Published online Dec 26, 2019. doi: 10.12998/wjcc.v7.i24.4254
Peer-review started: April 15, 2019
First decision: September 9, 2019
Revised: October 17, 2019
Accepted: November 26, 2019
Article in press: November 26, 2019
Published online: December 26, 2019
Processing time: 257 Days and 3.5 Hours
The literature suggests that there is a high degree of co-occurrence between chronic pain and posttraumatic stress disorder (PTSD). An association has been found between PTSD and substance abuse. PTSD is a severe disorder that should be taken into account when opioids are prescribed. It has been found that the prevalence of opioid use disorder (OUD) in chronic pain patients is higher among those with PTSD than those without this disorder.
To perform a systematic review on the association between PTSD, chronic non-cancer pain (CNCP), and opioid intake (i.e., prescription, misuse, and abuse).
We conducted a systematic review following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines. The Patient, Intervention, Comparator, and Outcomes (PICOS) criteria were formulated a priori in the protocol of the systematic review. A search was conducted of the PROSPERO database. In March 2019, searches were also conducted of 5 other databases: PubMed, MEDLINE, PsycINFO, Web of Science, and PILOTS. The Scottish Intercollegiate Guidelines Network checklist for cohort studies was used to assess the selected studies for their methodological quality and risk of bias. Each study was evaluated according to its internal validity, participant sampling, confounding variables, and the statistical analysis.
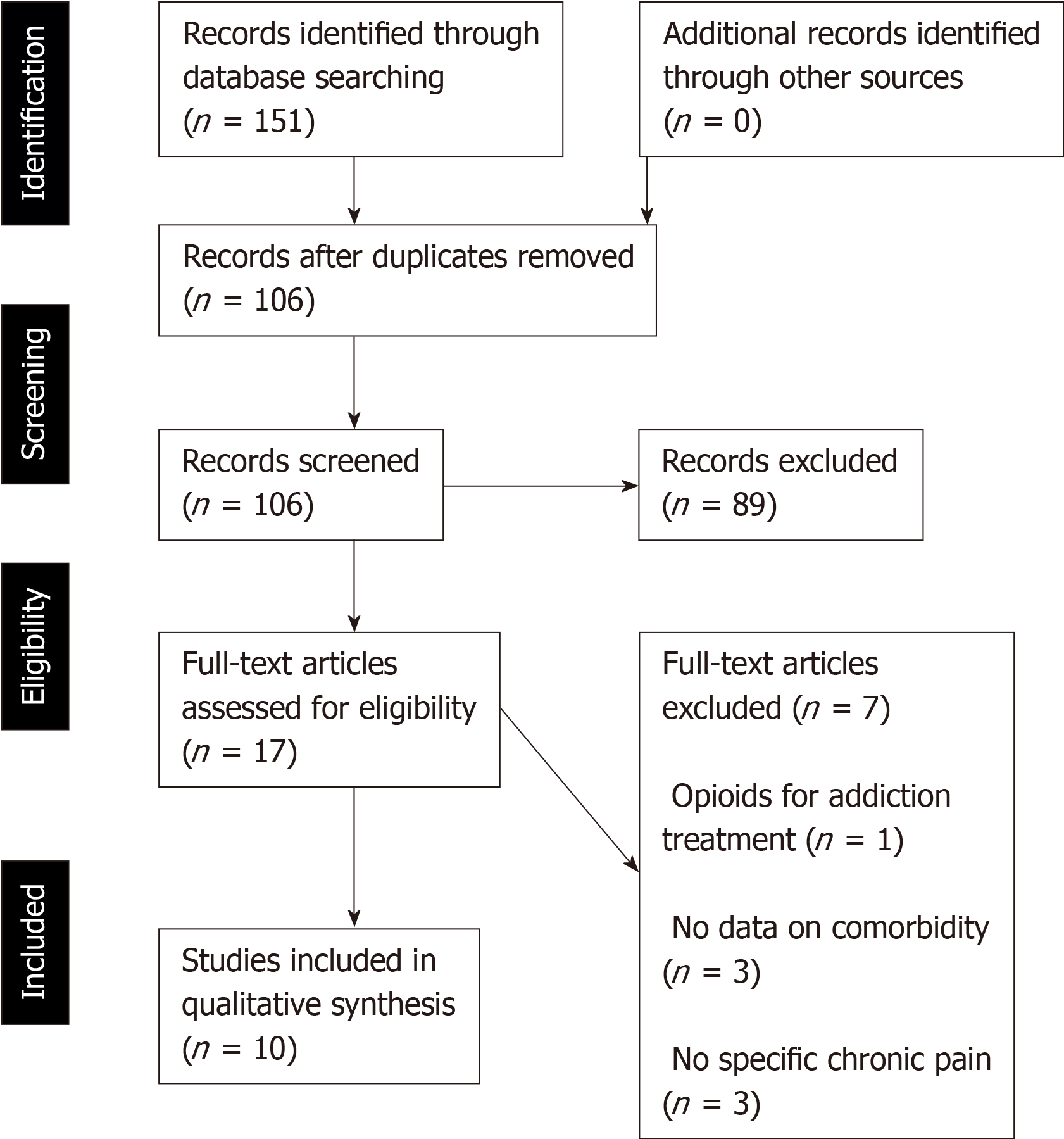
A total of 151 potentially eligible studies were identified of which 17 were retained for analysis. Only 10 met the selection criteria. All the studies were published between 2008 and 2018 and were conducted in the United States. The eligible studies included a total of 1622785 unique participants. Of these, 196516 had comorbid CNCP and PTSD and were consuming opiates. The participants had a cross-study mean age of 35.2 years. The majority of participants were men (81.6%). The most common chronic pain condition was musculoskeletal pain: back pain (47.14% across studies; range: 16%-60.6%), arthritis and joint pain (31.1%; range: 18%-67.5%), and neck pain (28.7%; range: 3.6%-63%). In total, 42.4% of the participants across studies had a diagnosis of PTSD (range: 4.7%-95%). In relation to opioid intake, we identified 2 different outcomes: opioid prescription and OUD. All the studies reported evidence of a greater prevalence of PTSD in CNCP patients who were receiving prescribed opioids and that PTSD was associated with OUD in CNCP patients.
Opioid analgesic prescription as the treatment of choice for CNCP patients should include screening for baseline PTSD to ensure that these drugs are safely consumed.
Core tip: In the management of chronic pain, co-morbid posttraumatic stress disorder (PTSD) plays a critical role in opioid misuse or abuse. The present systematic review found that the study participants with comorbid PTSD and chronic non-cancer pain received higher doses of opioids, received more than 1 type of opioid concurrently, and were more likely to receive chronic opioids.
- Citation: López-Martínez AE, Reyes-Pérez Á, Serrano-Ibáñez ER, Esteve R, Ramírez-Maestre C. Chronic pain, posttraumatic stress disorder, and opioid intake: A systematic review. World J Clin Cases 2019; 7(24): 4254-4269
- URL: https://www.wjgnet.com/2307-8960/full/v7/i24/4254.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v7.i24.4254
Chronic pain is a complex experience in which physical, emotional, cognitive, and social factors play a key role[1,2]. Thus, a multidisciplinary approach to treatment should be taken. Nevertheless, in reality, treatment is almost always pharmacological[3]. Over the last 2 decades, there has been a dramatic increase in prescribing opioid medications as a treatment strategy for patients with chronic non-cancer pain (CNCP), which has led to a large number of patients receiving chronic opioids[4]. However, the effectiveness of chronic opioid therapy in CNCP patients remains controversial[5]. It has been found that opioid misuse is common in these patients[6]. Smith et al[7] defined opioid misuse as the use of prescription opioids for non-medical purposes. There is a high rate of inappropriate opioid prescription[8]. Thus, it is crucial to identify individuals at risk of developing opioid use disorders (OUDs)[9]. Before starting opioid therapy, clinicians should identify any potential opioid abuse risk factors[10]. Mental health problems have been considered to be risk factors that may result in substance abuse over time[11]. Specifically, an association has been found between posttraumatic stress disorder (PTSD) and substance abuse and chronic pain conditions[12,13]. PTSD is a severe disorder that should be taken into account when opioids are prescribed.
