Published online Oct 26, 2019. doi: 10.12998/wjcc.v7.i20.3185
Peer-review started: June 18, 2019
First decision: July 30, 2019
Revised: August 24, 2019
Accepted: September 9, 2019
Article in press: September 9, 2019
Published online: October 26, 2019
Processing time: 137 Days and 13.8 Hours
Cervical cancer is the most common gynecological malignancy, ranking first in female reproductive malignancies with more than 500000 new cases and 275000 deaths each year. Traditionally, open radical hysterectomy is considered the standard surgical procedure for the treatment of resectable cervical cancer. The latest guidelines from the National Comprehensive Cancer Network and the European Society of Gynecological Oncology suggest that open surgery and laparoscopic surgery (using traditional laparoscopic or robotic techniques) are the main surgical approaches for radical hysterectomy for patients with stage IA2-IIA cervical cancer. Robotic surgery has been increasingly used in abdominal surgery and has shown more beneficial effects.
To analyse the perioperative conditions, complications, and short-term and long-term effects in patients undergoing robotic radical hysterectomy (RRH) and laparoscopic radical hysterectomy (LRH) to compare their clinical efficacy, safety, and feasibility.
The perioperative data of patients undergoing RRH and LRH were extracted and collected from the database of surgical treatments for cervical cancer for statistical analysis.
Of the patients, 342 underwent LRH for cervical cancer, and 216 underwent RRH. The total complication rate was 9.65% (20 patients) in the RRH group and 17.59% (60 patients) in the LRH group. The complication rate was significantly lower in the RRH group than in the LRH group. There was no significant difference in the follow-up period (P = 0.658). The total recurrence rates were 15.7% and 12% in the RRH and LRH groups, respectively. The progression-free survival time was 28.91 ± 15.68 mo and 28.34 ± 15.13 mo in the RRH and LRH groups, respectively (P = 0.669). The overall survival (OS) rates were 92.13% and 94.45% in the RRH and LRH groups, respectively (P = 0.292). The OS time was 29.87 ± 15.92 mo and 29.41 ± 15.14 mo in the RRH and LRH groups, respectively (P = 0.732). The survival curves and the progression-free survival curves were not statistically significantly different between the two groups (P = 0.407 and 0.28, respectively).
RRH is associated with significantly less operative time and blood loss than LRH. The two procedures have similar complication rates, OS, and progression-free survival time.
Core tip: The perioperative data of patients undergoing robotic radical hysterectomy (RRH) and laparoscopic radical hysterectomy (LRH) were extracted and collected from the database of surgical treatments for cervical cancer for statistical analysis. Of the patients, 342 underwent LRH for cervical cancer, and 216 underwent RRH. The operative time and blood loss were significantly less in the RRH group than in the LRH group. The two groups had similar complication rates, overall survival, and progression-free survival time.
- Citation: Chen L, Liu LP, Wen N, Qiao X, Meng YG. Comparative analysis of robotic vs laparoscopic radical hysterectomy for cervical cancer. World J Clin Cases 2019; 7(20): 3185-3193
- URL: https://www.wjgnet.com/2307-8960/full/v7/i20/3185.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v7.i20.3185
Cervical cancer is the most common gynecological malignancy, ranking first in female reproductive malignancies with more than 500000 new cases and 275000 deaths each year[1]. In China, the incidence of cervical cancer varies across different regions. Women between 40 and 50 years old have higher incidence rates. The incidence in rural and mountain areas is higher than that in urban areas and on the plains[2-4].
Surgical resection with adequate lymphadenectomy is the main treatment for improving the survival rate of patients with cervical cancer. Traditionally, open radical hysterectomy (ORH) is considered the standard surgical procedure for the treatment of resectable cervical cancer. In 1992, Nezhat et al[5] first reported laparoscopic radical hysterectomy (LRH) for the treatment of cervical cancer. Since then, LRH has been reported with satisfactory surgical outcomes, compared with traditional open surgery[6]. The latest guidelines from the National Comprehensive Cancer Network and the European Society of Gynecological Oncology suggest that open surgery and laparoscopic surgery (using traditional laparoscopic or robotic techniques) are the main surgical approaches for radical hysterectomy for patients with stage IA2-IIA cervical cancer[7-9]. Robotic surgery has been increasingly used in abdominal surgery and has shown more beneficial effects[10-12].
In 2006, Sert et al[13] first published the results of robotic radical hysterectomy (RRH) with lymphadenectomy. To date, there have been relatively more studies of RRH for cervical cancer in foreign countries[14-19]. Our center started to provide a service to perform RRH for cervical cancer relatively early in China, and it has also accumulated rich clinical experience.
