Published online Nov 6, 2019. doi: 10.12998/wjcc.v7.i21.3549
Peer-review started: May 21, 2019
First decision: August 1, 2019
Revised: September 20, 2019
Accepted: October 15, 2019
Article in press: October 15, 2019
Published online: November 6, 2019
Processing time: 182 Days and 10.8 Hours
Transcatheter aortic valve replacement (TAVR) is recommended in patients with severe aortic stenosis who have high surgical risk. However, in the pre-existing mechanical mitral valve prosthesis and natural pure aortic regurgitation, TAVR is relatively contraindicated. In this report, we described one case of TAVR with native aortic regurgitation in the presence of mechanical mitral valve prosthesis.
A 64-year-old man with a medical history of mitral valve replacement had severe dyspnea and was symptomatic even at rest for 3 mo. His echocardiography showed severe native pure aortic regurgitation. His euroscore was 15. A TAVR procedure with an evolut R was planned. A 34 mm evolut R was placed by transesophageal echocardiography. The mitral prosthesis was functioning normally, and mild-moderate paravalvular leakage was evident by transesophageal echocardiography. The patient recovered without any complication. At 1 mo follow up, the patient was well, and no paravalvular leakage was noted.
TAVR for pure aortic regurgitation in the presence of prosthetic mitral valve can be a safe procedure.
Core tip: Transcatheter aortic valve replacement (TAVR) is recommended in the treatment of severe aortic stenosis in patients who are at high or prohibitive surgical risk. Several patients with previous mitral valve surgery were reported to have been treated with TAVR. However, TAVR has been performed off-label to treat patients with pure aortic regurgitation due to the absence of calcium for device anchoring. There are few data showing that transfemoral TAVR is feasible and safe for the treatment aortic regurgitation in selected patients. Here, we report one case of transfemoral TAVR for native aortic regurgitation in the setting of a pre-existing mitral prosthesis.
- Citation: Erdem A, Esen Zencirci A, Ozden K, Terzi S. Transfemoral aortic valve implantation in the case of pre-existing mitral prosthesis and pure aortic regurgitation: A case report. World J Clin Cases 2019; 7(21): 3549-3552
- URL: https://www.wjgnet.com/2307-8960/full/v7/i21/3549.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v7.i21.3549
Transcatheter aortic valve replacement (TAVR) is recommended in the treatment of severe aortic stenosis in patients who are at high or prohibitive surgical risk[1]. Several patients with previous mitral valve surgery have been reported to be treated with TAVR[2-6]. However, TAVR has been performed off-label to treat patients with pure aortic regurgitation due to the absence of annular or leaflet calcification for device anchoring. There are few data showing that transfemoral TAVR is feasible and safe for the treatment aortic regurgitation in selected patients[7]. Here, we report one case of transfemoral TAVR for native aortic regurgitation in the setting of a pre-existing mitral prosthesis.
A 64-year-old man with a history of mitral valve replacement with a 27 St. Jude MedicalTM mechanical valve prosthesis for rheumatic mitral stenosis 3 years ago presented severe dyspnea for 3 mo.
The patient had severe aortic regurgitation and was symptomatic at mild exercise and rest (NYHA III-IV). His past history was unremarkable.
The family history was unremarkable.
Baseline echographic data included regurgitant jet width left ventricular outflow tract (LVOT) diameter of 70%, aortic valve pressure half time of 240, and left ventricular ejection fraction of 50. Euroscore was calculated to be 15.