As López-Martínez et al[12] reported, an association has been found between a history of trauma exposure and an increased risk of substance use disorders. Furthermore, individuals with a history of trauma engage in more adverse health practices than those without such a history[14]. Thus, there are increased rates of substance use disorders among PTSD patients[13,15]. Otis et al[16] found that approximately 10% of the patients who attended a pain care center also had PTSD, and that the rate of PTSD was considerably higher when the onset of pain corresponded to a traumatic event. The literature suggests that there is a high degree of co-occurrence between chronic pain and PTSD. It has also been suggested that the high comorbidity of these disorders is due to either shared vulnerability or mutual maintenance[17]. Thus, Friedman[18] hypothesized that PTSD patients may have endogenous opioid system dysregulation resulting in lower pain thresholds and lower endogenous opioid levels. In relation to this hypothesis, Phifer et al[19] suggested that PTSD and pain could share a vulnerability pathway including the endogenous opioid neurotransmission systems. According to the mutual-maintenance theory[17], PTSD and chronic pain may influence each other and lead to an exacerbation of symptoms[20]. This reciprocal cycle may lead to opioids being prescribed to control pain[16,21].
Several studies have drawn attention to some of the benefits of opioid use in PTSD patients with pain. For example, Bryant et al[22] found a significant association between the level of self-reported pain and the severity of PTSD symptoms in adults with trauma. These researchers suggested that opioid use had a significant protective effect against PTSD symptoms in this population. Holbrook et al[23] suggested that the use of opioids to reduce pain intensity could contribute to lowering the risk of the subsequent development of PTSD after severe injury. The results of these studies could explain the high rates of prescription opioid use in PTSD patients[24]. For example, Phifer et al[19] found that patients with a current diagnosis of PTSD were significantly more likely to have used prescribed opioid analgesics for pain control than those without this diagnosis.
However, it is noteworthy that CNCP patients with PTSD can be at risk of opioid abuse[18]. The prevalence of OUD in chronic pain patients has now been found to be higher among PTSD patients than among those without PTSD[13,25]. Hassan et al[9] suggested that PTSD increases the risk of developing OUD after exposure to opioid painkillers.
Similar results have been found in the empirical literature on veterans with PTSD. For example, several studies have found that this group has higher use rates of prescription opioids[26-28], higher use rates of health care services than veterans with pain or PTSD symptoms alone[29,30], and a much higher risk of opioid misuse[4,31].
Therefore, in the management of chronic pain, co-morbid PTSD plays a key role in opioid misuse or abuse. Although patients with co-morbid PTSD are at increased risk of substance abuse, it has been found that PTSD patients have higher rates of prescription opioid use[28]. As Ecker and Hundt[32] suggested, if a CNCP patient has PTSD, clinicians who decide to prescribe opioid analgesics should assess ongoing symptoms and, when possible, attempt to provide integrated care.
Despite this body of empirical evidence, to the best of our knowledge there are no published systematic reviews on the association between PTSD, CNCP, and opioid intake (i.e., prescription, misuse, and abuse). Therefore, the aim of this study was to systematically review this association and summarize the current body of scientific knowledge.
We conducted a systematic review of the literature. To ensure accuracy in the formulation of the research questions[33], the Patients, Intervention, Comparator, and Outcomes(PICOS) criteria were formulated a priori in the protocol of the systematic review. A search was conducted of the PROSPERO[34]. PROSPERO is an international database of prospectively registered systematic reviews of health-related topics. It records and maintains the key features of current review protocols as a permanent record. The present review followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines on quality standards for systematic reviews[35].
In March 2019, an electronic search was conducted of 5 databases: PubMed, MEDLINE, PsycINFO, Web of Science, and PILOTS. The search was limited to the English and Spanish languages, peer-reviews, and human studies. To collect all relevant articles, no limit was placed on the year of publication or publication status. Boolean logic operators were used to combine search terms. Specifically, the search included forms of the following terms: “chronic pain” AND “post-traumatic stress” AND “opioid”.
In order to be eligible for inclusion, the following criteria were applied: (1) The study was quantitative; (2) The study only included adults (> 18 years); (3) The study included participants with both PTSD and chronic pain; and (4) The study included patients receiving opioids for pain treatment. Articles were excluded if the study sample reported: (1) Cancer or postoperative or experimental pain; (2) Severe mental illness; and (3) The use of opioids for the treatment of addiction. We also excluded reviews, intervention studies, editorials, and conference abstract.
The selection process was conducted by 2 researchers (the second and third authors of this article). In a first step, each researcher independently examined the title and abstract of the articles according to the eligibility criteria. Those that did not meet the criteria were excluded. However, in case of doubt or disagreement between the researchers, the study was included as potentially relevant. In a second step, the full text of all potentially relevant studies was analyzed and disagreements over the issue of inclusion or exclusion were discussed with the other authors. In a final step, the 2 researchers extracted relevant data from each of the selected articles, reached a consensus on differences, and completed a table for publication.
The Scottish Intercollegiate Guidelines Network checklist for cohort studies was used to assess the selected studies for their methodological quality and risk of bias. Each study was evaluated according to its internal validity, the participant sampling method, confounding variables, and the type of statistical analysis. Internal validity was assessed according to whether the study addressed an appropriate and clearly focused question and whether the definitions of the variables (chronic pain, PTSD, and opioid intake) were clearly established. The participant sampling method was assessed according to whether: (1) The study groups were selected from the same source population (i.e., they were comparable in all respects other than the factor under investigation); (2) The methodology section indicated how many of the people asked to take part did so; and (3) The study indicated the percentage of individuals or clusters recruited that dropped out before the study was completed. Confounders were assessed according to whether the main potential confounders in each study were identified and taken into account in the design and analysis, and whether confidence intervals were provided in the results section. Finally, an overall assessment of each study was conducted and each was rated as having met “all or most of the criteria”, “some of the criteria” or “few or none of the criteria”.
Two authors (the second and third authors of this article) independently analyzed each article according to the foregoing criteria, compared their assessments, and reached consensus. Based on this strategy, each study was rated as having “little or no risk of bias”, “moderate risk of bias”, or “high risk of bias”.
Figure 1 shows the results of the study selection process according to the PRISMA flow diagram for published studies. The research strategy used identified 151 potentially eligible studies (18 from PubMed, 23 from MEDLINE, 21 from PsycINFO, 81 from Web of Science, and 8 from PILOTS). Forty-five eligible studies were excluded because they were duplicated across the 5 databases. Thus, a total of 106 articles were considered as potentially eligible on the basis of their title and the information contained in the abstract. Of these, 89 were rejected because they did not meet the selection criteria: (1) 11 studies included participants without chronic pain (e.g., they had postoperative pain), or the authors did not provide specific information on pain diagnosis; (2) 10 studies included chronic cancer pain patients; (3) 5 studies stated that their participants had PTSD and mental illness (e.g., delirium); (4) 6 studies used opioids (i.e., methadone or buprenorphine) as the treatment for abuse disorders; (5) 36 studies did not concurrently analyse the 3 conditions (i.e., chronic pain, PTSD, and opioids intake); (6) 13 studies were theoretical articles or systematic reviews of chronic pain and/or PTSD; (7) 12 studies assessed a treatment for one of the conditions; and (8) 8 studies were animal studies.
Finally, the authors read the full text of the 17 selected studies. Ten of these met the selection criteria and were retained for analysis in this review (for more details, please see Figure 1). The 10 studies were published between 2008 and 2018 and were conducted in the United States.
Table 1 shows the main characteristics of the 10 studies that were retrieved, including their objectives, sample size, average age, gender distribution, pain intensity measures, PTSD, and opioid intake. Table 1 also shows the main results and conclusions of each study. Seven studies used a retrospective cohort design, 1 was a prospective cohort study[36], and 2 were cross-sectional studies[37,38].