This study was retrospectively performed to analyze the perioperative conditions, complications, and short-term and long-term effects in patients undergoing RRH and LRH in our center from February 2014 to December 2018, with an aim to compare their clinical efficacy, safety, and feasibility.
The perioperative data of patients undergoing RRH and LRH were extracted and collected from the database of surgical treatments for cervical cancer (from February 2014 to December 2014) for statistical analysis.
The inclusion criteria included: (A) Patients with newly diagnosed cervical cancer [International Federation of Gynecology and Obstetrics (FIGO, 2009) stage IA-IIB]; (B) Patients with pathological diagnoses of squamous cell carcinoma, adenocarcinoma, or adenosquamous carcinoma; and (C) Patients with complete medical records who provided written informed consent for surgery. The exclusion criteria included: (A) Patients with uterine length greater than 12 cm; (B) Pregnant patients; (C) Patients with restrictions for creating a pneumoperitoneum; (D) Patients with clinical and radiological evidence showing lymph node and distant metastases; (E) Patients with histories of abdominal or pelvic chemoradiotherapy; (F) Patients without follow-up data available; and (G) Patients with histories of multiple primary malignancies.
The design of this study and the standardized regimen were approved by the Ethics Committee of the General Hospital of the People’s Liberation Army. After patient admission, the relevant examinations were completed; the patients were informed of the surgical risk and provided their written informed consent. The procedures were performed by surgical specialists and much the same team.
After general anesthesia with tracheal intubation, the patient was placed in the lithotomy position with 30° elevation of the feet. The pneumoperitoneum pressure was maintained at 14 mm Hg. Preparation of the Da Vinci robotic surgical system is as follows. Two trocars were placed in the site 8 cm above the umbilicus and 30° to 45° to the right and at a site 8 cm above the umbilicus in the middle line, respectively. After creation of the pneumoperitoneum and camera placement, the trocar for the robotic arms was placed under camera guidance. The trocars were located at sites 7 to 8 cm lateral to the umbilicus and formed fan-shaped distribution with the trocar for the camera. Robotic arms 1 and 2 were attached to the trocars. The monopolar electrosurgical knife/shovel and bipolar forceps were placed. Another 2 ancillary trocars were placed.
The pelvic and abdominal cavities were examined, and adhesions were lysed. The infundibulopelvic ligament and the round ligament on the right side were divided after high ligation. The anterior and posterior leaves of the broad ligament were opened gradually. The uterovesical fold was opened with scissors. The same procedure was performed on the left side for this step. The posterior peritoneum was opened to sequentially remove the bilateral common iliac lymph nodes, external iliac lymph nodes, deep inguinal lymph nodes, internal iliac lymph nodes, and obturator lymph nodes. The right uterine artery was divided to unroof the ureter and manage the sacrospinous ligament and cardinal ligament. The same procedure was performed on the left side for this step. The remaining parametrial tissues and the upper 1/3 of the vagina were resected. After specimen removal, the vaginal stump was closed with continuous sutures. The pelvic cavity was thoroughly evaluated. The pelvic cavity was irrigated, and hemostasis was performed if necessary. The drainage tube and urinary catheter were placed, and the procedure was completed. After the procedure, the patient’s pathological report was evaluated. Patients with lymph node metastasis, parametrial involvement, positive vaginal margins, lymphatic involvement, tumor invasion to deep interstitial regions, and large tumors (≥ 4 cm) underwent adjuvant therapy, such as radiotherapy and chemotherapy.
SPSS software (IBM statistics, version 22.0) was used for statistical analyses of the experimental data. The continuous parameters are expressed as the mean ± SD. The categorical variables are described as the positive rate (ratio). The paired t-test and the chi-square test were used for the statistical analysis. P < 0.05 was considered to be statistically significant between the two groups.
