Published online Jan 26, 2019. doi: 10.12998/wjcc.v7.i2.145
Peer-review started: August 14, 2018
First decision: October 5, 2018
Revised: November 13, 2018
Accepted: November 23, 2018
Article in press: November 24, 2018
Published online: January 26, 2019
Processing time: 165 Days and 13 Hours
To establish an appropriate N classification system for early gastric cancer (EGC).
Data from 10714 patients who underwent radical gastrectomy between 1988 and 2011 were retrieved from the National Cancer Institute’s Surveillance, Epidemiology, and End Result database. The overall survival (OS) based on the eighth edition and new tumor lymph node metastasis (TNM) staging systems were compared, and the analysis was repeated in an external validation set from the Fujian Medical University Union Hospital database.
There were no significant differences in OS between N1 and N2 cancers or between N3a and N3b cancers in cases of EGC. The X-tile program identified that the new staging system for EGC consisted of T1N0, T1N1’ [1-6 metastatic lymph nodes (LNs)], and T1N2’ ( ≥ 7 metastatic LNs). Compared with the eighth edition of the TNM staging system, the OS of patients in T1N1’ stage was similar to that of patients with stage IIA disease, whereas the OS of patients in T1N2’ stage was similar to that of patients with stage IIB disease. The new TNM staging system exhibited a slightly lower Akaike Information Criterion value and higher χ2 and c-statistic compared with the eighth edition of the TNM classification system. Similar results were found in the external validation dataset from the external validation set.
We have developed an optional new TNM staging system with a better predictive ability that can be used to accurately predict the 5-year OS of patients with EGC.
Core tip: The overall survival (OS) was not significantly different between N1 and N2 cancers or between N3a and N3b cancers in cases of early gastric cancer (EGC). We identified a new metastatic lymph node classification for EGC which consisted of T1N1’ (1-6 metastatic LNs) and T1N2’ ( ≥ 7 metastatic LNs). The OS of patients in T1N1’ stage was similar to that of the 8th edition American Joint Committee on Cancer stage IIA disease, while the OS of patients in T1N2’ stage was not significantly different from that of patients with stage IIB disease. The new TNM staging system has a better predictive ability of OS for EGC.
- Citation: Lin JX, Lin JP, Li P, Xie JW, Wang JB, Lu J, Chen QY, Cao LL, Lin M, Tu RH, Zheng CH, Huang CM. New metastatic lymph node classification for early gastric cancer should differ from those for advanced gastric adenocarcinoma: Results based on the SEER database. World J Clin Cases 2019; 7(2): 145-155
- URL: https://www.wjgnet.com/2307-8960/full/v7/i2/145.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v7.i2.145
Gastric cancer (GC) is still the fifth common malignant tumor with a high incidence of cancer-related death in the world[1,2]. In 2015, there were an estimated 24590 people who will be diagnosed with GC and more than 10000 patients will eventually die in the United States[3]. Radical gastrectomy with radical systemic lymphadenectomy is the only proven and potentially curative treatment for resectable GC[4-6]. Since the first American Joint Committee on Cancer (AJCC) staging of cancer was published in 1977, there have been eighth editions which have been changed a lot based on the treatment strategies and big database. However, as the founding editors adroitly noted that: “Staging of cancer is not an exact science. As new information becomes available about etiology and various methods of diagnosis and treatment, the classification and staging of cancer will change”[7]. Recently, the eighth edition of the AJCC tumor lymph node metastasis (TNM) staging system for gastric carcinoma was published[8]. This edition retains the same T, N, and M classifications as the seventh edition. However, early gastric cancer (EGC), which comprises of T1 tumors irrespective of lymph node metastasis, is a special type of cancer[9]. Although GC is often diagnosed at an advanced stage, the incidence of EGC may be increasing, especially in Japan and South Korea[10,11]. Variable rates of recurrence of EGC, which range from 2.1% to 12.4%, have been reported in several studies[11-14]. Al-Refaie et al[15] found that the AJCC TNM staging system was inadequate for EGC. Additionally, some studies showed that the survival of patients with EGC was more affected by the metastatic lymph nodes (LNs) than the depth of tumor spread into the stomach wall or its size[16,17]. Therefore, the current research was aimed to re-evaluate the long-term overall survival (OS) of EGC patients from the Surveillance, Epidemiology, and End Results (SEER) database who underwent radical gastrectomy between 1988 and 2011 in the context of the eighth edition of the AJCC staging system, and identify a better TNM classification to improve the prognostic prediction of patients with EGC after curative surgery. We also compared the discriminatory value of the new TNM classification with that of the eighth edition of the AJCC TNM staging system.