| Ref. | Study aims | Sample size (n) | Participants’ origin | Mean age (SD) | Women (%)/Men (%) | Chronic pain + PTSD + Opioid intake participants (%) | Variables and measures | Study design | Statistical analyses | Summary of results related to current study aims | Conclusions related to current study aims |
| Bilevicius et al[40], 2018 | To assess independent and combined contributions of PTSD and chronic pain conditions (digestive, nerve, and musculoskeletal pain) on opioid use disorder (OUD) | n = 36309 | Civilian, non-institutionalised United States residents | Not given | 44/56 | 3 | Chronic pain: ICD-10; PTSD: AUDADIS-5; Opioids (abuse): AUDADIS-5 | Retrospective cohort study | Multivariate logistic regression | PTSD was associated with OUD for musculoskeletal pain [adjusted odds ratio (AOR1): 4.2 95%CI: 2.54-7.12, P < 0.001) and nerve pain (AOR1: 3.1 95%CI: 1.93-5.10, P < 0.001), but not for digestive pain (AOR1: 1.8, 95%CI: 0.85-3.82, P = 0.124) | Comorbidity between PTSD and musculoskeletal/nerve chronic pain is a vulnerability risk factor for OUD. These patients should be carefully screened for opioid use, regardless of whether they are seeking a prescription |
| Han et al[41], 2017 | To analyse the characteristics (including PTSD) associated with long-term opioid dosing trends among veterans with chronic musculoskeletal pain | n = 79015 | Veterans’ health care | 29.80 (9.10) | 11/89 | 49 | Chronic pain: ICD-9CM + NRS; PTSD: (ICD-9-CM); Opioids: Dispensing pattern (daily dose, total number of days, and number of prescription episodes) | Retrospective cohort study | General estimating equation (GEE), GEE-logistic models | PTSD, major depression disorder, and substance abuse disorder were associated with 30% increased odds of high-dose opioid prescribing. Veterans with these disorders had both higher log (ME/d) opioid dose (adjusted mean difference: 0.038, 0.057, 0.063; all P values < 0.0001) and greater odds of high-dose prescribing (adjusted OR, 1.31; 1.36; 1.32; P = 0.008, 0.001, 0.002) than those without each diagnosis, respectively. Excluding PTSD did not modify the predicted dosing trends of the average log (βt = 0.03, P < 0.001; βt2 = −0.003, P = 0.013) or the odds of high-dose prescribing (βt = 0.19, P = 0.035; βt2 = −0.03, P = 0.060) | Veterans who were dispensed opioids for an extended duration or who had a mental health diagnoses tend to receive high opioid dose therapy regardless of PTSD diagnosis. Future studies are needed to assess the potential impact of opioid dosing trends on clinical outcomes and effectiveness of emerging intervention programs targeting high-risk opioid prescribing |
| Hudson et al[39], 2017 | To examine the pharmacoepidemiology of opioid use among veterans with chronic pain who are regular users of veterans' health care | n = 1397946 | Inpatients/or outpatients veterans' health care | 34.10 (9.70) | 12/88 | 10 | Chronic pain: ICD-9 + NRS; PTSD: NEPEC; Opioids: Dispensing pattern (PBM records) | Retrospective cohort study | Multivariate logistic regression | The percentage of chronic opioid users with PTSD (58%) was higher than nonchronic users (41%) or those with no opioid use (29%). Veterans with PTSD were more likely to receive opioids chronically (OR: 1.22; 95%CI: 1.20–1.25; P = 0.01) than those with a diagnosis of major depressive or tobacco use disorders | A diagnosis of PTSD was strongly associated with chronic prescription and use of opioids |
| Liebschutz et al[37], 2010 | To analyse the characteristics associated with prescription drug use disorder (PDUD) in patients with chronic pain | n = 597 | Primary care | 45.51 (9.16) | Not given | 14 | Chronic pain: GCPS PTSD: CIDI, v.2.1 (PTSD module); Opioids (abuse): CIDI v.2.1 (Drug disorders module) | Cross-sectional study | Multivariate logistic regression | The percentage of PTSD (28%) in the PDUD group was higher than in those with no substance use disorder (17%) but not in those with a substance use disorder (33%). PDUD patients had the highest percentage of PTSD (52%). Along with other variables, PTSD was associated with greater odds of both PDUD and substance abuse disorder (OR: 1.93; 95%CI: 1.09–3.43; P = 0.01) | PTSD was associated with a high probability of PDUD. Physicians treating patients with pain should screen for PTSD to help identify those at the highest risk of PDUD |
| Macey et al[42], 2011 | To examine variables (including PTSD) associated with opioid prescription in chronic pain patients | n = 762 | Veterans' health care service. | 34.00 (8.80) | 15/85 | 40 | Chronic pain: NRS; PTSD: ICD9-CM; Opioids: Dispensing pattern (days of prescription within 12 mo) | Retrospective cohort study | Multivariate logistic regression | Veterans prescribed opioids long-term had a greater prevalence of PTSD, major depressive disorder, and nicotine use disorder, but the proportion of users with both a short-term and long-term prescription was higher for those with PTSD (56% and 69%, respectively). A PTSD diagnosis was associated with increased opioid prescriptions (OR = 1.42, 95%CI: 1.04-1.96) | PTSD is associated with a greater likelihood of receiving a prescription for an opioid medication, which suggests the need for improvement in implementing guideline-level pain care for these veterans |
| Outcalt et al[43], 2013 | To examine health care utilization and dispensed medication (including opioids) among veterans with both PTSD and chronic pain | n = 40716 | Primary care, pain-related specialty or mental health pain veteran outpatients | Not given | 5/95 | Not given | Chronic pain: ICD-9; PTSD: ICD-9+PC-PTSD screen; Opioid: Pharmacy prescription dispensing data | Retrospective cohort study | Negative binomial regression and sensitivity analyses | Adjusted rates of opioid medication prescriptions were significantly higher for the chronic pain and PTSD group (mean = 1.47, SD = 0.86) than either of the comparison groups [pain only (mean = 0.92, SD = 0.51) and PTSD only (mean = 0.17, SD = 0.10) (P < 0.001)] | Opioids were more commonly prescribed for pain relief in veterans with both chronic pain and PTSD symptoms than in those with pain or PTSD alone. More integrated and streamlined treatments for this clinical population are needed |
| Seal et al[44], 2012 | To analyse the effect of mental health disorders, particularly PTSD, on patterns of opioid prescription, associated risks, and adverse outcomes | n = 141029 | Veterans’ health care | Not given | 11/89 | 11 | Chronic pain: ICD-9-CM; PTSD: ICD9-CM; Opioid: Dispensing pattern (≥ 20 consecutive days of prescription within 12 m | Retrospective cohort study | Poisson regression | Compared to veterans without a mental health diagnosis (6.5%) and veterans with mental health diagnoses other than PTSD (11.7%), 17.8% (adjusted RR, 2.58; 95%CI: 2.49-2.67) those with a PTSD diagnosis were significantly more likely to be prescribed opioids. Veterans with a drug use disorder and comorbid PTSD were more likely to be prescribed opioids than veterans with no mental health disorders (33.5% vs 6.5%; adjusted RR, 4.19; 95%CI: 3.84-4.57). Those with PTSD with prescription opioids were significantly more likely to be in the highest quintile by dose (22.7% vs 15.9%; adjusted RR, 1.42; 95%CI: 1.31-1.54), receive more than 1 type of opioid concurrently (19.8% vs 10.7%; adjusted RR, 1.87; 95%CI: 1.70-2.06), and obtain early opioid refills (33.8% vs 20.4%; adjusted RR, 1.64; 95%CI: 1.53-1.75) | Findings support further efforts to improve care of patients with comorbid pain and PTSD, given the heightened risk of self-medication with opioids and substance abuse in veterans with PTSD. Integrated treatments that simultaneously target mental health disorders and pain are effective for both problems and may decrease harm resulting from opioid therapy |
| Seal et al[45], 2018 | To test the hypothesis that among veterans with chronic pain diagnoses, greater traumatic brain injury severity and mental health comorbidity independently predict subsequent initiation of short- and long-term opioid therapy | n = 53124 | Veterans’ health care (veterans with traumatic brain injury) | 32.50 (8.50) | 7/93 | 17 | Chronic pain: not given; PTSD: ICD9-CM Opioids: Dispensing pattern (days of prescription within 12 mo) | Retrospective cohort study | Multivariate logistic regression | A PTSD diagnosis (69.4%) was significantly associated with subsequent long-term opioid therapy (RR, 1.98; 95%CI: 1.67-2.34) and, to a lesser extent, short-term opioid therapy (RR, 1.23; 95%CI: 1.15-1.31) after controlling for sex, race/ethnicity, marital status, rank and education, military component, branch of service, number of deployments, antidepressant medication use, alcohol disorders, drug disorders, and self-rated pain disability | Comorbid mental health problems substantially increase risk when initiating short- and long-term opioid therapy in veterans with moderate to severe traumatic brain injury, PTSD, and depression. There is a need to provide enhanced education and interdisciplinary behavioural health support to primary care providers who care for veterans with complex chronic pain that includes TBI and comorbid mental health problems |
| Trevino et al[36], 2013 | To examine opioid use and psychological characteristic (including PTSD) associated in traumatically injured individuals with chronic pain (secondary to physical injuries) 4 months post trauma | n = 101 | Trauma surgery inpatients' service | Not given | Not given | Not given | Chronic pain: BPI-SF; PTSD: PCL-C; Opioid: Opiates use (yes/no) | Prospective cohort study | Multivariate analysis of covariance | There was a statistically significant difference between those using narcotics and those not using narcotics at 4 mo posttrauma on the combined dependent variables (pain, life interference, depression, anxiety, PTSD, and length of hospital stay [F (6, 68) = 2.7, P = 0.02; Wilks’ lambda = 0.81; partial eta squared = 0.2 Bonferroni adjusted level = 0.007). PTSD was higher in patients with chronic pain and opioid use (mean = 44, SD = 3.6) than in the non-opioid use groups (mean = 32, SD = 2.7) | Opioid prescription should be used carefully in traumatically injured patients, especially in individuals with comorbid psychological pathology such as PTSD. Viewing chronic pain as a disease that requires further diagnostic workup through assessments of psychological disorders can help to identify those who may not benefit from opioid use and identify those at higher risk for adverse outcomes |
| Wilsey et al[38], 2008 | To analyse psychological factors (including PTSD) that are correlated with the propensity for prescription opioid abuse among patients with chronic pain | n = 113 | Patients visiting emergency department or urgent care centre | Not given | 42/58 | 34 | Chronic pain: not given; PTSD: SCID; Opioid (propensity abuse): SOAPP | Cross-sectional study | Multiple regression analysis | A total of 81% of the patients were positive according to SOAPP for propensity for prescription opioid abuse. PTSD (34%) was significantly correlated with the SOAPP score (r = 0.26, P < 0.005) but did not significantly predict this score (β = 1.2, P = 0.39) | Improving outcomes for patients with marked psychopathology will require treatments that independently address chronic pain and psychopathology |
The 10 eligible studies included 1622785 unique participants. Sample sizes ranged from 101 participants[36] to 1397946 participants[39]. In total, 196516 participants had comorbid CNCP and PTSD and were consuming opiates. Five studies reported an age range (18-70 years; mean 29.8-45.5 years). The cross-study mean age was 35.2 years. The majority of participants were men (81.6% across studies; range: 56%-95%). The majority of participants were of Caucasian origin (54.9% across studies; range: 21.6%-87%) in all but one of the studies[37]. The majority of the studies recruited the samples from the veterans' health care system. The remaining studies recruited participants from other health care sources (i.e., primary care centres, pain management clinics, gynaecology clinics, trauma surgery inpatient services, emergency departments, urgent care centres)[36-38] and from the noninstitutionalized civilian population[40].