A total of 558 cervical cancer patients who were admitted between February 2014 and December 2018 were included in this study. Of the patients, 342 underwent LRH for cervical cancer, and 216 underwent RRH. The age of the patients was 47.49 ± 9.81 years old and 48.90 ± 9.65 years old in the LRH and RRH groups, respectively. The body mass index was 23.74 ± 2.96 kg/m2 and 24.20 ± 3.37 kg/m2 in the LRH and RRH groups, respectively (Table 1).
| RRH (n = 216) | LRH (n = 342) | P value | |
| Age (yr) | 48.90 ± 9.65 | 47.49 ± 9.81 | 0.773 |
| BMI (kg/m2) | 24.20 ± 3.37 | 23.74 ± 2.96 | 0.925 |
| FIGO stage | |||
| IA1-IB1 | 133 (61.57%) | 208 (60.82%) | |
| IB2-IIA1 | 67 (35.65%) | 105 (30.70%) | 0.902 |
| IIA2-IIB | 16 (2.78%) | 29 (8.48%) | |
| Pathological type | |||
| Squamous cell carcinoma | 196 (90.74%) | 316 (92.40%) | |
| Adenocarcinoma | 19 (8.80%) | 22 (6.43%) | 0.504 |
| Adenosquamous carcinoma | 1 (0.46%) | 2 (0.58%) |
Stage IA1-IB1 patients accounted for 60.82% and 61.57% of all patients in the LRH and RRH groups, respectively. The stage IB2-IIA1 patients accounted for 36.55% and 35.65% of all patients in the LRH and RRH groups, respectively. Squamous cell carcinoma (92.40% and 90.74%) and adenocarcinoma (7.02% and 8.80%) were the main pathological types in the LRH and RRH groups, and there were two patients with adenosquamous carcinoma in the LRH group (P = 0.504).
In the comparison of the main parameters of the procedure, the operative time and estimated intraoperative blood loss were significantly less in the RRH group than in the LRH group (operative time: 197.16 ± 57.76 vs 233.50 ± 59.76 min, P < 0.001; blood loss: 163.09 ± 320.95 vs 233.50 ± 59.76 min, P < 0.001; Table 2). The number of dissected lymph nodes during surgery (23.51 ± 9.31 vs 25.09 ± 11.41, P = 0.748), length of postoperative hospital stay (12.28 ± 5.06 vs 10.87 ± 4.44, P = 0.772), and total length of hospital stay (18.57 ± 5.61 vs 17.19 ± 6.29, P = 0.777) were not statistically significantly different between the RRH and LRH groups. The proportion of blood transfusions in the RRH group (3.80%, 8 cases) was significantly less than that in the LRH group (11.57%, 39 cases) (P < 0.001).
| LRH (n = 342) | RRH (n = 216) | P value | |
| Operative time (min) | 233.50 ± 59.76 | 197.16 ± 57.76 | 0.001 |
| Intraoperative blood loss (mL) | 280.74 ± 246.66 | 163.09 ± 320.95 | 0.001 |
| Intraoperative blood vessel and organ injuries (cases) | 3 (0.87%) | 5 (2.31%) | 0.153 |
| Total number of dissected lymph nodes (pieces) | 25.09 ± 11.41 | 23.51 ± 9.31 | 0.748 |
| Intraoperative blood transfusion (cases) | 39 (11.57%) | 8 (3.80%) | 0.001 |
| Total length of hospital stay (d) | 17.19 ± 6.29 | 18.57 ± 5.61 | 0.777 |
| Length of postoperative hospital stay (d) | 10.87 ± 4.44 | 12.28 ± 5.06 | 0.772 |
| Postoperative chemotherapy (cases) | 172 (50.29%) | 102 (47.22%) | 0.268 |
| Postoperative radiotherapy (cases) | 42 (12.28%) | 25 (11.57%) | 0.457 |
There was no statistically significant difference in the intra-operative injury rate (2.31% vs 0.87%, P > 0.05) between the two groups. There were four cases of vascular injury and one case of bladder injury in the RRH group and three cases of vascular injury in the LRH group.
The postoperative radiotherapy rates were 47.22% (102 patients) and 50.29% (172 patients), respectively; the postoperative chemotherapy rates were 11.57% (25 patients) and 12.28% (42 patients) in the two groups. There was no significant difference in the postoperative radiotherapy or chemotherapy rates between the two groups (P = 0.268 and P = 0.457, respectively).
The total complication rate was 9.65% (20 patients) in the RRH group and 17.59% (60 patients) in the LRH group (Table 3). The complication rate was significantly lower in the RRH group than in the LRH group.