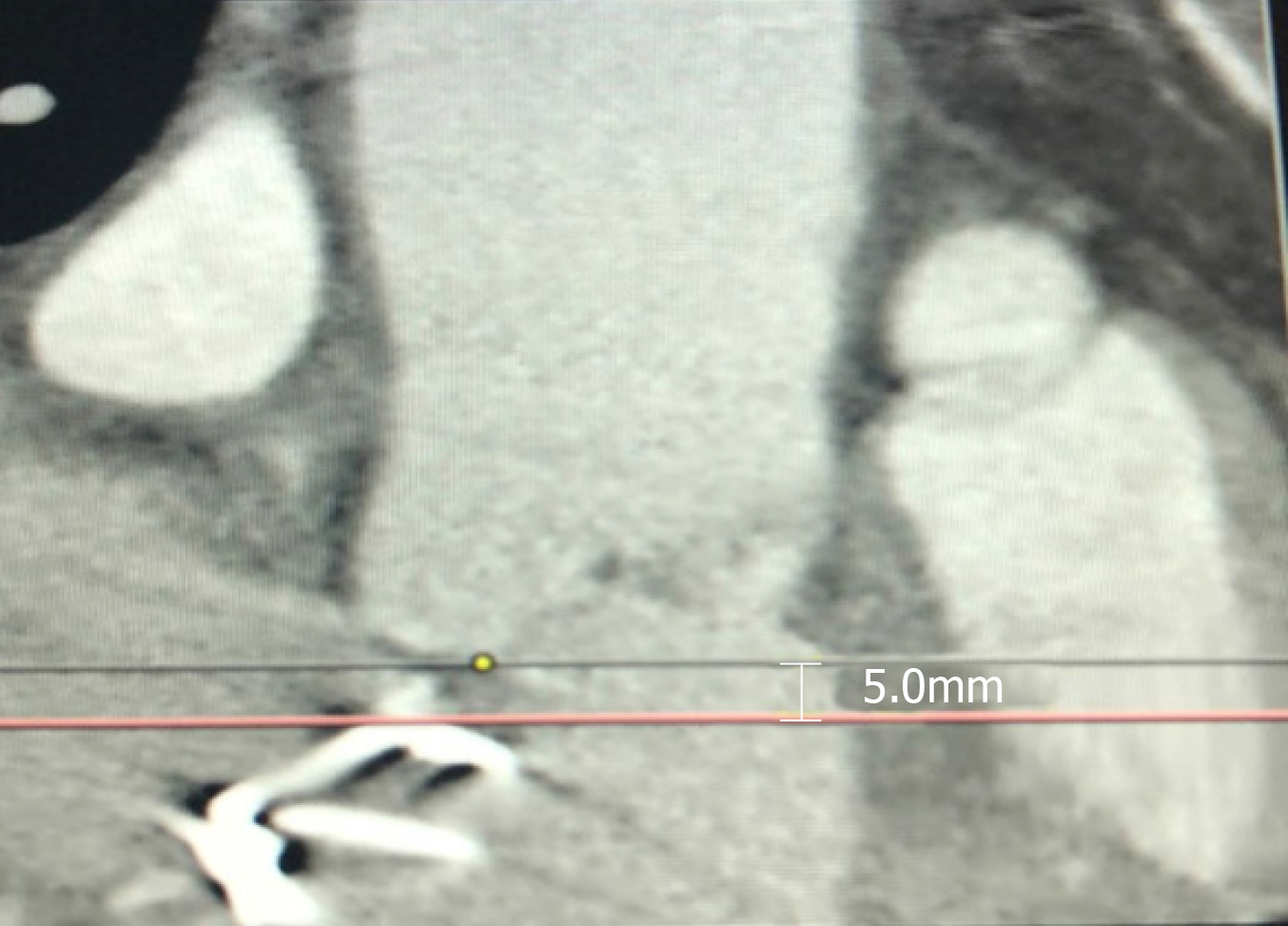
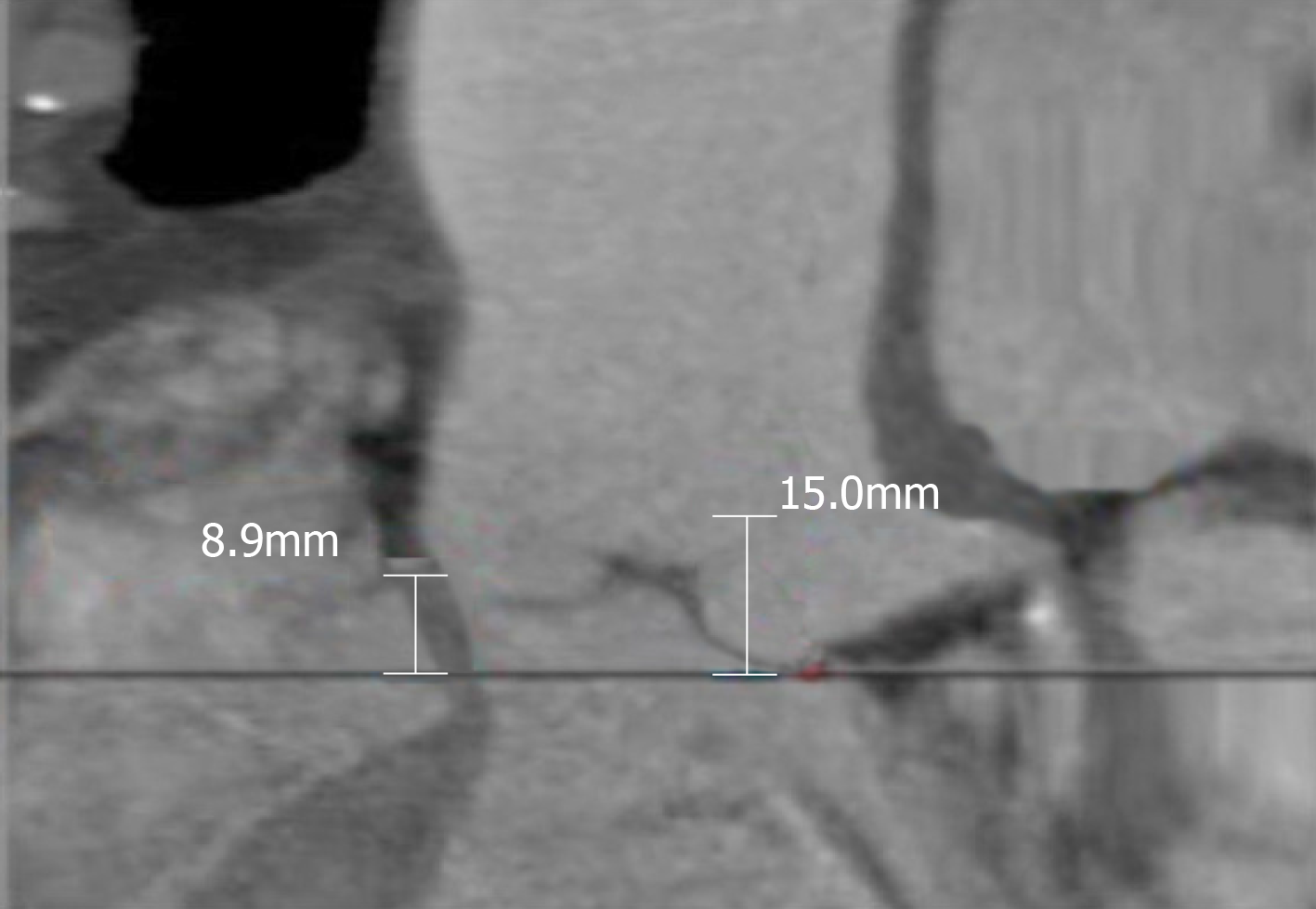
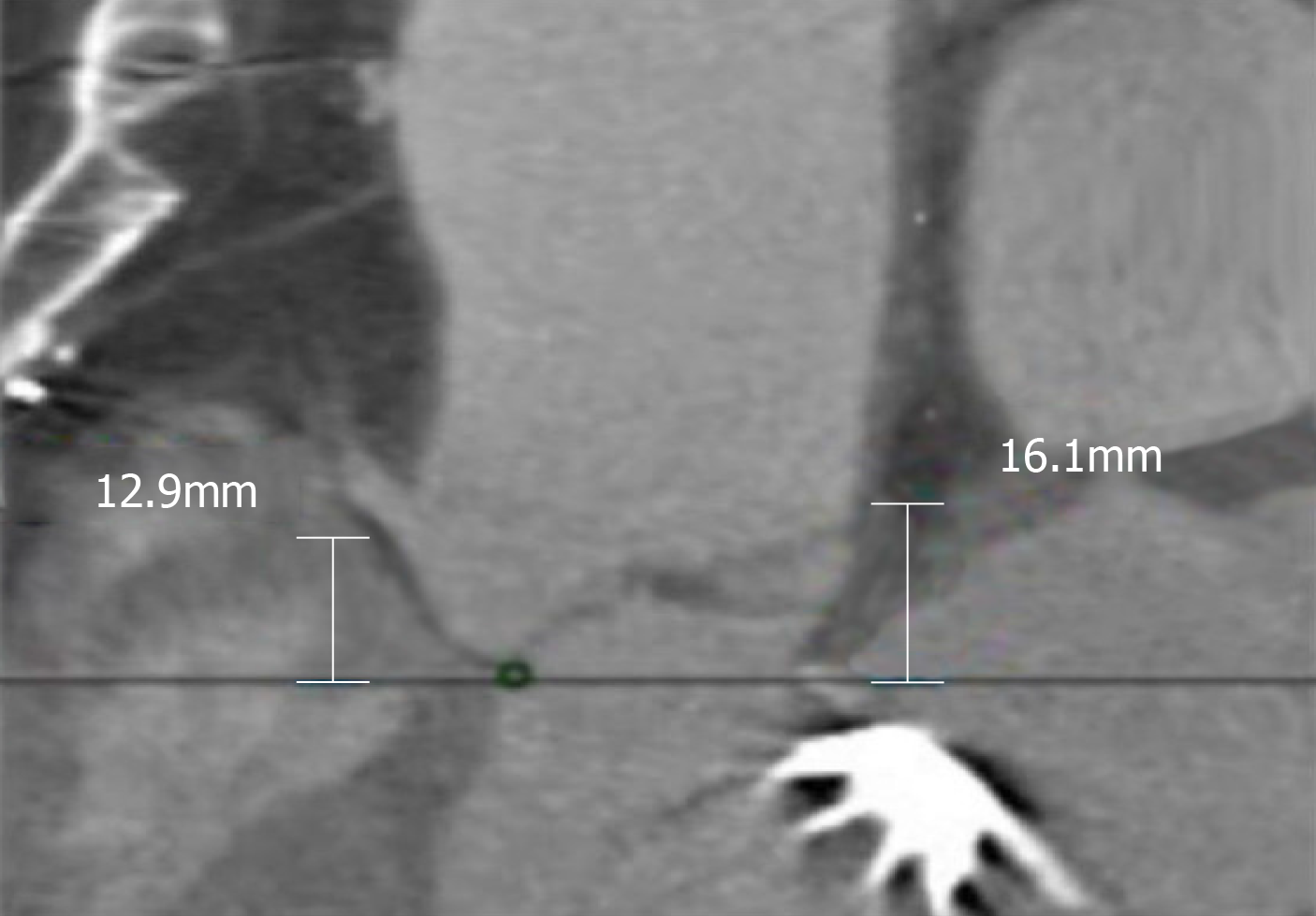
The minimum aortic annulus to mitral valve distance was measured to be 5 mm by multislice computed tomography (Figure 1). Computed tomography showed an aortic annulus perimeter of 83.3 and a cross sectional area of 515.1 mm2, and the right and left coronary ostia height were 12.9 mm and 8.9 mm, respectively (Figure 2, Figure 3).
Severe aortic stenosis.
Heart team at our hospital decided to recommend TAVR. A TAVR procedure with an evolut R (Medtronic, Brampton, ON, Canada) through femoral access was planned. The patient was administered general anesthesia. Intraoperative transesophageal echocardiography during TAVR was used. A 34 mm evolut R valve was deployed during rapid ventricular pacing. Aortic root angiography was performed immediately after valve deployment. We confirmed that the coronary arteries were patent. By arteriography, mild aortic regurgitation was demonstrated. The mitral prosthesis was functioning normally by transesophageal echocardiography (TEE). Mild to moderate paravalvular leakage was evident on TEE.
The patient was discharged at 4 d. He recovered without any complication. At 1 mo follow up, the patient was well and his New York Heart Association Class was 1 and 2. Doppler transthoracic echocardiography revealed maximal/mean gradients of 16/8 mmHg. No paravalvular leakage was noted. The left ventricular ejection fraction was 55.