The 10 selected studies were assessed for their methodological quality in relation to risk of bias. In total, 7 studies fulfilled all or most of the criteria (little risk of bias), whereas 3 studies[36-39] fulfilled some of the criteria (moderate risk of bias).
Regarding internal validity, all but 1 study[37] clearly defined CNCP, and only 4 studies specified the diagnosis[39,41-43]. All studies clearly defined the presence of PTSD. Although the studies used different measures to assess CNCP and PTSD, all of these measures were validated assessment tools or were well-known international classifications of diseases (e.g., International Classification of Diseases, Ninth Revision). However, 1 study[44] did not include a separate category for PTSD; instead, this diagnosis was aggregated with other mental health conditions (including alcohol and non-alcohol drug use disorders), thus affecting the results. Given that PTSD has high co-morbidity with other disorders, such as mood, anxiety, and substance use disorders, these variables should be considered as potential confounders. In fact, all the 10 studies controlled for both categories of disorders in their design and analyses. In addition, substance abuse disorder is a confounding variable of particular relevance when considering opioid intake as the outcome variable and must be controlled for in the analyses. All 10 studies fulfilled this aspect.
Regarding opioid classes, 3 studies provided a list of specific opioids[41,43,44], 2 studies[42,44] used a standard formula to calculate morphine equivalents to compare opioid doses across classes, and 3 studies[39,43,45] specified opioid replacement therapy for OUD (i.e., methadone and buprenorphine) as an exclusion criterion. With regard to opioid intake, 7 studies defined opioid prescription as the outcome variable. Three of these studies[40,42,44] converted daily opioid dose into average daily morphine equivalents in milligrams (ME/d) and used established conversion factors to identify opioid overdose. One of these studies[40] assessed opioid abuse or dependence, which were defined according to the DSM-5 criteria for prescription opiate use disorder. Liebschutz et al[37] used prescription drug use disorder (PDUD) as the criterion for abuse and also took into account the social, physical, or legal consequences of PDUD. The criteria for dependence also included compulsive use, health consequences, and physical dependence. Of note, this study not only included opioids in PDUD but also sedative drugs. Only 1 study[38] used a screening tool to determine the propensity for prescription opioid abuse. Trevino et al[36] only compared those who were taking opioids to those who were not taking them. These differences in the definition of outcome may affect the interpretation of the results and become a risk of bias.
The majority of the study samples were selected from the same source population and were comparable in all respects other than the factor under investigation. However, Wilsey et al[38] selected patients presenting to the emergency department seeking opioid refills for chronic pain instead of randomly selecting the sample from the target population. In the study by Trevino et al[36] the sample was small and only included participants from a single level-1 trauma centre.
All but 2 studies[38,39] statistically controlled for demographic characteristics such as age and sex. Three studies also controlled for race/ethnicity[36,37,41]. Of these, 2 also controlled for educational level[37,41] and 2 also controlled for marital status[40,45].
The only study that used a self-report tool to assess PTSD[40] failed to describe the psychometric properties of the scale. Furthermore, studies that use self-reports may be at risk of potential bias compared to studies that use databases in which PTSD is diagnosed by clinical interviews based on DSM diagnostic criteria.
The recommended dropout rate of 20% was only exceeded in 1 study, which applied dropout analysis[36]. When appropriate, confidence intervals were provided by all but 1 study[43]. To some degree all studies included the risk of confounding in the study design and discussed this issue in the appropriate sections. However, several studies were retrospective cohort studies using health records that had already been collected, which would have introduced some bias[46]. Thus, it could have been the case that many different healthcare professionals would have been involved in patient care, and so the measurement of risk factors and outcome(s) using such databases would probably be less accurate and consistent than that achieved with a prospective cohort study design. In addition, some of the studies had long follow-up times, which makes it difficult to ensure that outcomes were consistently measured or used the same criteria. Furthermore, long follow-up times entail the possibility of changes over time in the association between some risk factors and outcomes or conditions.
Most of the studies investigated variables associated with the prescription of opioids in CNCP. The majority of these variables were psychological. However, 2 studies[37,38] specifically examined prescribed OUD and only 1[40] assessed OUD with or without a prescription.
Musculoskeletal pain was the most common chronic pain condition in the participants: back pain (47.14% across studies; range: 16%-60.6%), arthritis and joint pain (31.1% across studies; range: 18%-67.5%), and neck pain (28.7% across studies, range: 3.6%-63%). Several studies[39,41,43,44] defined chronic pain according to the list of codes provided in the International Statistical Classification of Diseases and Related Health Problems 9th revision (ICD-9) and 1 study[40] used the 10th revision (ICD-10). Some of the studies[39,41,42] measured pain intensity, pain interference, and pain disability using self-reports, such as the Numerical Rating Scale(NRS), the Graded Chronic Pain Scale(GCPS)[37], and the Brief Pain Inventory Short Form (BPI-SF)[36].
In total, 42.4% of the participants across studies had a diagnosis of PTSD (range: 4.7%-95%). The majority of studies used the ICD-9 to define PTSD. However, 1 study[38] used the Structured Clinical Interview for DSM-IV Axis I Disorders, 1 study[37] used the Composite International Diagnostic Interview 2.1 (CIDI 2.1), and 1 study[36] used the PTSD Checklist - Civilian Version(PCL-C). It must be highlighted that only 2 studies[40,43] specifically investigated comorbid PTSD and chronic pain, whereas the other studies investigated a variety of psychological disorders including PTSD.
In relation to opioid intake, 2 different outcome variables were identified: opioid prescriptions and OUD. Opioid prescriptions were generally assessed according to the medical prescription-dispensing pattern, although this pattern was defined in different ways, such as total number of prescription days[41,42,44,45] and pharmacy records of opioid prescriptions[39,43]. One study[37] only indicated whether or not patients consumed prescribed opioids. Two studies used diagnostic interviews, such as the Alcohol Use Disorder and Associated Disabilities Interview Schedule, 5th edition (AUDADIS-5)[40] and the CIDI V.2.1[37] to determine OUD, whereas Wilsey et al[38] used the Screener and Opioid Assessment for Patients with Pain (SOAPP) to determine the propensity for prescribed opioid abuse.