| RRH (n = 216) | LRH (n = 342) | P value | |
| Total complications (cases) | 20 (9.65) | 60 (17.59) | 0.012 |
| Early postoperative complications (within 4 wk) | |||
| Infection (cases) | 3 (1.46) | 12 (3.70) | 0.007 |
| Fever (including infection with fever) (cases) | 9 (4.39) | 22 (6.48) | 0.017 |
| Venous thrombosis (cases) | 1 (0.46) | 0 (0) | 0.387 |
| Other | 1 (0.46) | 2 (0.58) | 0.667 |
| Long-term postoperative complications (after 4 wk) | |||
| Lower extremity edema | 8 (3.80) | 16 (4.63) | 0.036 |
| Inguinal lymphatic cyst | 3 (1.39) | 3 (0.88) | 0.429 |
| Other | 1 (0.46) | 0 (0) | 0.387 |
Early postoperative complications (within 4 wk after surgery) were mainly infections and fever (body temperature ≥ 38.0 °C). The early infection rate and fever rate were significantly lower in the RRH group than in the LRH group (infection: 1.46% vs 3.70%, P = 0.007; fever: 4.39% vs 6.48%, P = 0.017). The infections mainly included wound infections (2 cases and 9 cases) and urinary tract infections (1 case and 3 cases). A case of lower extremity venous thrombosis occurred after surgery in the RRH group. A urethral fistula was reported in a patient in the RRH group, and intestinal obstruction was reported in two patients in the LRH group.
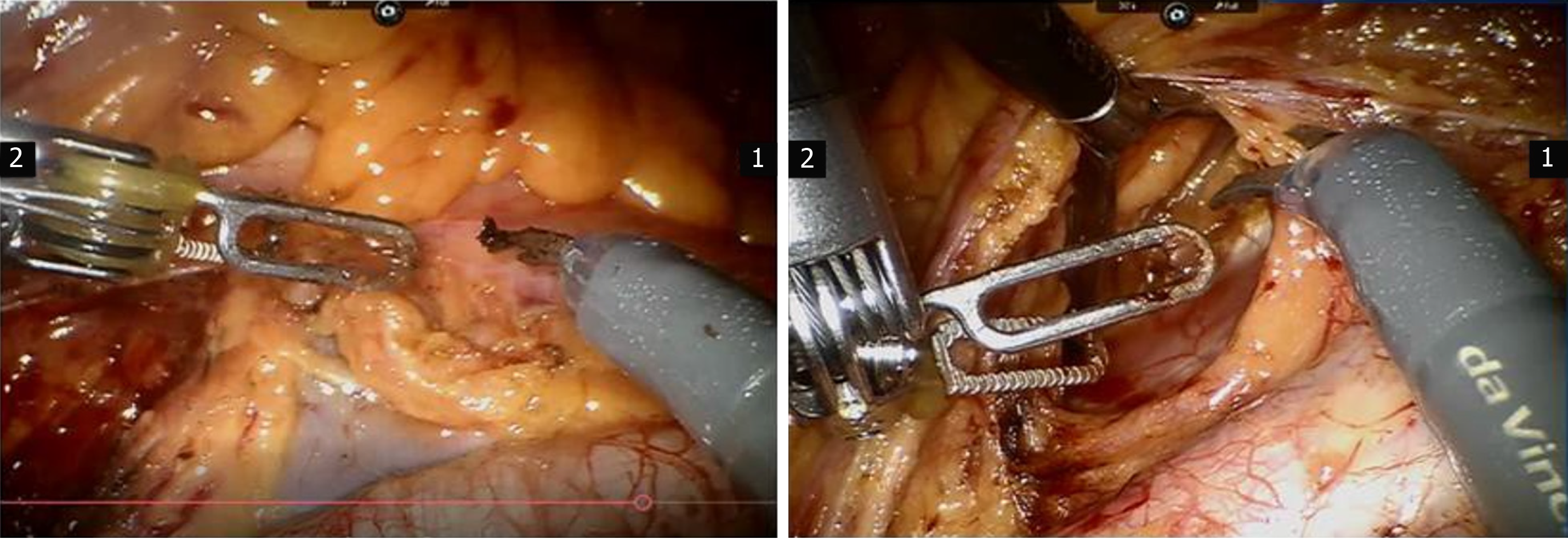
Long-term postoperative complications (after 4 wk postoperatively) were mainly associated with lymphatic drainage disorder after lymphadenectomy (Figure 1). The incidence of lower extremity edema was significantly lower in the RRH group than in the LRH group (3.8% vs 4.63%, P = 0.036). There was no statistically significant difference in the incidence of inguinal lymphocysts (1.39% vs 0.88%, P = 0.429).
