| Kuzmicki et al[14], 2009 | 1 | 0 | 0 | 1 | 2 | 1 | 1 | NI | 6 |
| Palik et al[17], 2007 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | NI | 5 |
| Ref. | Representativeness of the exposed cohort | Selection of the non-exposed cohort | Ascertainment of exposure | Demonstration that outcome of interest was not present at start of study | Comparability of cohorts | Assessment of outcome | Follow-up long enough for outcomes to occur | Adequacy of follow -up of cohorts | Total quality score |
| Khanam et al[37], 2017 | 0 | 1 | 1 | 1 | 2 | 1 | 1 | 0 | 7 |
| Vitoratos et al[18], 2011 | 0 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 8 |
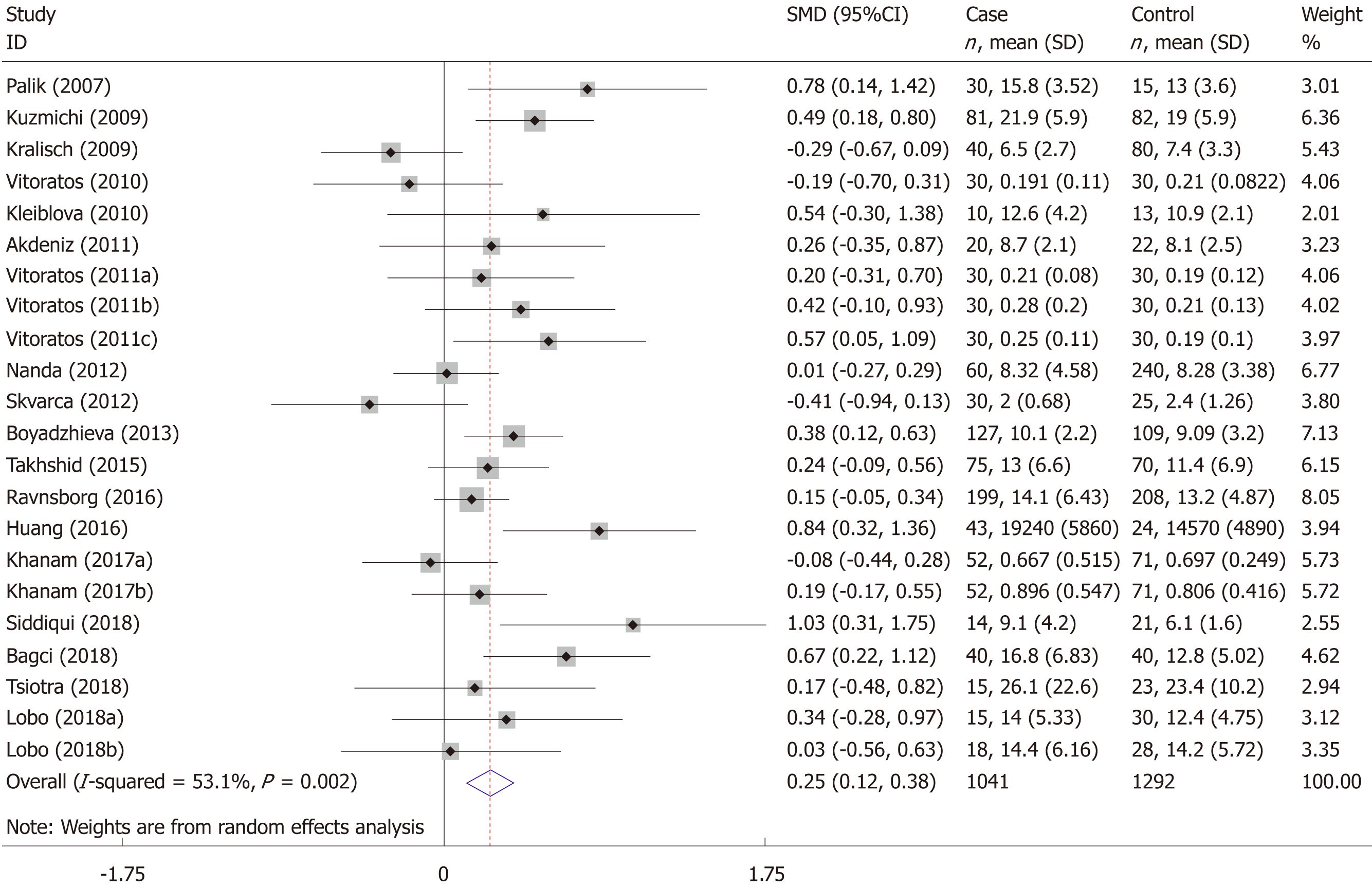
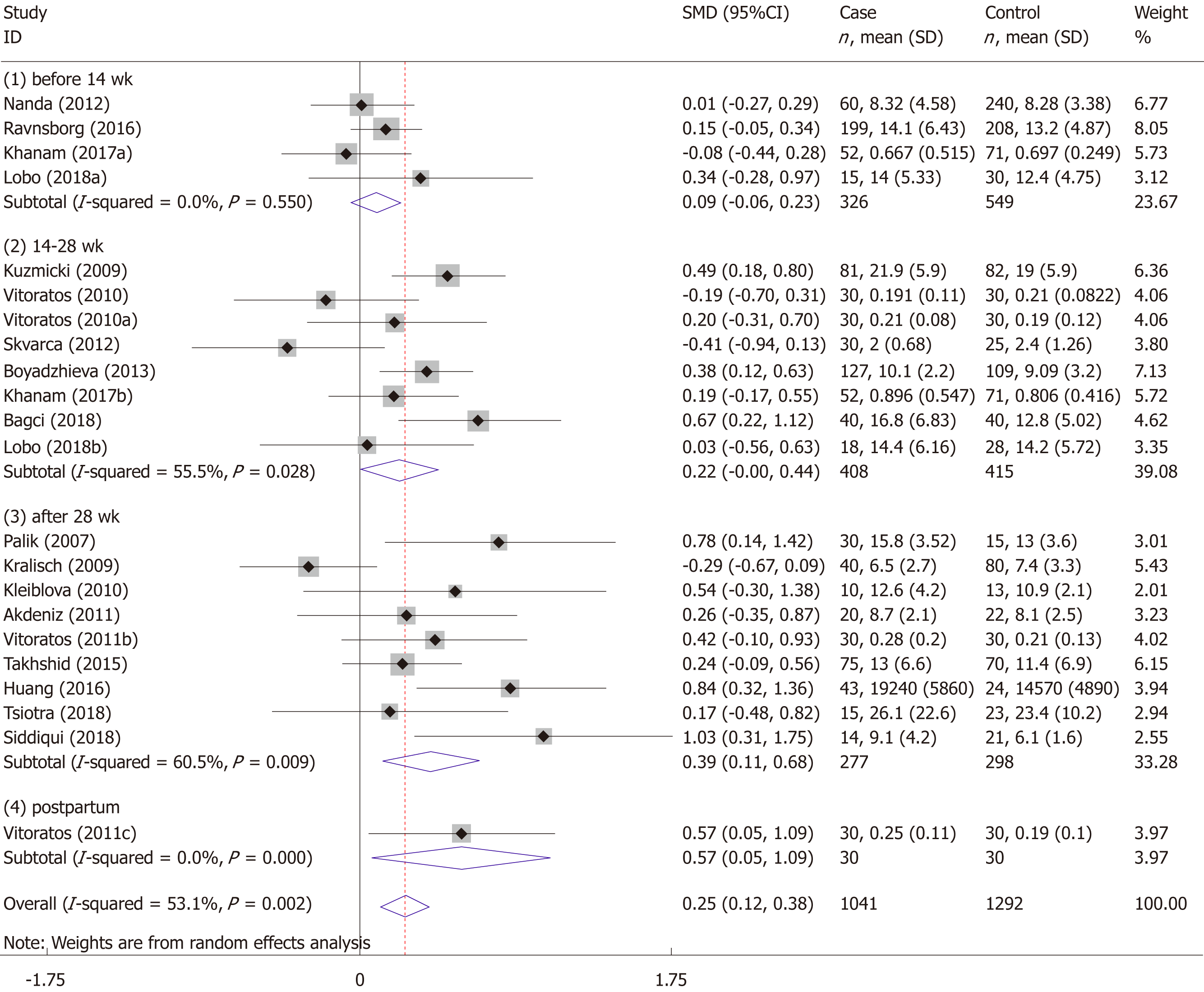
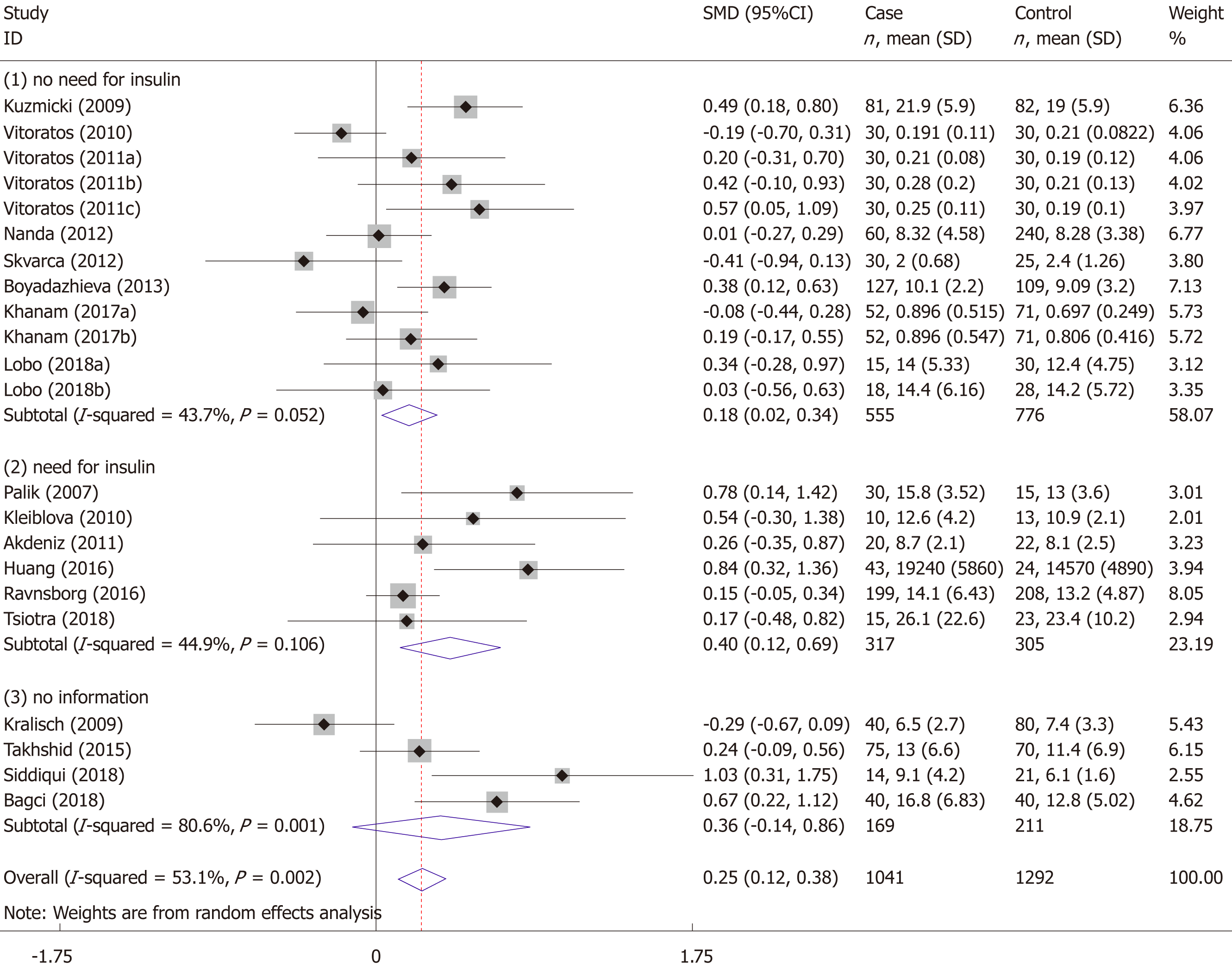
The meta-analysis included 18 studies (22 comparisons) with 1041 cases and 1292 controls. The total results showed that higher serum resistin was associated with the risk of GDM (SMD = 0.250, 95%CI: 0.116, 0.384) (Table 3 and Figure 2). The “after 28 wk”, “no need for insulin”, and “need for insulin” subgroups indicated that higher serum resistin was related to the risk of GDM (“after 28 wk” subgroup: SMD = 0.394, 95%CI: 0.108, 0.680; “no need for insulin” subgroup: SMD = 0.177, 95%CI: 0.018, 0.336; “need for insulin” subgroup: SMD = 0.403, 95%CI: 0.119, 0.687). The “before 14 wk” subgroup, “14-28 wk” subgroup, and “no information of need for insulin” subgroup showed a nonsignificant association between serum resistin level and GDM risk (“before 14 wk” subgroup: SMD = 0.087, 95%CI: -0.055, 0.230; “14-28 wk” subgroup: SMD = 0.217, 95%CI: -0.003, 0.436; “no information of need for insulin” subgroup: SMD = 0.356, 95%CI: -0.143, 0.855). The postpartum subgroup included only one study and showed that higher serum resistin level was related to GDM risk (SMD = 0.571, 95%CI: 0.054, 1.087) (Table 3, Figures 3 and 4).