All the studies reported evidence of a greater prevalence of PTSD in CNCP patients who had been prescribed opioids. Outcalt et al[43] found higher adjusted rates of opioid medication prescriptions in a CNCP plus PTSD group than in a CNCP-only and a PTSD-only group [means (SD) were 1.47 (0.8), 0.92 (0.51), and 0.17 (0.10), P < 0.0001, respectively). Likewise, Seal et al[44] found that after controlling for sex, race/ethnicity, and military rank, the likelihood of being prescribed opioids was significantly higher in veterans with PTSD than in veterans with or without a mental health diagnosis other than PTSD [odds ratio (OR) 2.74 and 4.65, P < 0.001, respectively). Those with PTSD who had been prescribed opioids were significantly more likely to be in the highest quintile by dose (22.7% vs 15.9%; adjusted RR, 1.42; 95%CI: 1.31-1.54), receive more than 1 type of opioid concurrently (19.8% vs 10.7%; adjusted RR, 1.87; 95%CI: 1.70-2.06), receive sedative hypnotics concurrently (40.7% vs 7.6%; adjusted RR, 5.46; 95%CI: 4.91-6.07), and obtain early opioid refills (33.8% vs 20.4%; adjusted RR, 1.64; 95%CI: 1.53-1.75). Opioids were more likely to be prescribed to veterans with comorbid PTSD and a drug use disorder than to veterans with no mental health disorders (33.5% vs 6.5%; adjusted RR, 4.19; 95%CI: 3.84-4.57).
Seal et al[45] found that, after controlling for numerous variables such as antidepressant medication use, alcohol and non-alcohol drug disorders, and self-informed pain disability, a diagnosis of PTSD was significantly associated with opioid therapy, and particularly with a long-term therapy (adjusted RR, 2.32; 95%CI: 2.05–2.63). Similarly, Macey et al[42] found that the proportion of users with PTSD who had been prescribed opioids short- and long-term was higher than those with major depressive disorder and those with nicotine use disorder. After adjusting for age, sex, and pain scores, a diagnosis of PTSD (OR = 1.42, 95%CI: 1.04–1.96) was associated with an increased likelihood of being prescribed opioids. Likewise, Hudson et al[39] found that the likelihood of receiving opioids chronically (OR, 1.22, P < 0.0001) was higher in veterans with a diagnosis of PTSD than in those with a diagnosis of major depressive or tobacco use disorders.
The only study[41] that investigated a group of psychological disorders (i.e., major depression disorder, substance abuse disorder, and PTSD) found that veterans with these diagnoses had higher log (ME/d) opioid doses (adjusted mean difference: 0.038, 0.057, 0.063; P < 0.0001) than those without such diagnoses. They also had greater odds of high-dose prescribing (adjusted OR, 1.31; 1.36; 1.32; P = 0.008, 0.001, 0.002 for PTSD, major depression disorder, and substance abuse disorder, respectively).
Trevino et al[36] found that the combination of pain interference, depression, anxiety, and PTSD (among other variables) statistically differentiated participants using opioids from those not using opioids [F (6,68) = 2.7, P = 0.02; Wilks’ lambda = 0.81; partial eta squared = 0.2 using a Bonferroni adjusted level of 0.007]. PTSD scores were higher in the opioid use group than in the non-opioid use group [44 (3.6), 32 (2.7), P < 0.0001, mean (SD), respectively].
The studies that investigated OUD[37,38,40] found that PTSD was associated with OUD in CNCP patients. Liebschutz et al[37] found that a PDUD group had a higher percentage of participants with current PTSD (31%) and that PTSD was independently and significantly associated with PDUD (OR = 1.93, 95%CI: 1.09–3.43). Bilevicius et al[40] found a significant association between having a chronic pain condition, PTSD, or a comorbid PTSD/chronic pain condition and OUD across musculoskeletal pain, digestive pain, and nerve pain conditions in an unadjusted model. After controlling for sociodemographic characteristics and mental health conditions, PTSD was associated with OUD in the digestive pain condition [adjusted OR (AOR1): 1.8, 95%CI: 1.23-2.76]. Digestive pain conditions alone and comorbid PTSD/digestive pain conditions were not significantly associated with OUD in the most stringent model. However, musculoskeletal pain and nerve pain conditions were significantly associated with OUD in the comorbid PTSD/chronic pain condition, with the largest effect sizes in the comorbid PTSD/chronic musculoskeletal pain condition group (musculoskeletal pain + PTSD: AOR1: 4.2, 95%CI: 2.54-7.12; nerve pain + PTSD: AOR1: 3.1, 95%CI: 1.93-5.10). However, Wilsey et al[38] found that PTSD did not significantly predict the propensity for prescribed opioid abuse as measured with the SOAPP (β = 1.2, SE = 1.4, P = 0.39).
This systematic review of the literature on the association between PTSD, CNCP, and opioid intake (i.e., prescription, misuse, and abuse) identified 10 studies with acceptable methodological quality and risk of bias.
A synthesis of the results showed that the prevalence of PTSD was higher in CNCP patients who had been prescribed opioids than in CNCP patients without opioid prescriptions. Comorbidity between CNCP conditions (in particular, chronic musculoskeletal pain conditions) and psychiatric conditions (e.g., PTSD) has been well documented in military samples[47,48] and in civilian samples across several countries[49]. Our systematic review suggests that participants with comorbid PTSD and CNCP receive higher doses of opioids, receive more than 1 type of opioid concurrently, and are more likely to be receiving opioids chronically. The results suggest that PTSD is associated with OUD in CNCP patients. It should be noted that these results derive from studies using samples of veterans.
Previous results have demonstrated that a diagnosis of OUD is more common among veterans than among non-veterans. In fact, 1 study found a striking increase in the percentage of a diagnosis of OUD among veterans receiving opioids (37% relative increase)[50]. However, the studies reviewed that included civilian samples also suggest that participants with both PTSD and CNCP[36,37,40] are more frequently prescribed opioids and have increased rates of OUD. The only exception was the study by Wilsey et al[38], which was also the only study that assessed the propensity to abuse using a self-report measure (i.e., SOAPP). However, the European Pain Federation (EFIC)[51] has recently recommended the use of this instrument, among others, in its revised form (i.e., SOAPP-R) to assess chronic pain patients at risk of drug abuse when initiating opioid therapy.
It has been found that a diagnosis of PTSD significantly increases the odds of having an OUD diagnosis and that baseline PTSD increases the risk of developing OUD after exposure to opioid analgesics in different populations[9]. There are several explanations for the increased risk of substance abuse among PTSD patients. These explanations refer to either biological aspects or to psychological aspects.
On the one hand, an association has been found between abnormalities in the endogenous opioid system and PTSD[23,52]. It has also been hypothesized that these abnormalities would manifest in lower pain thresholds and lower endogenous opioid levels[18]. It is well-established that stress plays a critical variable in drug abuse vulnerability. Exposure to trauma and stress causes corticotropin-releasing hormone to trigger the hypothalamic pituitary adrenal axis and acts centrally to mediate fear-related behaviours, thereby triggering other neurochemical responses to stress such as the noradrenergic system via the brainstem locus coeruleus[53]. Moreover, it has been found that noradrenergic overstimulation in individuals with PTSD may also increase their predisposition to illicit drug use for self-medication, especially for substances with sedative properties, such as alcohol, opioids, and benzodiazepines[54]. It has been recently demonstrated in a rodent model[55] that abnormal dopamine signals in the prefrontal cortex may underlie the ability of traumatic memories to predispose individuals to addiction by increasing their sensitivity to the rewarding effects of drugs such as opioids.
On the other hand, PTSD is characterized by avoidance of thoughts associated with the trauma as well as by increased arousal. Chronic arousal results in elevated physiologic responses, such as hypervigilance and an exaggerated startle response, leading to discomfort that many PTSD patients attempt to control by the use of drugs (e.g., opioids)[21]. Phifer et al[19] suggested that individuals with high-avoidance PTSD symptoms were more likely to seek opiates as a means to avoid thoughts pertaining to traumatic events. The opioid susceptibility model suggests that problematic opioid use emerges from an effort to medicate intolerable affect states and symptoms of stress[56]. Some results suggest that the use of opioids during acute trauma care could reduce the risk of the subsequent development of PTSD. In particular, it has been found that the use of morphine was associated with a significantly reduced risk of PTSD development in injured military personnel[23]. Similarly, some researchers have hypothesized that the beta-adrenergic mechanism activated by opiate intake could interfere with or prevent memory consolidation[57].