The follow-up periods were 30.07 ± 15.67 mo and 29.48 ± 15.07 mo in the RRH and LRH groups, respectively (Table 4). There was no significant difference in follow-up period (P = 0.658). The total recurrence rates were 15.7% and 12% in the RRH and LRH groups, respectively. The progression-free survival time was 28.91 ± 15.68 mo and 28.34 ± 15.13 mo in the RRH and LRH groups, respectively, and the difference in progression-free survival time was not statistically significant (P = 0.669). The overall survival (OS) rates were 92.13% and 94.45% in the RRH and LRH groups, respectively, and there was no significant difference between them (P = 0.292). The OS time was 29.87 ± 15.92 mo and 29.41 ± 15.14 mo in the RRH and LRH groups, respectively (P = 0.732).
| RRH(n = 216) | LRH (n = 342) | P value | |
| Average follow-up period (mo) | 30.07 ± 15.67 | 29.48 ± 15.07 | 0.658 |
| Median follow-up period (mo) | 29.0 (1.0-58.0) | 30.0 (3.0-58.0) | |
| Recurrence | |||
| Total recurrence rate | 15.7% | 12.00% | 0.206 |
| Number of patients with recurrence | 34 | 41 | |
| Median recurrence time (mo) | 20.0 (7.0-49.0) | 20.0 (3.0-48.0) | |
| Survival | |||
| Progression-free survival rate | 81.02% | 85.67% | 0.157 |
| Progression-free survival time | 28.91 ± 15.68 | 28.34 ± 15.13 | 0.669 |
| Overall survival rate | 92.13% | 94.45% | 0.292 |
| Overall survival time | 29.87 ± 15.92 | 29.41 ± 15.14 | 0.732 |
The survival curves and the progression-free survival curves were not statistically significantly different between the RRH and LRH groups (P = 0.407 and P = 0.28, respectively).
According to statistics from the World Health Organization, more than 90% of new cases and deaths of patients with cervical cancer worldwide were reported in developing countries[7,20-23]. To date, the treatments for cervical cancer include surgery, radiation therapy, and chemotherapy.
As one of the important approaches to cervical cancer treatment, surgical treatment has evolved from transabdominal surgery, transvaginal surgery, and laparoscopic surgery to robotic surgery. Compared with ORH, LRH has advantages including cosmetic incisions, less trauma, quick healing, a clear and accurately magnified surgical field, rapid recovery, and fewer complications[11,24,25]. However, it has many shortcomings: The poor accuracy of the surgical field on two-dimensional imaging; the limited range of instrument flexibility, which is not conducive to fine surgical operations; and the long learning curve, which means that extensive practice is needed to master the procedure[26]. The da Vinci robotic surgery system somewhat overcomes these shortcomings and has greater accuracy and stability for the operation. Thus, it has been widely used in the treatment of gynecological diseases[27-32].
A study by Boggess et al[33] compared the results of 51 patients undergoing RRH and 49 patients undergoing LRH and showed that type III pelvic lymphadenectomy is feasible during RRH and might be superior to that during LRH in patients with early cervical cancer.
The current study included more patients than the previous studies, including 216 patients undergoing RRH and 342 patients undergoing LRH. There were no differences in the basic characteristics between the two groups. However, the operative time and blood loss were significantly less in the RRH group than in the LRH group.
According to the literature, the incidences of urinary and vascular injuries were 3.3% and 6%, respectively, in patients undergoing open radical hysterectomy[34]. In the current study, a patient in the RRH group experienced intraoperative urinary tract injury and a ureterovaginal fistula after surgery. The incidence of urinary injury was 0.46%. There were 4 and 3 cases of vascular injury in the RRH and LRH groups, respectively. The incidences were 1.85% and 0.87%, respectively. The incidence of intraoperative injury was significantly lower than that reported in the previous ORH study.
The main evaluation parameters of the surgical efficacy of malignant tumors are the recurrence rate and survival rate of patients after surgery. To date, there have been few reports about the recurrence rate and survival rate in patients undergoing RRH and LRH. Kawal et al[35] performed a follow-up in 109 patients undergoing RRH. The recurrence rate was 16.5%. The 2-year and 5-year OS rates were 96% and 89%, respectively. The 2-year and 5-year DFS rates were 88% and 72%, respectively.
With the publication of a large, prospective, multicenter randomized, controlled trial of minimally invasive (laparoscopic and robotic surgery) radical hysterectomy for cervical cancer and open radical hysterectomy for cervical cancer in New England Journal of Medicine in 2018, the postoperative efficacy of minimally invasive surgery has once again drawn widespread attention[36]. The study showed that, compared with open surgery, minimally invasive surgery had a significantly higher recurrence rate and a poorer survival rate. It remains a problem widely acknowledged by the medical community whether minimally invasive surgery, especially laparoscopic surgery, can reduce residual tumors through technological innovations.