| Category | No. of comparisons | No. of cases | No. of controls | SMD (95%CI) | Z | P | I2% | Phet | |
| Overall | 22 | 1041 | 1292 | 0.250 (0.116, 0.384) | 3.65 | 0.000 | 53.1 | 0.002 | |
| Gestational age at blood sampling | Before 14 wk | 4 | 326 | 549 | 0.087 (-0.055, 0.230) | 1.20 | 0.229 | 0.0 | 0.550 |
| 14-28 wk | 8 | 408 | 415 | 0.217 (-0.003, 0.436) | 1.93 | 0.053 | 55.5 | 0.028 | |
| After 28 wk | 9 | 277 | 298 | 0.394 (0.108, 0.680) | 2.70 | 0.007 | 60.5 | 0.009 | |
| Postpartum | 1 | 30 | 30 | 0.571 (0.054, 1.087) | - | - | - | - | |
| Need for insulin | No need for insulin | 12 | 555 | 776 | 0.177 (0.018, 0.336) | 2.18 | 0.029 | 43.7 | 0.052 |
| Need for insulin | 6 | 317 | 305 | 0.403 (0.119, 0.687) | 2.78 | 0.005 | 44.9 | 0.106 | |
| No information | 4 | 169 | 211 | 0.356 (-0.143, 0.855) | 1.40 | 0.162 | 80.6 | 0.001 |
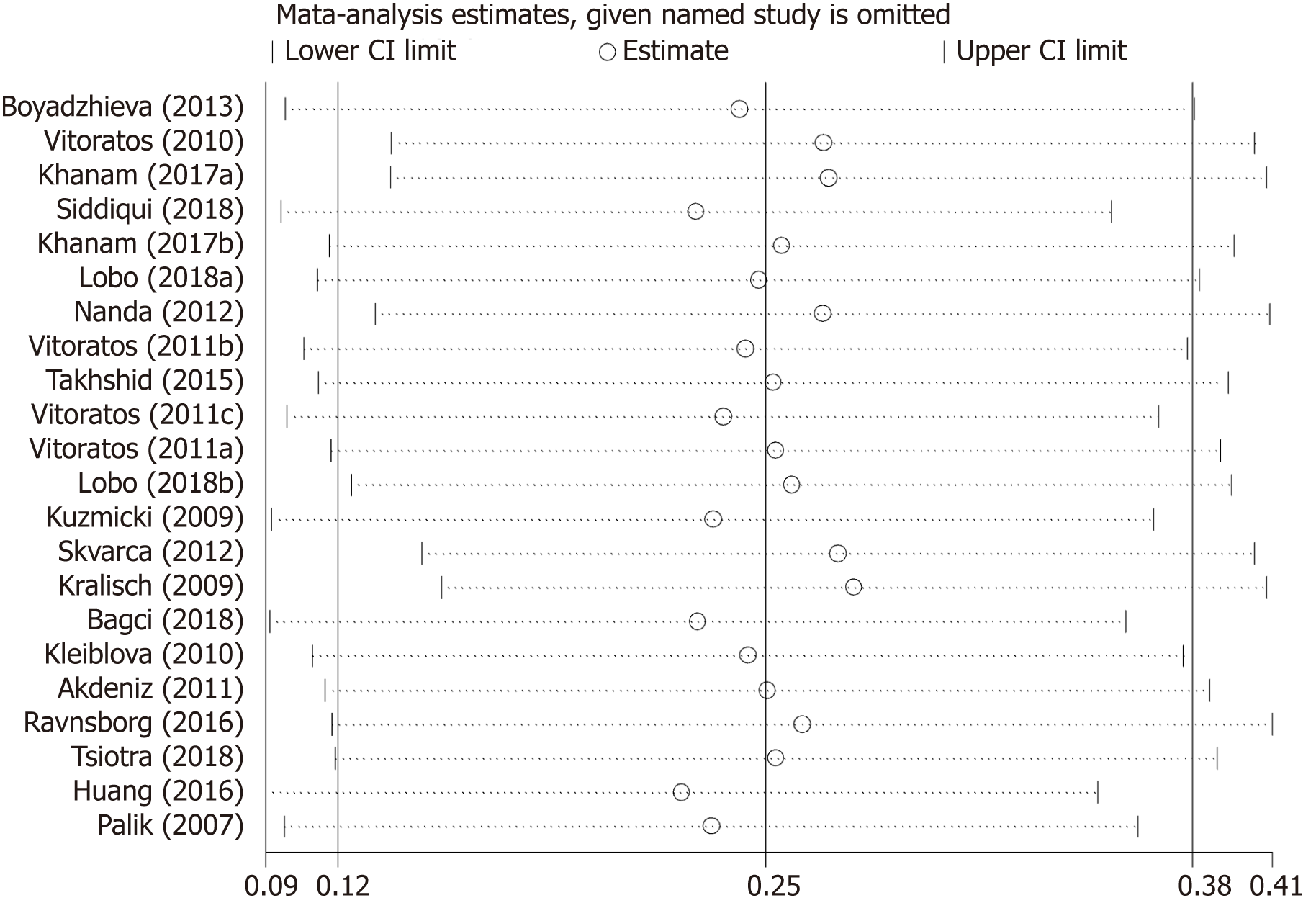
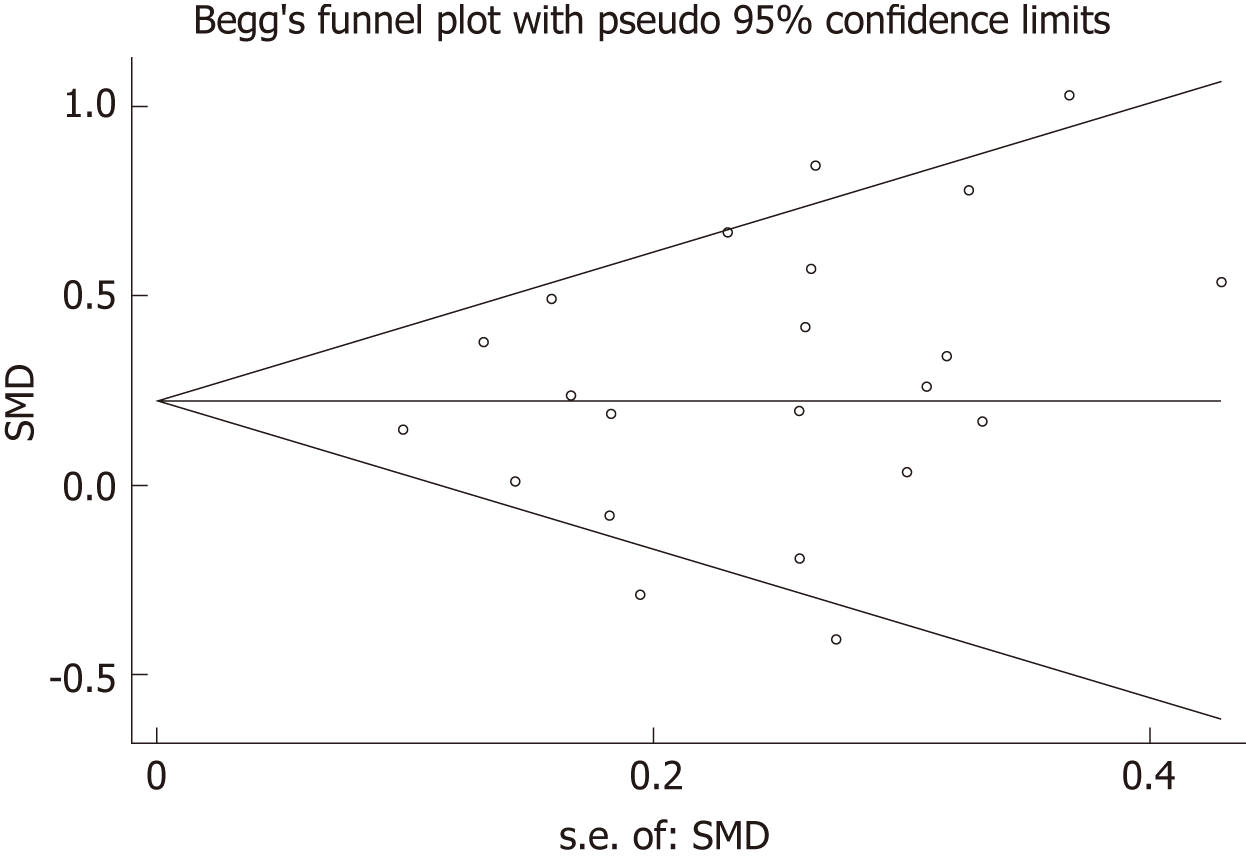
Table 4 summarizes results of meta-regression; “no need for insulin in GDM patients”, “age distribution similar between cases and controls”, and ELISA all had a significant impact on between-study heterogeneity. REML estimate of between-study variance tau2 decreased from 0.0482 to 0.02687, indicating that these variables can account for 44.3% of heterogeneity sources. No association was statistically significant when meta-regression was performed on the three strategies considered one by one. In the sensitivity analysis, no changes were observed in the significance of the results