There is further evidence that the use of opiates for pain relief as part of trauma care has a protective effect against the subsequent development of PTSD. Thus, it has been suggested[21] that chronic pain patients who are using opioids do so to self-medicate to avoid experiencing PTSD symptoms. Of note, it has been suggested that PTSD and pain could share vulnerability pathways including the endogenous opioid neurotransmission systems[19]. For example, a significant and strong association has been found between self-reported pain levels within 48 hours after severe injury and a subsequent risk of PTSD[58]. A significant association has also been found between post-injury pain and an increased risk of PTSD 1 year after hospitalization among severely injured patients[59]. Sharp and Harvey’s Mutual Maintenance Model[60] suggests that PTSD and chronic pain are mutually maintaining, and that they may be involved in the escalation of symptoms. According to this model, certain aspects of the 2 conditions feed into each another, hence maintaining both conditions and escalating the levels of disability and distress.
The results of the present review suggested that there is high co-occurrence between PTSD, CNCP, and other psychological disorders such as depression, anxiety, and substance abuse. It is noteworthy that problems associated with opioid use have highlighted the serious unmet need for improved recognition and treatment of common mental health problems in patients with CNCP. Moreover, consistent screening practices and coordinated treatment have not been routine in the treatment of comorbid CNCP and psychological disorders such as PTSD[13]. In this regard, the recent EFIC position paper for primary care physicians and other non-specialist healthcare professionals in Europe[51] has provided a practical step-by-step guide to the clinical processes and considerations involved in initiating analgesic therapy with opioids. This position paper emphasised that patient suitability for opioid treatment should be assessed prior to initiating therapy. It also suggested that this assessment should address psychosocial issues such as mood, psychiatric morbidities, and addiction risk (including previous opioid use, if any).
It is also noteworthy that 6 of the reviewed studies reported the combined use of opioids and benzodiazepines. The prescription of benzodiazepines is justified by the aforementioned comorbidity between CNCP and certain mental health problems. However, the International Association for the Study of Pain stated that opioid analgesics should be used with caution when combined with benzodiazepines[61]. Likewise, the aforementioned EFIC position paper[51] warned that caution is required when prescribing opioids in patients using other centrally acting drugs, and that the co-prescription of opioids and benzodiazepines should be avoided whenever possible.
The conclusions of this review should be considered in the light of several limitations. Firstly, although the majority of studies considered opioid prescription as the outcome variable, 3 studies used opioid abuse (with or without prescription) as the outcome variable. This aspect limits comparability between the studies and the conclusions that may be drawn from this review. Secondly, caution is warranted in interpreting the results given that the studies differ in the nature and size of the samples, the methods used for assessing PTSD, the DSM criteria employed (DSM-IV or DSM-5), and the spectrum of other psychological disorders considered. Thirdly, in this respect, it should be noted that most of the reviewed studies used samples in which there was a predominance of men. The difference in pain experience between genders is well-documented in the literature, and there is consensus that both sexes should be included in medication trials in greater numbers to detect gender effects[62]. Empirical evidence suggests that the risk of opioid abuse is greater among women than among men[63] and that women are more likely to be treated with opioids, to use prescription opioids for longer periods, and to be prescribed higher doses[64]. Furthermore, the prevalence of PTSD, anxiety, and depression is higher among women than among men[65]. Fourthly, 2 studies included samples of patients with chronic pain due to a traumatic injury. In such patients, the onset of PTSD and chronic pain may coincide temporally. This aspect could lead to differences in prescriptions between these patients and patients whose diagnosis of PTSD was prior to the pain condition. Fifthly, several of the reviewed studies collected their data from administrative databases, and so no information was provided on the effectiveness of these opioid therapies or why some patients were ultimately prescribed opioids. Finally, half of the studies included in the review were conducted with samples from United States veterans, which limits the generalizability of the results to other populations of people with CNCP and comorbid PTSD.
Despite these limitations, it can be concluded that patients with CNCP should be screened for existing baseline PTSD to ensure that they can safely consume opioid analgesics when these drugs are prescribed as the treatment of choice.
The prescription of opioid drugs as a treatment strategy for patients with chronic pain unrelated to cancer has increased considerably. However, the effectiveness of this drug treatment has not been fully demonstrated and opioid misuse is common in these patients. In addition, substance abuse has been shown to be common in people with mental health problems. These include post-traumatic stress disorder. Additionally, it has been detected that patients with posttraumatic stress have higher rates of opioid prescriptions. Since this disorder has a high co-morbidity with chronic pain, chronic non-cancer pain (CNCP) patients with posttraumatic stress disorder (PTSD) can be at risk due to the wrong use of opioid.
The identification of individuals in danger of developing opioid use disorder (OUD) is certainly relevant in the field of medical intervention for patients with CNCP. Despite this, there are no published systematic reviews on the association between posttraumatic stress, chronic pain of benign origin, and opioid intake (i.e., prescription, misuse, and abuse).
A systematic review was accomplished in order to offer a comprehensive overview on currently available data regarding opioid intake in CNCP patients with comorbid PTSD.
We organized a systematic review according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses 2009 Checklist. The Patients, Intervention, Comparator, and Outcomes(PICOS) criteria were formulated a priori in the protocol of the systematic review. A search was conducted of the PROSPERO database. PubMed, MEDLINE, PsycINFO, Web of Science, and PILOTS was extensively investigated in March 2019 for any papers reporting the following terms: chronic pain, post-traumatic stress AND opioid. The search was limited to English and Spanish languages, peer-reviews, and human studies. No time limits were applied. Duplicate articles were removed. Remainder were screened out reading titles and abstracts. Manuscripts were assessed in full text if the study was quantitative, only included adults (> 18 years) and participants with both CNCP and posttraumatic stress receiving opioids for pain treatment. Selected studies were also assessed for methodological quality using the Scottish Intercollegiate Guidelines Network checklist for cohort studies. Given the nature of the studies, we decided not to meta-analyze data. To the best of our knowledge, this is the first systematic review on the topic.
In the preliminary search, 151 articles were identified. After duplicate were removed (n = 45), a pool of 106 manuscripts were remained for further evaluation. Reviewing articles by title and abstract, 86 were rejected because they did not meet the selection criteria and 10 were retained for analysis. The 10 eligible studies included 1622785 unique participants. In total, 196516 participants had comorbid CNCP and a PTSD, and were consuming opiates. The cross-study mean age was 35.2 years. The majority of participants were men of Caucasian origin, recruited from the veterans' health care system. Musculoskeletal pain was the most common chronic pain condition in the participants. In total, 42.4% of the participants across studies had a diagnosis of PTSD. All the studies reported evidence of a greater prevalence of this disorder in those patients who had been prescribed opioids. Regarding to opioid intake, 2 different outcome variables were identified across studies: opioid prescriptions and OUD. Opioid prescriptions were generally assessed according to the medical prescription-dispensing pattern, although this pattern was defined in different ways, such as total number of prescription days and pharmacy records of opioid prescriptions. The studies that investigated OUD shown that this disorder was associated with a diagnosis of posttraumatic stress in CNCP patients.
Our systematic review shows that participants with comorbid posttraumatic stress and CNCP obtain higher doses of opioids, receive more than 1 type of opioid concurrently, and are more likely to be receiving opioids chronically. Furthermore, the findings suggest that PTSD is associated with OUD in CNCP patients. In addition, the results of this review show that there is high co-occurrence between CNCP, PTSD, and other psychological disorders such as depression, anxiety, and substance abuse. The choice of opioid treatment for CNCP patients should include screening for baseline PTSD, particularly when combined with benzodiazepines.