Corrado et al[17] demonstrated possible differences in perioperative outcomes and complications between mLRH and RRH in patients with early-stage cervical cancer. Their studies showed that the surgical efficiency of microlaparoscopic surgery is comparable to that of robotic surgery. Therefore, this result suggests room for improvement in the surgical accuracy of and reduced surgical trauma with laparoscopic techniques.
The da Vinci robotic surgery system somewhat overcomes the shortcomings of LRH and has greater accuracy and stability for the operation. Thus, it has been widely used in the treatment of gynecological diseases
This study was retrospectively performed to analyze the perioperative conditions, complications, and short-term and long-term effects in patients undergoing robotic radical hysterectomy (RRH) and laparoscopic radical hysterectomy (LRH) at our center from February 2014 to December 2018.
To analyze the perioperative conditions, complications, and short-term and long-term effects in patients undergoing RRH and LRH.
The clinical efficacy, safety, and feasibility of RRH and LRH were analyzed and compared.
The complication rate was significantly lower in the RRH group than in the LRH group. There was no significant difference in follow-up period (P = 0.658). The total recurrence rates were 15.7% and 12% in the RRH and LRH groups, respectively. The progression-free survival time was 28.91 ± 15.68 mo and 28.34 ± 15.13 mo in the RRH and LRH groups, respectively (P = 0.669). The overall survival (OS) rates were 92.13% and 94.45% in the RRH and LRH groups, respectively (P = 0.292). The OS time was 29.87 ± 15.92 mo and 29.41 ± 15.14 mo in the RRH and LRH groups, respectively (P = 0.732). The survival curves and the progression-free survival curves were not statistically significantly different between the two groups.
The operative time and blood loss were significantly less in the RRH group than in the LRH group. The two groups had similar complication rats, OS, and progression-free survival time.
RRH can achieve similar long-term outcome to LRH with less operative time and less blood loss.
| 1. | Dizon DS, Mackay HJ, Thomas GM, Werner TL, Kohn EC, Hess D, Rose PG, Covens AL. State of the science in cervical cancer: where we are today and where we need to go. Cancer. 2014;120:2282-2288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 36] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 2. | Practice Bulletin No. 168 Summary: Cervical Cancer Screening and Prevention. Obstet Gynecol. 2016;128:923-925. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 25] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 3. | Practice Bulletin No. 157: Cervical Cancer Screening and Prevention. Obstet Gynecol. 2016;127:e1-e20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 92] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 4. | Herbert A, Bryant TN, Campbell MJ, Smith J. Investigation of the effect of occult invasive cancer on progress towards successful cervical screening. J Med Screen. 1998;5:92-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 5. | Nezhat CR, Burrell MO, Nezhat FR, Benigno BB, Welander CE. Laparoscopic radical hysterectomy with paraaortic and pelvic node dissection. Am J Obstet Gynecol. 1992;166:864-865. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 336] [Cited by in RCA: 312] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 6. | Angelopoulos G, Etman A, Cruickshank DJ, Twigg JP. Total laparoscopic radical hysterectomy: a change in practice for the management of early stage cervical cancer in a U.K. cancer center. Eur J Gynaecol Oncol. 2015;36:711-715. [PubMed] |
| 7. | Vaccarella S, Lortet-Tieulent J, Plummer M, Franceschi S, Bray F. Worldwide trends in cervical cancer incidence: impact of screening against changes in disease risk factors. Eur J Cancer. 2013;49:3262-3273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 273] [Cited by in RCA: 364] [Article Influence: 28.0] [Reference Citation Analysis (0)] |
| 8. | Saslow D, Solomon D, Lawson HW, Killackey M, Kulasingam SL, Cain J, Garcia FA, Moriarty AT, Waxman AG, Wilbur DC, Wentzensen N, Downs LS, Spitzer M, Moscicki AB, Franco EL, Stoler MH, Schiffman M, Castle PE, Myers ER; ACS-ASCCP-ASCP Cervical Cancer Guideline Committee. American Cancer Society, American Society for Colposcopy and Cervical Pathology, and American Society for Clinical Pathology screening guidelines for the prevention and early detection of cervical cancer. CA Cancer J Clin. 2012;62:147-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 854] [Cited by in RCA: 824] [Article Influence: 58.9] [Reference Citation Analysis (0)] |