or in the corresponding pooled SMD (Figure 5). In the publication bias analysis, the results of Begg’s funnel plot and the modified Egger linear regression test (Figure 6) showed no publication bias (Z = 1.30, P = 0.195; t = 1.10, P = 0.284).
| Covariate | Coefficient | Std. error | t | P | 95%CI lower | 95%CI upper |
| No need for insulin | -0.482 | 0.183 | -2.64 | 0.017 | -0.868 | -0.097 |
| Need for insulin | -0.169 | 0.190 | -0.89 | 0.388 | -0.570 | 0.233 |
| Age distribution similar | 0.298 | 0.126 | 2.37 | 0.030 | 0.033 | 0.563 |
| ELISA | -0.399 | 0.160 | -2.50 | 0.023 | -0.737 | -0.062 |
The results of the studies focused on the association of maternal serum resistin level and GDM risk varied widely due to differences in study design type, ethnicity, diagnostic criteria for GDM, insulin use in GDM patients, inclusion criteria of the control group, the distribution equilibrium of maternal age, BMI, gestational age at sampling, sample size, serum/plasma selection, sample storage methods, and assay methods. To obtain a more reliable meta-analysis result, we set a threshold for the diagnostic criteria of GDM. To explore the possible influencing factors of the results, we performed subgroup analyses according to the gestational age at sampling and need for insulin in GDM patients. The total results showed that serum resistin level was associated with GDM. The results of the third trimester, “no need for insulin”, and “need for insulin” subgroups were consistent with the total result.