Overall results of this systematic review are encouraging, but there is a lack of civilian population studies and also with female participants. Likewise, there are differences between the studies included in this review relating to the nature, the size of the samples and the criteria employed for assessing PTSD. More research is therefore needed in this area.
| 1. | Turk DC, Okifuji A. Psychological factors in chronic pain: evolution and revolution. J Consult Clin Psychol. 2002;70:678-690. [PubMed] |
| 2. | Gatchel RJ, Peng YB, Peters ML, Fuchs PN, Turk DC. The biopsychosocial approach to chronic pain: scientific advances and future directions. Psychol Bull. 2007;133:581-624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2285] [Cited by in RCA: 1994] [Article Influence: 104.9] [Reference Citation Analysis (1)] |
| 3. | Broekmans S, Dobbels F, Milisen K, Morlion B, Vanderschueren S. Medication adherence in patients with chronic non-malignant pain: is there a problem? Eur J Pain. 2009;13:115-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 78] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 4. | Bohnert AS, Valenstein M, Bair MJ, Ganoczy D, McCarthy JF, Ilgen MA, Blow FC. Association between opioid prescribing patterns and opioid overdose-related deaths. JAMA. 2011;305:1315-1321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1075] [Cited by in RCA: 1177] [Article Influence: 78.5] [Reference Citation Analysis (0)] |
| 5. | Chou R, Fanciullo GJ, Fine PG, Adler JA, Ballantyne JC, Davies P, Donovan MI, Fishbain DA, Foley KM, Fudin J, Gilson AM, Kelter A, Mauskop A, O'Connor PG, Passik SD, Pasternak GW, Portenoy RK, Rich BA, Roberts RG, Todd KH, Miaskowski C; American Pain Society-American Academy of Pain Medicine Opioids Guidelines Panel. Clinical guidelines for the use of chronic opioid therapy in chronic noncancer pain. J Pain. 2009;10:113-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1400] [Cited by in RCA: 1487] [Article Influence: 87.5] [Reference Citation Analysis (0)] |
| 6. | Vowles KE, McEntee ML, Julnes PS, Frohe T, Ney JP, van der Goes DN. Rates of opioid misuse, abuse, and addiction in chronic pain: a systematic review and data synthesis. Pain. 2015;156:569-576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 707] [Cited by in RCA: 859] [Article Influence: 78.1] [Reference Citation Analysis (0)] |
| 7. | Smith KZ, Smith PH, Cercone SA, McKee SA, Homish GG. Past year non-medical opioid use and abuse and PTSD diagnosis: Interactions with sex and associations with symptom clusters. Addict Behav. 2016;58:167-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 38] [Article Influence: 3.8] [Reference Citation Analysis (1)] |
| 8. | Couto JE, Romney MC, Leider HL, Sharma S, Goldfarb NI. High rates of inappropriate drug use in the chronic pain population. Popul Health Manag. 2009;12:185-190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 40] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 9. | Hassan AN, Foll BL, Imtiaz S, Rehm J. The effect of post-traumatic stress disorder on the risk of developing prescription opioid use disorder: Results from the National Epidemiologic Survey on Alcohol and Related Conditions III. Drug Alcohol Depend. 2017;179:260-266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 53] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 10. | Hamill-Ruth RJ, Larriviere K, McMasters MG. Addition of objective data to identify risk for medication misuse and abuse: the inconsistency score. Pain Med. 2013;14:1900-1907. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 11. | Sullivan MD, Edlund MJ, Zhang L, Unützer J, Wells KB. Association between mental health disorders, problem drug use, and regular prescription opioid use. Arch Intern Med. 2006;166:2087-2093. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 284] [Cited by in RCA: 335] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 12. | López-Martínez AE, Serrano-Ibáñez ER, Ruiz-Párraga GT, Gómez-Pérez L, Ramírez-Maestre C, Esteve R. Physical Health Consequences of Interpersonal Trauma: A Systematic Review of the Role of Psychological Variables. Trauma Violence Abuse. 2018;19:305-322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 56] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 13. | Montaño M, Bernardy NC, Sherrieb K. Cultivating change door to door: Educational outreach to improve prescribing practices in rural veterans with posttraumatic stress disorder. Subst Abus. 2017;38:129-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 14. | McCall-Hosenfeld JS, Winter M, Heeren T, Liebschutz JM. The association of interpersonal trauma with somatic symptom severity in a primary care population with chronic pain: exploring the role of gender and the mental health sequelae of trauma. J Psychosom Res. 2014;77:196-204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 54] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 15. | Pietrzak RH, Goldstein RB, Southwick SM, Grant BF. Prevalence and Axis I comorbidity of full and partial posttraumatic stress disorder in the United States: results from Wave 2 of the National Epidemiologic Survey on Alcohol and Related Conditions. J Anxiety Disord. 2011;25:456-465. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 732] [Cited by in RCA: 654] [Article Influence: 43.6] [Reference Citation Analysis (0)] |
| 16. | Otis JD, Keane TM, Kerns RD. An examination of the relationship between chronic pain and post-traumatic stress disorder. J Rehabil Res Dev. 2003;40:397-405. [PubMed] |
| 17. | Asmundson GJ, Coons MJ, Taylor S, Katz J. PTSD and the experience of pain: research and clinical implications of shared vulnerability and mutual maintenance models. Can J Psychiatry. 2002;47:930-937. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 447] [Cited by in RCA: 447] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 18. | Friedman MJ. What might the psychobiology of posttraumatic stress disorder teach us about future approaches to pharmacotherapy? J Clin Psychiatry. 2000;61 Suppl 7:44-51. [PubMed] |
| 19. | Phifer J, Skelton K, Weiss T, Schwartz AC, Wingo A, Gillespie CF, Sands LA, Sayyar S, Bradley B, Jovanovic T, Ressler KJ. Pain symptomatology and pain medication use in civilian PTSD. Pain. 2011;152:2233-2240. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 80] [Cited by in RCA: 69] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 20. | Gibson CA. Review of posttraumatic stress disorder and chronic pain: the path to integrated care. J Rehabil Res Dev. 2012;49:753-776. [PubMed] |
| 21. | Shorter D, Hsieh J, Kosten TR. Pharmacologic management of comorbid post-traumatic stress disorder and addictions. Am J Addict. 2015;24:705-712. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 50] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 22. | Bryant RA, Creamer M, O'Donnell M, Silove D, McFarlane AC. A study of the protective function of acute morphine administration on subsequent posttraumatic stress disorder. Biol Psychiatry. 2009;65:438-440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 145] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 23. | Holbrook TL, Galarneau MR, Dye JL, Quinn K, Dougherty AL. Morphine use after combat injury in Iraq and post-traumatic stress disorder. N Engl J Med. 2010;362:110-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 294] [Cited by in RCA: 279] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 24. | Schwartz AC, Bradley R, Penza KM, Sexton M, Jay D, Haggard PJ, Garlow SJ, Ressler KJ. Pain medication use among patients with posttraumatic stress disorder. Psychosomatics. 2006;47:136-142. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 74] [Cited by in RCA: 77] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 25. | Barry DT, Cutter CJ, Beitel M, Kerns RD, Liong C, Schottenfeld RS. Psychiatric Disorders Among Patients Seeking Treatment for Co-Occurring Chronic Pain and Opioid Use Disorder. J Clin Psychiatry. 2016;77:1413-1419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 78] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 26. | Dobscha SK, Morasco BJ, Duckart JP, Macey T, Deyo RA. Correlates of prescription opioid initiation and long-term opioid use in veterans with persistent pain. Clin J Pain. 2013;29:102-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 111] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 27. | Sullivan MD. Who gets high-dose opioid therapy for chronic non-cancer pain? Pain. 2010;151:567-568. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 44] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 28. | Wu PC, Lang C, Hasson NK, Linder SH, Clark DJ. Opioid use in young veterans. J Opioid Manag. 2010;6:133-139. [PubMed] |
| 29. | Rosenheck R, Fontana A. Do Vietnam-era veterans who suffer from posttraumatic stress disorder avoid VA mental health services? Mil Med. 1995;160:136-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 24] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 30. | Taylor BC, Hagel EM, Carlson KF, Cifu DX, Cutting A, Bidelspach DE, Sayer NA. Prevalence and costs of co-occurring traumatic brain injury with and without psychiatric disturbance and pain among Afghanistan and Iraq War Veteran V.A. users. Med Care. 2012;50:342-346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 223] [Cited by in RCA: 257] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 31. | Zedler B, Xie L, Wang L, Joyce A, Vick C, Kariburyo F, Rajan P, Baser O, Murrelle L. Risk factors for serious prescription opioid-related toxicity or overdose among Veterans Health Administration patients. Pain Med. 2014;15:1911-1929. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 184] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 32. | Ecker AH, Hundt N. Posttraumatic stress disorder in opioid agonist therapy: A review. Psychol Trauma. 2018;10:636-642. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 38] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 33. | Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;6:e1000100. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11206] [Cited by in RCA: 11298] [Article Influence: 664.6] [Reference Citation Analysis (0)] |