| 9. | Saslow D, Solomon D, Lawson HW, Killackey M, Kulasingam SL, Cain J, Garcia FA, Moriarty AT, Waxman AG, Wilbur DC, Wentzensen N, Downs LS, Spitzer M, Moscicki AB, Franco EL, Stoler MH, Schiffman M, Castle PE, Myers ER; American Cancer Society; American Society for Colposcopy and Cervical Pathology; American Society for Clinical Pathology. American Cancer Society, American Society for Colposcopy and Cervical Pathology, and American Society for Clinical Pathology screening guidelines for the prevention and early detection of cervical cancer. Am J Clin Pathol. 2012;137:516-542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 541] [Cited by in RCA: 563] [Article Influence: 40.2] [Reference Citation Analysis (0)] |
| 10. | Chen DX, Hou YH, Jiang YN, Shao LW, Wang SJ, Wang XQ. Removal of pediatric stage IV neuroblastoma by robot-assisted laparoscopy: A case report and literature review. World J Clin Cases. 2019;7:1499-1507. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 7] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 11. | Willows K, Kupets R, Diong C, Vicus D, Covens A, Gien LT. Rates over time and regional variation of radical minimally invasive surgery for cervical cancer: A population based study. Gynecol Oncol. 2019;154:338-344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 12. | Corrado G, Vizza E, Legge F, Pedone Anchora L, Sperduti I, Fagotti A, Mancini E, Gallotta V, Zampa A, Chiofalo B, Scambia G. Comparison of Different Surgical Approaches for Stage IB1 Cervical Cancer Patients: A Multi-institution Study and a Review of the Literature. Int J Gynecol Cancer. 2018;28:1020-1028. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 74] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 13. | Sert BM, Abeler VM. Robotic-assisted laparoscopic radical hysterectomy (Piver type III) with pelvic node dissection--case report. Eur J Gynaecol Oncol. 2006;27:531-533. [PubMed] |
| 14. | Jin YM, Liu SS, Chen J, Chen YN, Ren CC. Robotic radical hysterectomy is superior to laparoscopic radical hysterectomy and open radical hysterectomy in the treatment of cervical cancer. PLoS One. 2018;13:e0193033. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 43] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 15. | Shazly SA, Murad MH, Dowdy SC, Gostout BS, Famuyide AO. Robotic radical hysterectomy in early stage cervical cancer: A systematic review and meta-analysis. Gynecol Oncol. 2015;138:457-471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 136] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 16. | Corrado G, Fanfani F, Ghezzi F, Fagotti A, Uccella S, Mancini E, Sperduti I, Stevenazzi G, Scambia G, Vizza E. Mini-laparoscopic versus robotic radical hysterectomy plus systematic pelvic lymphadenectomy in early cervical cancer patients. A multi-institutional study. Eur J Surg Oncol. 2015;41:136-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 30] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 17. | Corrado G, Cutillo G, Saltari M, Mancini E, Sindico S, Vici P, Sergi D, Sperduti I, Patrizi L, Pomati G, Baiocco E, Vizza E. Surgical and Oncological Outcome of Robotic Surgery Compared With Laparoscopic and Abdominal Surgery in the Management of Locally Advanced Cervical Cancer After Neoadjuvant Chemotherapy. Int J Gynecol Cancer. 2016;26:539-546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 23] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 18. | Vizza E, Corrado G, Mancini E, Vici P, Sergi D, Baiocco E, Patrizi L, Saltari M, Pomati G, Cutillo G. Laparoscopic versus robotic radical hysterectomy after neoadjuvant chemotherapy in locally advanced cervical cancer: a case control study. Eur J Surg Oncol. 2015;41:142-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 29] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 19. | Chong GO, Lee YH, Hong DG, Cho YL, Park IS, Lee YS. Robot versus laparoscopic nerve-sparing radical hysterectomy for cervical cancer: a comparison of the intraoperative and perioperative results of a single surgeon's initial experience. Int J Gynecol Cancer. 2013;23:1145-1149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 43] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 20. | Santesso N, Mustafa RA, Schünemann HJ, Arbyn M, Blumenthal PD, Cain J, Chirenje M, Denny L, De Vuyst H, Eckert LO, Forhan SE, Franco EL, Gage JC, Garcia F, Herrero R, Jeronimo J, Lu ER, Luciani S, Quek SC, Sankaranarayanan R, Tsu V, Broutet N; Guideline Support Group. World Health Organization Guidelines for treatment of cervical intraepithelial neoplasia 2-3 and screen-and-treat strategies to prevent cervical cancer. Int J Gynaecol Obstet. 2016;132:252-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 140] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 21. | Davis M, Feldman S. Making Sense of Cervical Cancer Screening Guidelines and Recommendations. Curr Treat Options Oncol. 2015;16:55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 22. | Coppell K, Paul C, Cox B. An evaluation of the National Cervical Screening Programme Otago site. N Z Med J. 2000;113:48-51. [PubMed] |