Transcatheter aortic valve replacement in the presence of mechanical mitral valve prosthesis and pure native aortic regurgitation is an off-label indication. This is because the absence of annular or leaflet calcification in pure aortic regurgitation is an associated risk for valve dislocation[1-7]. The stiff mitral cage can affect the TAVR device’s stability. Balloon expansion and device instability can increase the risk of malposition and embolization. The LVOT distance below the aortic annulus has to be more than 2 mm for safe positioning[5]. In our patient, the distance between aortic annulus and mitral valve prosthesis was 5 mm.
Despite good positioning of the aortic valve prosthesis, it can interfere with the mitral valve prosthesis[4]. This is especially true for first generation devices that are larger prosthesis that may interfere with the stiff mitral valve. Our device evolut R is a second generation device[8]. Coronary height is important for coronary obstruction during the procedure, and it is predicted to be less than 12 mm[6]. In our patient, left coronary ostia height was lower than 12 mm. Therefore, we implanted the prosthesis slightly lower into the LVOT by TEE guidance.
According to our experience, transcatheter aortic valve implantation in the presence of mitral valve prosthesis and pure aortic regurgitation can be a safe procedure. However, preprocedural screening of the patient by multi slides tomography and interprocedural TEE are essential for the safe procedure.
| 1. | Clavel MA, Webb JG, Pibarot P, Altwegg L, Dumont E, Thompson C, De Larochellière R, Doyle D, Masson JB, Bergeron S, Bertrand OF, Rodés-Cabau J. Comparison of the hemodynamic performance of percutaneous and surgical bioprostheses for the treatment of severe aortic stenosis. J Am Coll Cardiol. 2009;53:1883-1891. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 285] [Cited by in RCA: 278] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 2. | Amat-Santos IJ, Cortés C, Nombela Franco L, Muñoz-García AJ, Suárez De Lezo J, Gutiérrez-Ibañes E, Serra V, Larman M, Moreno R, De La Torre Hernandez JM, Puri R, Jimenez-Quevedo P, Hernández García JM, Alonso-Briales JH, García B, Lee DH, Rojas P, Sevilla T, Goncalves R, Vera S, Gómez I, Rodés-Cabau J, San Román JA. Prosthetic Mitral Surgical Valve in Transcatheter Aortic Valve Replacement Recipients: A Multicenter Analysis. JACC Cardiovasc Interv. 2017;10:1973-1981. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 31] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 3. | Squiers JJ, Hebeler KR, DiMaio JM, Ogbue P, Szerlip M, Brinkman WT. Impingement of Single-Tilting Disc Mitral Prosthesis During Transcatheter Aortic Valve Replacement. Ann Thorac Surg. 2016;102:e529-e531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 4. | Vavuranakis M, Vrachatis DA, Kariori MG, Moldovan C, Kalogeras K, Lavda M, Aznaouridis K, Stefanadis C. TAVI in the case of preexisting mitral prosthesis: tip and tricks and literature review. J Invasive Cardiol. 2014;26; 609-13. |
| 5. | Wachter K, Ahad S, Rustenbach CJ, Franke UF, Baumbach H. Transapical aortic valve implantation in patients with pre-existing mitral valve prostheses: a case report. J Cardiothorac Surg. 2016;11:133. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 6. | Bagur R, Pestrichella V, Montesanti R, Alemanni R, Cassese M. Transfemoral transcatheter ACURATE-neo™ aortic valve replacement in a patient with a previous mechanical mitral valve. J Card Surg. 2017;32:358-360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 7. | Spina R, Anthony C, Muller DW, Roy D. Transcatheter Aortic Valve Replacement for Native Aortic Valve Regurgitation. Interv Cardiol. 2015;10:49-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 8. | Mahtta D, Elgendy IY, Bavry AA. From CoreValve to Evolut PRO: Reviewing the Journey of Self-Expanding Transcatheter Aortic Valves. Cardiol Ther. 2017;6:183-192. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 37] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See:
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, Research and Experimental
Country of origin: Turkey
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Erkut B, Ueda H S-Editor: Zhang L L-Editor: Filipodia E-Editor: Qi LL