| 34. | PROSPERO 2017. Available from: https://www.crd.york.ac.uk/prospero/. |
| 35. | Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52948] [Cited by in RCA: 48540] [Article Influence: 2855.3] [Reference Citation Analysis (3)] |
| 36. | Trevino CM, deRoon-Cassini T, Brasel K. Does opiate use in traumatically injured individuals worsen pain and psychological outcomes? J Pain. 2013;14:424-430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 29] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 37. | Liebschutz JM, Saitz R, Weiss RD, Averbuch T, Schwartz S, Meltzer EC, Claggett-Borne E, Cabral H, Samet JH. Clinical factors associated with prescription drug use disorder in urban primary care patients with chronic pain. J Pain. 2010;11:1047-1055. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 84] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 38. | Wilsey BL, Fishman SM, Tsodikov A, Ogden C, Symreng I, Ernst A. Psychological comorbidities predicting prescription opioid abuse among patients in chronic pain presenting to the emergency department. Pain Med. 2008;9:1107-1117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 67] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 39. | Hudson TJ, Painter JT, Martin BC, Austen MA, Williams JS, Fortney JC, Sullivan MD, Edlund MJ. Pharmacoepidemiologic analyses of opioid use among OEF/OIF/OND veterans. Pain. 2017;158:1039-1045. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 40] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 40. | Bilevicius E, Sommer JL, Asmundson GJG, El-Gabalawy R. Posttraumatic stress disorder and chronic pain are associated with opioid use disorder: Results from a 2012-2013 American nationally representative survey. Drug Alcohol Depend. 2018;188:119-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 40] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 41. | Han L, Allore H, Goulet J, Bathulapali H, Skanderson M, Brandt C, Haskell S, Krebs E. Opioid dosing trends over eight years among US Veterans with musculoskeletal disorders after returning from service in support of recent conflicts. Ann Epidemiol. 2017;27:563-569.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 42. | Macey TA, Morasco BJ, Duckart JP, Dobscha SK. Patterns and correlates of prescription opioid use in OEF/OIF veterans with chronic noncancer pain. Pain Med. 2011;12:1502-1509. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 49] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 43. | Outcalt SD, Yu Z, Hoen HM, Pennington TM, Krebs EE. Health care utilization among veterans with pain and posttraumatic stress symptoms. Pain Med. 2014;15:1872-1879. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 35] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 44. | Seal KH, Shi Y, Cohen G, Cohen BE, Maguen S, Krebs EE, Neylan TC. Association of mental health disorders with prescription opioids and high-risk opioid use in US veterans of Iraq and Afghanistan. JAMA. 2012;307:940-947. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 321] [Cited by in RCA: 381] [Article Influence: 27.2] [Reference Citation Analysis (0)] |
| 45. | Seal KH, Bertenthal D, Barnes DE, Byers AL, Gibson CJ, Rife TL, Yaffe K; Chronic Effects of Neurotrauma Consortium Study Group. Traumatic Brain Injury and Receipt of Prescription Opioid Therapy for Chronic Pain in Iraq and Afghanistan Veterans: Do Clinical Practice Guidelines Matter? J Pain. 2018;19:931-941. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 41] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 46. | Sedgwick P. Retrospective cohort studies: advantages and disadvantages. BMJ. 2014;348:g2276. [DOI] [Full Text] |
| 47. | Kelsall HL, McKenzie DP, Forbes AB, Roberts MH, Urquhart DM, Sim MR. Pain-related musculoskeletal disorders, psychological comorbidity, and the relationship with physical and mental well-being in Gulf War veterans. Pain. 2014;155:685-692. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 35] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 48. | McGeary D, Moore M, Vriend CA, Peterson AL, Gatchel RJ. The evaluation and treatment of comorbid pain and PTSD in a military setting: an overview. J Clin Psychol Med Settings. 2011;18:155-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 31] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 49. | Demyttenaere K, Bruffaerts R, Lee S, Posada-Villa J, Kovess V, Angermeyer MC, Levinson D, de Girolamo G, Nakane H, Mneimneh Z, Lara C, de Graaf R, Scott KM, Gureje O, Stein DJ, Haro JM, Bromet EJ, Kessler RC, Alonso J, Von Korff M. Mental disorders among persons with chronic back or neck pain: results from the World Mental Health Surveys. Pain. 2007;129:332-342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 382] [Cited by in RCA: 399] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 50. | Shiner B, Leonard Westgate C, Bernardy NC, Schnurr PP, Watts BV. Trends in Opioid Use Disorder Diagnoses and Medication Treatment Among Veterans With Posttraumatic Stress Disorder. J Dual Diagn. 2017;13:201-212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 43] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 51. | O'Brien T, Christrup LL, Drewes AM, Fallon MT, Kress HG, McQuay HJ, Mikus G, Morlion BJ, Perez-Cajaraville J, Pogatzki-Zahn E, Varrassi G, Wells JC. European Pain Federation position paper on appropriate opioid use in chronic pain management. Eur J Pain. 2017;21:3-19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 171] [Cited by in RCA: 152] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 52. | Rasmusson AM, Shalev AY, Friedman MJ, Keane TM, Resick PA. Integrating the neuroendocrinology, neurochemistry, and neuroimmunology of PTSD to date and the challenges ahead. In: Friedman MJ, Keane TM, Resick PA. Handbook of PTSD: Science and practice. Friedman MJ, Keane TM, Resick PA. New York, NY: Guilford Press 2014; 275-299. |
| 53. | Patel RS, Elmaadawi A, Nasr S, Haskin J. Comorbid Post-Traumatic Stress Disorder and Opioid Dependence. Cureus. 2017;9:e1647. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 54. | Bremner JD, Southwick SM, Darnell A, Charney DS. Chronic PTSD in Vietnam combat veterans: course of illness and substance abuse. Am J Psychiatry. 1996;153:369-375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 273] [Cited by in RCA: 259] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 55. | Jing Li J, Szkudlarek H, Renard J, Hudson R, Rushlow W, Laviolette SR. Fear Memory Recall Potentiates Opiate Reward Sensitivity through Dissociable Dopamine D1 versus D4 Receptor-Dependent Memory Mechanisms in the Prefrontal Cortex. J Neurosci. 2018;38:4543-4555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 56. | Danovitch I. Post-traumatic stress disorder and opioid use disorder: A narrative review of conceptual models. J Addict Dis. 2016;35:169-179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 38] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 57. | Morgan CA, Krystal JH, Southwick SM. Toward early pharmacological posttraumatic stress intervention. Biol Psychiatry. 2003;53:834-843. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 32] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 58. | Norman SB, Stein MB, Dimsdale JE, Hoyt DB. Pain in the aftermath of trauma is a risk factor for post-traumatic stress disorder. Psychol Med. 2008;38:533-542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 139] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 59. | Zatzick DF, Galea S. An epidemiologic approach to the development of early trauma focused intervention. J Trauma Stress. 2007;20:401-412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 60. | Sharp TJ, Harvey AG. Chronic pain and posttraumatic stress disorder: mutual maintenance? Clin Psychol Rev. 2001;21:857-877. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 498] [Cited by in RCA: 516] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 61. | International Association for the Study of Pain. Pain: Clinical Updates 2015; 23. Available from: http://www.iasp-pain.org/PublicationsNews/NewsletterIssue.aspx?ItemNumber= 4962. |
| 62. | Greenspan JD, Craft RM, LeResche L, Arendt-Nielsen L, Berkley KJ, Fillingim RB, Gold MS, Holdcroft A, Lautenbacher S, Mayer EA, Mogil JS, Murphy AZ, Traub RJ; Consensus Working Group of the Sex, Gender, and Pain SIG of the IASP. Studying sex and gender differences in pain and analgesia: a consensus report. Pain. 2007;132 Suppl 1:S26-S45. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 779] [Cited by in RCA: 760] [Article Influence: 40.0] [Reference Citation Analysis (11)] |
| 63. | Beaudoin FL, Baird J, Liu T, Merchant RC. Sex Differences in Substance Use Among Adult Emergency Department Patients: Prevalence, Severity, and Need for Intervention. Acad Emerg Med. 2015;22:1307-1315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 64. | Simoni-Wastila L. The use of abusable prescription drugs: the role of gender. J Womens Health Gend Based Med. 2000;9:289-297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 119] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 65. | Cole J, Logan TK. Nonmedical use of sedative-hypnotics and opiates among rural and urban women with protective orders. J Addict Dis. 2010;29:395-409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See:
Manuscript source: Invited manuscript
Specialty type: Medicine, Research and Experimental
Country of origin: Spain
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Vyshka G S-Editor: Dou Y L-Editor: A E-Editor: Xing YX