| 23. | Cuzick J. Screening for cancer: future potential. Eur J Cancer. 1999;35:1925-1932. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 13] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 24. | Wang Y, Deng L, Cao L, Xu H, Liang Z. The Outcome of Laparoscopy Versus Laparotomy for the Management of Early Stage Cervical Cancer-Meta Analysis. J Minim Invasive Gynecol. 2015;22:S4-S5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 25. | Barletta F, Corrado G, Vizza E. A Comparison Between Laparotomy, Laparoscopy and Robotic-Assisted Radical Hysterectomy in Surgical Treatment of Early Stage Cervical Cancer. J Minim Invasive Gynecol. 2015;22:S48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 26. | Nezhat F, Mahdavi A, Nagarsheth NP. Total laparoscopic radical hysterectomy and pelvic lymphadenectomy using harmonic shears. J Minim Invasive Gynecol. 2006;13:20-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 42] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 27. | Medlin EE, Kushner DM, Barroilhet L. Robotic surgery for early stage cervical cancer: Evolution and current trends. J Surg Oncol. 2015;112:772-781. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 28. | Morelli L, Furbetta N, Gianardi D, Palmeri M, Di Franco G, Bianchini M, Stefanini G, Guadagni S, Di Candio G. Robot-assisted trans-gastric drainage and debridement of walled-off pancreatic necrosis using the EndoWrist stapler for the da Vinci Xi: A case report. World J Clin Cases. 2019;7:1461-1466. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 8] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (1)] |
| 29. | Satkunasivam R, Tallman CT, Taylor JM, Miles BJ, Klaassen Z, Wallis CJD. Robot-assisted Radical Cystectomy Versus Open Radical Cystectomy: A Meta-analysis of Oncologic, Perioperative, and Complication-related outcomes. Eur Urol Oncol. 2019;2:443-447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 64] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 30. | Gil-Moreno A, Carbonell-Socias M, Salicrú S, Centeno-Mediavilla C, Franco-Camps S, Colas E, Oaknin A, Pérez-Benavente A, Díaz-Feijoo B. Radical Hysterectomy: Efficacy and Safety in the Dawn of Minimally Invasive Techniques. J Minim Invasive Gynecol. 2019;26:492-500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 37] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 31. | Bartos P, Struppl D, Trhlík M, Czudek S, Skrovina M, Adamcík L, Soumarová R. [Da vinci robotic surgery in gynaecological oncology: a critical interim appraisal]. Ceska Gynekol. 2007;72:354-359. [PubMed] |
| 32. | Raspagliesi F, Bogani G, Martinelli F, Signorelli M, Scaffa C, Sabatucci I, Lorusso D, Ditto A. 3D vision improves outcomes in early cervical cancer treated with laparoscopic type B radical hysterectomy and pelvic lymphadenectomy. Tumori. 2017;103:76-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 33. | Boggess JF, Gehrig PA, Cantrell L, Shafer A, Ridgway M, Skinner EN, Fowler WC. A case-control study of robot-assisted type III radical hysterectomy with pelvic lymph node dissection compared with open radical hysterectomy. Am J Obstet Gynecol. 2008;199:357.e1-357.e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 163] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 34. | Park DA, Yun JE, Kim SW, Lee SH. Surgical and clinical safety and effectiveness of robot-assisted laparoscopic hysterectomy compared to conventional laparoscopy and laparotomy for cervical cancer: A systematic review and meta-analysis. Eur J Surg Oncol. 2017;43:994-1002. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 74] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 35. | Kawal T, Sahadev R, Srinivasan A, Chu D, Weiss D, Long C, Van Batavia J, Bodar Y, Shah J, Shukla AR. Robotic surgery in infants and children: an argument for smaller and fewer incisions. World J Urol. 2019;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 36. | Melamed A, Margul DJ, Chen L, Keating NL, Del Carmen MG, Yang J, Seagle BL, Alexander A, Barber EL, Rice LW, Wright JD, Kocherginsky M, Shahabi S, Rauh-Hain JA. Survival after Minimally Invasive Radical Hysterectomy for Early-Stage Cervical Cancer. N Engl J Med. 2018;379:1905-1914. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 496] [Cited by in RCA: 537] [Article Influence: 67.1] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See:
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Hoda A, Ichiro S S-Editor: Zhang L L-Editor: Wang TQ E-Editor: Liu JH