The SEER program of the National Cancer Institute is an authoritative source of information on cancer incidence and survival data which cover approximately 26 percent of the United States population[18]. Its registries routinely collect data on patient demographics, primary tumor site, tumor morphology and stage at diagnosis, treatment, and survival. The inclusion criteria included: (1) patients in the SEER database who were at least 20 years of age with gastric adenocarcinoma confirmed by histology (ICD-0-3M-8140/3, M-8142/3 through M-8145/3, M-8210/3, M-8211/3, M-8255/3, M-8260/3 through M-8263/3, M-8310/3, M-8323/3, M-8480/3, M-8481/3, and M-8490/3) and who underwent radical gastrectomy between 1988 and 2011 were eligible for this study; (2) only patients with at least 15 LNs examined were included; (3) we then obtained data from the following categories: “Race recode (White, Black, and Other)”, “Age at diagnosis”, “Grade”, “ICD-O-3 Hist/behav”, “Primary Site - labeled”, “Derived AJCC Stage Group, 6th ed (2004+)”, “Derived AJCC Stage Group, 7th ed (2010+)”, “EOD 10 - extent (1988-2003)”, “CS extension (2004+)”, “Derived AJCC T, 6th ed (2004+)”, “Derived AJCC T, 7th ed (2010+)”, “EOD 10 - nodes (1988-2003)”, “CS lymph nodes (2004+)”, “Derived AJCC N, 6th ed (2004+)”, “Derived AJCC N, 7th ed (2010+)”, “CS mets at dx (2004+)”, “Derived AJCC M, 6th ed (2004+)”, “Derived AJCC M, 7th ed (2010+)”, “Site specific surgery (1973-1997 varying detail by year and site)”, “RX Summ--Surg Prim Site (1998+)”, “Regional nodes examined (1988+)”, and “Regional nodes positive (1988+)”. Patients were excluded due to: (1) pT4b stage; (2) when distant metastasis was apparent (M1); (3) unknown T and/or N category; (4) patients with previous malignancies; and (5) patients received radiotherapy or chemotherapy prior to surgery. Patients with tumors located in the esophagogastric junction (sit code 160) were also excluded, as these may be classified as either GC or esophageal cancer, depending on the extent of the disease[19]. The tumor stage was reclassified according to the eighth edition of the AJCC/UICC TNM classification[8]. All patients with incomplete medical records or whose records lacked certain information were eliminated from the dataset. For the re-classification of the TNM stages for early GC, the T1 patients were excluded from stage IIA and IIB. The median follow-up for SEER data sets was 121 mo.
Additional external validation was performed using the dataset from Fujian Medical University Union Hospital (FMUUH), China. Patients with GC in the FMUUH database who were older than 18 years of age and who underwent radical gastrectomy between January 1997 and December 2014 were eligible for this study. The inclusion criteria were defined as: (1) histologically confirmed primary gastric adenocarcinoma; (2) at least 15 LNs examined; (3) no distant metastasis; and (4) radical gastrectomy with R0 resection and regional lymphadenectomy. Patients in the validation data set were excluded if they had fewer than 15 examined LNs, T4b stage, and if the T stage, number of positive LNs, or status of distant metastases were unknown. The final FMUUH cohort included in this study comprised 4407 patients. The adjuvant chemotherapy generally consisted of 5-fluorouracil (5-FU)-based regimens (commonly Oxaliplatin with either Xeloda or S1) for advanced GC in the FMUUH data sets[20,21]. The follow-up data were obtained from the follow-up office established by the Department of Gastric Surgery, FMUUH or from the National Statistical Office data. The survival time was calculated from the date of operation to the date of last follow-up or death. All patients were followed until death or date of December 2016, whichever occurred first. The median follow-up period was 66.0 mo. This project was approved by the local ethics committee of the FMUUH, China.