The effect of resistin on blood glucose was confirmed in animal studies by highly evidence-based studies[6,65]. In a mouse model, Steppan et al[6] found that resistin levels were increased in diet-induced obesity, as well as in genetic models of obesity and insulin resistance. Neutralization of resistin reversibly reduced hyperglycemia in this model of diet-induced insulin resistance. The ability of recombinant resistin to produce glucose intolerance and insulin resistance is consistent with the opposite effects of immunoneutralization of endogenous resistin. Similar effects of resistin, i.e., decreasing insulin-stimulated glucose uptake, were confirmed in vitro by using 3T3-L1 adipocytes[6]. Banerjee et al[65] studied the mechanism by which resistin regulates blood glucose, and they found that mice lacking resistin exhibited low blood glucose levels after fasting due to the impairment of hepatic glucose output. Resistin normally acts on the liver to inhibit the activation of adenosine monophosphate-activated protein kinase (AMPK). The key gluconeogenic enzymes glucose 6-phosphatase (G6Pase) and phosphoenolpyruvate carboxykinase (PEPCK) genes are both downregulated by activation of AMPK. Gene expression of G6Pase and PEPCK was markedly decreased in the liver of the resistin-null mice[65]. There have been no animal studies related to circulating resistin level and GDM risk.
The association between elevated serum resistin level and GDM was only found in the third-trimester subgroup when we divided the studies into four subgroups according to the gestational age. This may be because the serum level of resistin increases with gestational age[17-19,23,37,40,42,44], and the gap in resistin levels between GDM and the controls may also increase with increasing gestational age. Figure 3 shows this trend. Therefore, a study focusing on the association between the serum resistin level and GDM risk in the first and second trimesters would require a larger sample size than one focusing only on the third trimester. Choi et al[12] found higher circulating resistin levels in women with pGDM than in women with normal glucose tolerance during pregnancy and one year after delivery, suggesting that in addition to the placenta, other secretory organs such as adipose tissue may also contribute to the occurrence of GDM. A study on circulating resistin and fat mass compartments in pregnancy revealed that resistin levels are not related to BMI, total body fat mass, or abdominal subcutaneous fat mass but are related to abdominal visceral fat mass[66]. In this meta-analysis, the postpartum subgroup included only one study. Further research may be needed to focused on the postpartum. The results of the subgroup analysis, according to the need for insulin, showed that the pooled SMD of “need for insulin in GDM patients” was higher than the pooled SMD of “no need for insulin in GDM patients”, suggesting that the serum level of resistin may be related to the severity of GDM. The research of Huang et al[13] showed that among pregnant women with GDM, the serum resistin levels of women with satisfactory glycemic control were lower than those in women with unsatisfactory glycemic control[13].
Studies on RETN (gene encoded resistin) mRNA and SNPs have also suggested that resistin is associated with GDM risk. The mRNA expression of RETN was increased in adipose tissue from GDM when compared to a non-GDM group, was related to insulin resistance level, and could be regulated by adrenomedullin and an adrenomedullin antagonist[13,67,68]. RETN rs1423096 and -420 C/G were found to be associated with GDM risk[48,69], and it has been reported that the G allele of -420 C/G in the RETN gene promoter was associated with an increase in circulating resistin[70,71].
In summary, our meta-analysis showed that higher maternal serum resistin level is related to GDM risk and suggested that the serum level of resistin may be related to the severity of GDM. Studies focusing on the association between the serum resistin level and GDM risk in the first and second trimesters would require a larger sample size than ones focusing only on the third trimester.
Resistin is most likely involved in the pathogenesis of gestational diabetes mellitus (GDM), but the existing findings are inconsistent.
To explore the sources of heterogeneity in the existing literature, we made the literature heterogeneity within an acceptable range by setting reasonable inclusion criteria. Based on this, we aimed to explore the relationship between serum level of resistin and GDM risk.
This article aims to review the studies investigating the association of GDM risk with serum resistin level.
A systematic literature search was performed using MEDLINE, EMBASE, and Web of Science (all databases). We did subgroup analysis according to the need for insulin in GDM patients and gestational age at blood sampling. Meta-regression with restricted maximum likelihood estimation was performed to assess the potentially important covariate exerting substantial impact on between-study heterogeneity.
The meta-analysis included 18 studies (22 comparisons) with 1041 cases and 1292 controls. The total results showed that the risk of GDM was associated with serum resistin level. The results of subgroup are consistent with the total results. The meta-regression revealed that no need for insulin in GDM patients, age distribution similar between cases and controls, and ELISA all had a significant impact on between-study heterogeneity.
This meta-analysis supports that the maternal serum resistin level is associated with GDM risk.
In summary, our meta-analysis showed that higher maternal serum resistin level is related to GDM risk and suggested that the serum level of resistin may be related to the severity of GDM.