Statistical analyses were performed with SPSS statistics software (version 18.0, SPSS Inc, Chicago, IL, United States) and STATA version 12.0 (StataCorp, College Station, TX, United States). OS was analyzed using the Kaplan-Meier method, and log-rank tests were used for statistical comparisons of the survival curves. X-tile program (http://www.tissuearray.org/rimmlab/) was used to produce the LN cutoff points with the minimum P-values from log-rank χ2 statistics for the categorical metastatic LNs in terms of survival. The Akaike Information Criterion (AIC) and the Harrell’s concordance index (c-statistic) were used to assess the relative discriminatory abilities of the different TNM staging systems. In general, a predictive model with a low AIC indicates a better model fit, and a high c-statistic represents a better discriminatory ability[22-24]. Statistical significant differences were assumed as a two-side P < 0.05.
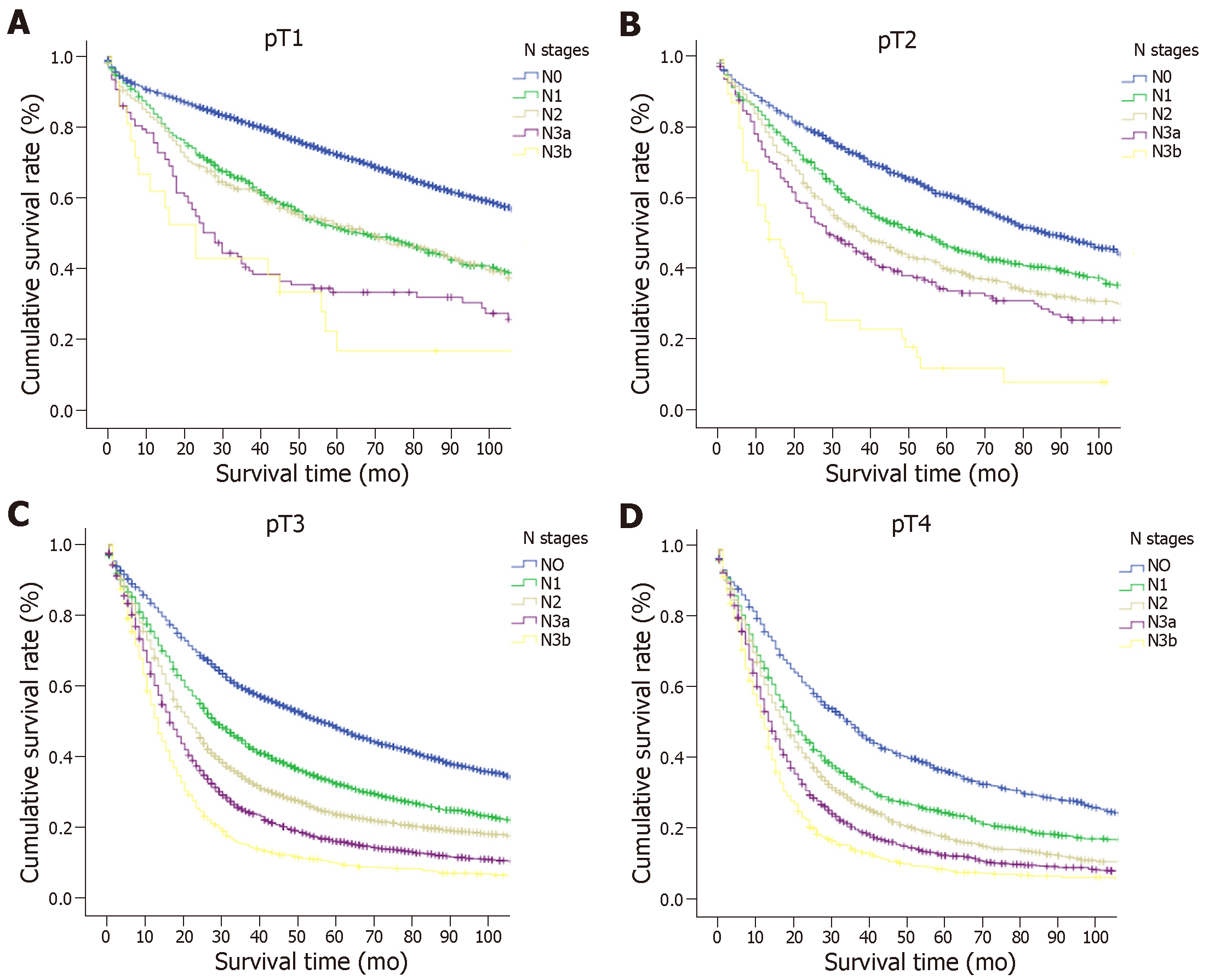
In all, 10714 patients in the SEER database fulfilled the selection criteria (Table 1). There were 6497 (60.6%) males and 4217 (39.4%) females aged between 20 and 101 years (67.1 ± 13.1 years). The median number of nodes examined was 22, and the 25% and 75% interquartile ranges were 18.0 and 29.0, respectively. Based on the eighth edition of the TNM classification[7], 1814 (16.9%) patients had stage pT1 (T1N0, 1353 patients; T1N1, 235 patients; T1N2, 139 patients; and T1N3, 87 patients), 1167 (10.9%) had pT2, 4417 (41.2%) had pT3, and 3316 (30.9%) had pT4a disease. A total of 3169 (29.6%) patients showed no LN metastasis, whereas 1530 (14.3%) had pN1, 1820 (17.0%) had pN2, 2596 (24.2%) had pN3a, and 1599 (14.9%) had pN3b disease. According to the TNM classification, there were 1353 (12.6%) patients in stage IA, 754 (7.0%) in stage IB, 1323 (12.3%) in stage IIA, 1213 (11.3%) in stage IIB, 1328 (12.4%) in stage IIIA, 2569 (24.0%) in stage IIIB, and 2174 (20.3%) in stage IIIC.
| Characteristic | SEER set (10714 patients) | FMUUH set (4407 patients) | P-valus | ||
| mean or n | SD or % | mean or n | SD or % | ||
| Age (SD) | 67.1 | 13.1 | 59.8 | 11.3 | 0.000 |
| Sex | 0.000 | ||||
| Male | 6497 | 60.6 | 3321 | 75.4 | |
| Female | 4217 | 39.4 | 1086 | 24.6 | |
| Race | - | ||||
| White | 6539 | 61.0 | - | - | |
| Black | 1297 | 12.1 | - | - | |
| Other | 2848 | 26.6 | - | - | |
| Unknown | 30 | 0.3 | - | - | |
| No. of examined nodes (SD) | 24.99 | 10.7 | 30.9 | 13.0 | 0.000 |
| pT category | 0.000 | ||||
| pT1 | 1814 | 16.9 | 899 | 20.4 | |
| pT2 | 1167 | 10.9 | 534 | 12.1 | |
| pT3 | 4417 | 41.2 | 954 | 21.6 | |
| pT4a | 3316 | 30.9 | 2020 | 45.8 | |
| pN category | 0.000 | ||||
| pN0 | 3169 | 29.6 | 1485 | 33.7 | |
| pN1 | 1530 | 14.3 | 630 | 14.3 | |
| pN2 | 1820 | 17.0 | 781 | 17.7 | |
| pN3a | 2596 | 24.2 | 928 | 21.1 | |
| pN3b | 1599 | 14.9 | 583 | 13.2 | |
| TNM stage | 0.000 | ||||
| IA | 1353 | 12.6 | 738 | 16.7 | |
| IB | 754 | 7.0 | 372 | 8.4 | |
| IIA | 1323 | 12.3 | 387 | 8.8 | |
| IIB | 1213 | 11.3 | 537 | 12.2 | |
| IIIA | 1328 | 12.4 | 921 | 20.9 | |
| IIIB | 2569 | 24.0 | 882 | 20.0 | |
| IIIC | 2174 | 20.3 | 570 | 12.9 | |
The 5-year OS rate of the entire cohort was 39.3%. The OS was compared with each N classification in the same T category (Figure 1 and Supplemental Table 1). For advanced GC (pT2-4a) patients, the survival curves of different N classifications demonstrated significant differences in the same T classification (P < 0.05). However, the OS between N1 and N2 cancers and between N3a and N3b cancers in cases of EGC did not differ significantly (P > 0.05), although a marked difference was seen in survival between the N0 and N1, and between the N2 and N3a cancer groups (P < 0.05).