| 1. | Buchanan TA, Xiang AH. Gestational diabetes mellitus. J Clin Invest. 2005;115:485-491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 578] [Cited by in RCA: 578] [Article Influence: 27.5] [Reference Citation Analysis (0)] |
| 2. | Schneider S, Bock C, Wetzel M, Maul H, Loerbroks A. The prevalence of gestational diabetes in advanced economies. J Perinat Med. 2012;40:511-520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 90] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 3. | Li X, Tan H, Huang X, Zhou S, Hu S, Wang X, Xu X, Liu Q, Wen SW. Similarities and differences between the risk factors for gestational hypertension and preeclampsia: A population based cohort study in south China. Pregnancy Hypertens. 2016;6:66-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 43] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 4. | He XJ, Qin FY, Hu CL, Zhu M, Tian CQ, Li L. Is gestational diabetes mellitus an independent risk factor for macrosomia: a meta-analysis? Arch Gynecol Obstet. 2015;291:729-735. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 77] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 5. | Hartling L, Dryden DM, Guthrie A, Muise M, Vandermeer B, Donovan L. Benefits and harms of treating gestational diabetes mellitus: a systematic review and meta-analysis for the U.S. Preventive Services Task Force and the National Institutes of Health Office of Medical Applications of Research. Ann Intern Med. 2013;159:123-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 245] [Cited by in RCA: 268] [Article Influence: 20.6] [Reference Citation Analysis (1)] |
| 6. | Steppan CM, Bailey ST, Bhat S, Brown EJ, Banerjee RR, Wright CM, Patel HR, Ahima RS, Lazar MA. The hormone resistin links obesity to diabetes. Nature. 2001;409:307-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3205] [Cited by in RCA: 3246] [Article Influence: 129.8] [Reference Citation Analysis (1)] |
| 7. | Srinivasan M, Meadows ML, Maxwell L. Assessment of Salivary Adipokines Resistin, Visfatin, and Ghrelin as Type 2 Diabetes Mellitus Biomarkers. Biochem Res Int. 2018;2018:7463796. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 24] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 8. | Tokuyama Y, Osawa H, Ishizuka T, Onuma H, Matsui K, Egashira T, Makino H, Kanatsuka A. Serum resistin level is associated with insulin sensitivity in Japanese patients with type 2 diabetes mellitus. Metabolism. 2007;56:693-698. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 33] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 9. | Heidemann C, Sun Q, van Dam RM, Meigs JB, Zhang C, Tworoger SS, Mantzoros CS, Hu FB. Total and high-molecular-weight adiponectin and resistin in relation to the risk for type 2 diabetes in women. Ann Intern Med. 2008;149:307-316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 159] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 10. | Majdi MA, Mohammadzadeh NA, Lotfi H, Mahmoudi R, Alipour FG, Shool F, Moghanloo MN, Porfaraj S, Zarghami N. Correlation of Resistin Serum Level with Fat Mass and Obesity-Associated Gene (FTO) rs9939609 Polymorphism in Obese Women with Type 2 Diabetes. Diabetes Metab Syndr. 2017;11 Suppl 2:S715-S720. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 11. | Santilli F, Liani R, Di Fulvio P, Formoso G, Simeone P, Tripaldi R, Ueland T, Aukrust P, Davì G. Increased circulating resistin is associated with insulin resistance, oxidative stress and platelet activation in type 2 diabetes mellitus. Thromb Haemost. 2016;116:1089-1099. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 48] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 12. | Choi SH, Kwak SH, Youn BS, Lim S, Park YJ, Lee H, Lee N, Cho YM, Lee HK, Kim YB, Park KS, Jang HC. High plasma retinol binding protein-4 and low plasma adiponectin concentrations are associated with severity of glucose intolerance in women with previous gestational diabetes mellitus. J Clin Endocrinol Metab. 2008;93:3142-3148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 53] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 13. | Huang H, Xiao L, Wang A, Gu J, Ke H, Yan Y, Yang X. Correlation of resistin expression in maternal serum and subcutaneous adipose tissue with insulin resistance in gestational diabetes mellitus. Int J Clin Exp Med. 2016;9:18721-18727. |
| 14. | Kuzmicki M, Telejko B, Szamatowicz J, Zonenberg A, Nikolajuk A, Kretowski A, Gorska M. High resistin and interleukin-6 levels are associated with gestational diabetes mellitus. Gynecol Endocrinol. 2009;25:258-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 109] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 15. | Liu DY, Yu J, Zhou SX, Zhou Y, Li QM, Shi XY, Lin F, Shi QF. Study of levels of resistin in patients with gestational diabetes mellitus. Dalian Yike Daxue Xuebao. 2008;30:334-335, 343. |
| 16. | Oncul M, Tuten A, Erman H, Gelisgen R, Benian A, Uzun H. Maternal and cord blood apelin, resistin and visfatin levels in gestational diabetes mellitus. Minerva Med. 2013;104:527-535. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 17. | Palik E, Baranyi E, Melczer Z, Audikovszky M, Szöcs A, Winkler G, Cseh K. Elevated serum acylated (biologically active) ghrelin and resistin levels associate with pregnancy-induced weight gain and insulin resistance. Diabetes Res Clin Pract. 2007;76:351-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 76] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 18. | Vitoratos N, Deliveliotou A, Dimitrakaki A, Hassiakos D, Panoulis C, Deligeoroglou E, Creatsas GK. Maternal serum resistin concentrations in gestational diabetes mellitus and normal pregnancies. J Obstet Gynaecol Res. 2011;37:112-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 19. | Chen D, Fang Q, Chai Y, Wang H, Huang H, Dong M. Serum resistin in gestational diabetes mellitus and early postpartum. Clin Endocrinol (Oxf). 2007;67:208-211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 38] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 20. | Akdeniz FT, Akbulut Z, Sarı H, Aydın H, Dalan AB, Şit D, İspir T, Demirel GY. Effect of pro-inflammatory markers on gestational diabetes. Turkish J Immunology. 2017;5:45-50. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 21. | Bagci H, Melekoglu R, Gursu MF, Akyol A, Bulmus FG. Associations between serum levels of adiponectin and resistin and metabolic parameters in pregnant women with gestational diabetes mellitus. Clin Exp Obstet Gynecol. 2018;45:539-543. [RCA] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 22. | Oh ES, Han JH, Han SM, Im JA, Rhee EJ, Park CY, Oh KW, Lee WY. Adipokine Concentrations in Pregnant Korean Women with Normal Glucose Tolerance and Gestational Diabetes Mellitus. Korean Diabetes J. 2009;33:279-288. [RCA] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 23. | Noureldeen AF, Qusti SY, Al-Seeni MN, Bagais MH. Maternal leptin, adiponectin, resistin, visfatin and tumor necrosis factor-alpha in normal and gestational diabetes. Indian J Clin Biochem. 2014;29:462-470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 39] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 24. | Shang M, Dong X, Hou L. Correlation of adipokines and markers of oxidative stress in women with gestational diabetes mellitus and their newborns. J Obstet Gynaecol Res. 2018;44:637-646. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 53] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 25. | Siddiqui K, George TP, Nawaz SS, Shehata N, El-Sayed AA, Khanam L. Serum adipokines (adiponectin and resistin) correlation in developing gestational diabetes mellitus: pilot study. Gynecol Endocrinol. 2018;34:502-506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 26. | Zheng L, Zhang H, Du J. Study on the Correlation of Serum Adiponectin and Resistin with Gestational Diabetes Mellitus Pregnant Women. Zhongguo Yike Daxue Xuebao. 2011;40:645-648. [RCA] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 27. | McManus R, Summers K, de Vrijer B, Cohen N, Thompson A, Giroux I. Maternal, umbilical arterial and umbilical venous 25-hydroxyvitamin D and adipocytokine concentrations in pregnancies with and without gestational diabetes. Clin Endocrinol (Oxf). 2014;80:635-641. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 38] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 28. | Megia A, Vendrell J, Gutierrez C, Sabaté M, Broch M, Fernández-Real JM, Simón I. Insulin sensitivity and resistin levels in gestational diabetes mellitus and after parturition. Eur J Endocrinol. 2008;158:173-178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 46] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 29. | Park S, Kim MY, Baik SH, Woo JT, Kwon YJ, Daily JW, Park YM, Yang JH, Kim SH. Gestational diabetes is associated with high energy and saturated fat intakes and with low plasma visfatin and adiponectin levels independent of prepregnancy BMI. Eur J Clin Nutr. 2013;67:196-201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 70] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 30. | Hossein-nezhad A, Mirzaei K, Maghbooli Z, Rahmani M, Larijani B. Resistin, adiponectin and visfatin; can adipocytokines predict gestational diabetes mellitus and early post partum metabolic syndrome? Iranian J Diabetes Lipid Disorders. 2010;9:1-8. |
| 31. | Akdeniz N, Kuyumcuoğlu U, Kale A, Arikan S, Kale E, Erdemoğlu M. Resistin may not associate with gestational diabetes mellitus although insulin resistance. Clin Exp Obstet Gynecol. 2011;38:236-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 32. | Boyadzhieva M, Atanasova I, Zacharieva S, Kedikova S. Adipocytokines during pregnancy and postpartum in women with gestational diabetes and healthy controls. J Endocrinol Invest. 2013;36:944-949. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 33. | Cortelazzi D, Corbetta S, Ronzoni S, Pelle F, Marconi A, Cozzi V, Cetin I, Cortelazzi R, Beck-Peccoz P, Spada A. Maternal and foetal resistin and adiponectin concentrations in normal and complicated pregnancies. Clin Endocrinol (Oxf). 2007;66:447-453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 154] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 34. | Fugmann M, Uhl O, Hellmuth C, Hetterich H, Kammer NN, Ferrari U, Parhofer KG, Koletzko B, Seissler J, Lechner A. Differences in the serum nonesterified Fatty Acid profile of young women associated with a recent history of gestational diabetes and overweight/obesity. PLoS One. 2015;10:e0128001. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 35. | Georgiou HM, Lappas M, Georgiou GM, Marita A, Bryant VJ, Hiscock R, Permezel M, Khalil Z, Rice GE. Screening for biomarkers predictive of gestational diabetes mellitus. Acta Diabetol. 2008;45:157-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 111] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 36. | Karatas A, Tunçay Işikkent N, Ozlü T, Demirin H. Relationship of maternal serum resistin and visfatin levels with gestational diabetes mellitus. Gynecol Endocrinol. 2014;30:355-358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 37. | Guelfi KJ, Ong MJ, Li S, Wallman KE, Doherty DA, Fournier PA, Newnham JP, Keelan JA. Maternal circulating adipokine profile and insulin resistance in women at high risk of developing gestational diabetes mellitus. Metabolism Clinical And Experimental. 2017;75:54-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 38. | Kleiblova P, Dostalova I, Bartlova M, Lacinova Z, Ticha I, Krejci V, Springer D, Kleibl Z, Haluzik M. Expression of adipokines and estrogen receptors in adipose tissue and placenta of patients with gestational diabetes mellitus. Mol Cell Endocrinol. 2010;314:150-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 87] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 39. | Kralisch S, Stepan H, Kratzsch J, Verlohren M, Verlohren HJ, Drynda K, Lössner U, Blüher M, Stumvoll M, Fasshauer M. Serum levels of adipocyte fatty acid binding protein are increased in gestational diabetes mellitus. Eur J Endocrinol. 2009;160:33-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 46] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 40. | Kulik-Rechberger B, Mora-Janiszewska O. [Serum resistin concentrations in cases of gestational diabetes mellitus with good glycemic control and in cord blood]. Ginekol Pol. 2009;80:432-436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 41. | Lain KY, Daftary AR, Ness RB, Roberts JM. First trimester adipocytokine concentrations and risk of developing gestational diabetes later in pregnancy. Clin Endocrinol (Oxf). 2008;69:407-411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 88] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 42. | Lobo TF, Torloni MR, Mattar R, Nakamura MU, Alexandre SM, Daher S. Adipokine levels in overweight women with early-onset gestational diabetes mellitus. J Endocrinol Invest. 2018;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 43. | Nanda S, Poon LC, Muhaisen M, Acosta IC, Nicolaides KH. Maternal serum resistin at 11 to 13 weeks' gestation in normal and pathological pregnancies. Metabolism. 2012;61:699-705. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 44. | Pagán A, Sabater-Molina M, Olza J, Prieto-Sánchez MT, Blanco-Carnero JE, Parrilla JJ, Gil Á, Larqué E. A gene variant in the transcription factor 7-like 2 (TCF7L2) is associated with an increased risk of gestational diabetes mellitus. Eur J Obstet Gynecol Reprod Biol. 2014;180:77-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 28] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 45. | Ravnsborg T, Andersen LL, Trabjerg ND, Rasmussen LM, Jensen DM, Overgaard M. First-trimester multimarker prediction of gestational diabetes mellitus using targeted mass spectrometry. Diabetologia. 2016;59:970-979. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 30] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 46. | Rottenkolber M, Ferrari U, Holland L, Aertsen S, Kammer NN, Hetterich H, Fugmann M, Banning F, Weise M, Sacco V, Kohn D, Freibothe I, Hutter S, Hasbargen U, Lehmann R, Grallert H, Parhofer KG, Seissler J, Lechner A. The Diabetes Risk Phenotype of Young Women With Recent Gestational Diabetes. J Clin Endocrinol Metab. 2015;100:E910-E918. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 42] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 47. | Skvarca A, Tomazic M, Krhin B, Blagus R, Janez A. Adipocytokines and insulin resistance across various degrees of glucose tolerance in pregnancy. J Int Med Res. 2012;40:583-589. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 48. | Takhshid MA, Zare Z. Resistin - 420 C/G polymorphism and serum resistin level in Iranian patients with gestational diabetes mellitus. J Diabetes Metab Disord. 2015;14:37. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 49. | Tsiotra PC, Halvatsiotis P, Patsouras K, Maratou E, Salamalekis G, Raptis SA, Dimitriadis G, Boutati E. Circulating adipokines and mRNA expression in adipose tissue and the placenta in women with gestational diabetes mellitus. Peptides. 2018;101:157-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 51] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 50. | Vitoratos N, Dimitrakaki A, Vlahos NF, Gregoriou O, Panoulis K, Christopoulos P, Creatsas G. Maternal and umbilical resistin levels do not correlate with infant birth weight either in normal pregnancies and or in pregnancies complicated with gestational diabetes. J Matern Fetal Neonatal Med. 2010;23:1019-1023. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 51. | Lappas M, Yee K, Permezel M, Rice GE. Release and regulation of leptin, resistin and adiponectin from human placenta, fetal membranes, and maternal adipose tissue and skeletal muscle from normal and gestational diabetes mellitus-complicated pregnancies. J Endocrinol. 2005;186:457-465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 184] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 52. | Verhaeghe J, van Bree R, Lambin S, Caluwaerts S. Adipokine profile and C-reactive protein in pregnancy: effects of glucose challenge response versus body mass index. J Soc Gynecol Investig. 2005;12:330-334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 53. | Minn AH, Patterson NB, Pack S, Hoffmann SC, Gavrilova O, Vinson C, Harlan DM, Shalev A. Resistin is expressed in pancreatic islets. Biochem Biophys Res Commun. 2003;310:641-645. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 63] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 54. | Sheng CH, Di J, Jin Y, Zhang YC, Wu M, Sun Y, Zhang GZ. Resistin is expressed in human hepatocytes and induces insulin resistance. Endocrine. 2008;33:135-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 45] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 55. | Street ME, Viani I, Ziveri MA, Volta C, Smerieri A, Bernasconi S. Impairment of insulin receptor signal transduction in placentas of intra-uterine growth-restricted newborns and its relationship with fetal growth. Eur J Endocrinol. 2011;164:45-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 42] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 56. | Hauguel-de Mouzon S, Guerre-Millo M. The placenta cytokine network and inflammatory signals. Placenta. 2006;27:794-798. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 208] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 57. | Zhou Y, Zhang M, Guo W, Yu M, Xue K, Huang S, Chen Y, Zhu H, Xu L, Guo T. Expression of resistin protein in normal human subcutaneous adipose tissue and pregnant women subcutaneous adipose tissue and placenta. J Huazhong Univ Sci Technolog Med Sci. 2006;26:288-291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 58. | Lobo TF, Torloni MR, Gueuvoghlanian-Silva BY, Mattar R, Daher S. Resistin concentration and gestational diabetes: a systematic review of the literature. J Reprod Immunol. 2013;97:120-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 29] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 59. | Wells G, Shea B, O'Connell J. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. 2018; Available from: URL: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. |
| 60. | Zhang T, Zhong W, editors. Applied methodology for evidence-based medicine. Changsha, China: Central South University Press. 2011;. |
| 61. | Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions version 5.1.0. The Cochrane Collaboration. 2011;. |
| 62. | Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. 2005;5:13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4895] [Cited by in RCA: 7287] [Article Influence: 347.0] [Reference Citation Analysis (1)] |
| 63. | Whitehead A, Whitehead J. A general parametric approach to the meta-analysis of randomized clinical trials. Stat Med. 1991;10:1665-1677. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 515] [Cited by in RCA: 538] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 64. | Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539-1558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21630] [Cited by in RCA: 27085] [Article Influence: 1128.5] [Reference Citation Analysis (0)] |
| 65. | Banerjee RR, Rangwala SM, Shapiro JS, Rich AS, Rhoades B, Qi Y, Wang J, Rajala MW, Pocai A, Scherer PE, Steppan CM, Ahima RS, Obici S, Rossetti L, Lazar MA. Regulation of fasted blood glucose by resistin. Science. 2004;303:1195-1198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 516] [Cited by in RCA: 519] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 66. | Ozias MK, Li S, Hull HR, Brooks WM, Carlson SE. Relationship of circulating adipokines to body composition in pregnant women. Adipocyte. 2014;4:44-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 67. | Dong Y, Chauhan M, Betancourt A, Belfort M, Yallampalli C. Adipose Tissue Inflammation and Adrenomedullin Overexpression Contribute to Lipid Dysregulation in Diabetic Pregnancies. J Clin Endocrinol Metab. 2018;103:3810-3818. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 68. | Houshmand-Oeregaard A, Hansen NS, Hjort L, Kelstrup L, Broholm C, Mathiesen ER, Clausen TD, Damm P, Vaag A. Differential adipokine DNA methylation and gene expression in subcutaneous adipose tissue from adult offspring of women with diabetes in pregnancy. Clin Epigenetics. 2017;9:37. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 48] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 69. | Urbanek M, Hayes MG, Lee H, Freathy RM, Lowe LP, Ackerman C, Jafari N, Dyer AR, Cox NJ, Dunger DB, Hattersley AT, Metzger BE, Lowe WL. The role of inflammatory pathway genetic variation on maternal metabolic phenotypes during pregnancy. PLoS One. 2012;7:e32958. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 70. | Cho YM, Youn BS, Chung SS, Kim KW, Lee HK, Yu KY, Park HJ, Shin HD, Park KS. Common genetic polymorphisms in the promoter of resistin gene are major determinants of plasma resistin concentrations in humans. Diabetologia. 2004;47:559-565. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 93] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 71. | Osawa H, Yamada K, Onuma H, Murakami A, Ochi M, Kawata H, Nishimiya T, Niiya T, Shimizu I, Nishida W, Hashiramoto M, Kanatsuka A, Fujii Y, Ohashi J, Makino H. The G/G genotype of a resistin single-nucleotide polymorphism at -420 increases type 2 diabetes mellitus susceptibility by inducing promoter activity through specific binding of Sp1/3. Am J Hum Genet. 2004;75:678-686. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 183] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country of origin: China
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See:
P- Reviewer: Das U, Kai K S- Editor: Ji FF L- Editor: Wang TQ E- Editor: Bian YN