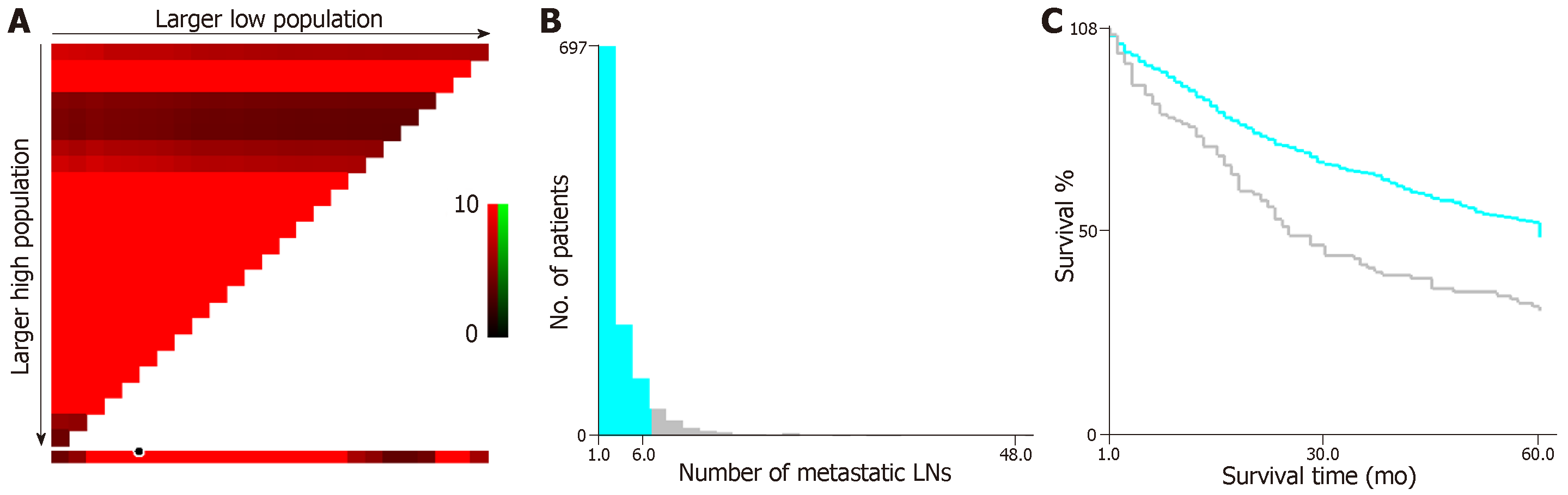
We applied X-tile analysis to determine the optimal cut-off N classification in order to predict the OS according to the different numbers of metastatic LNs. The result showed that 6 was the optimal cut-off for the number of metastatic LNs in patients with EGC (P < 0.05, Figure 2). Therefore, this value was used to divide the patients with EGC with LN metastasis into two subsets as follow: N1’, 1-6 LNs and N2’, ≥ 7 LNs.
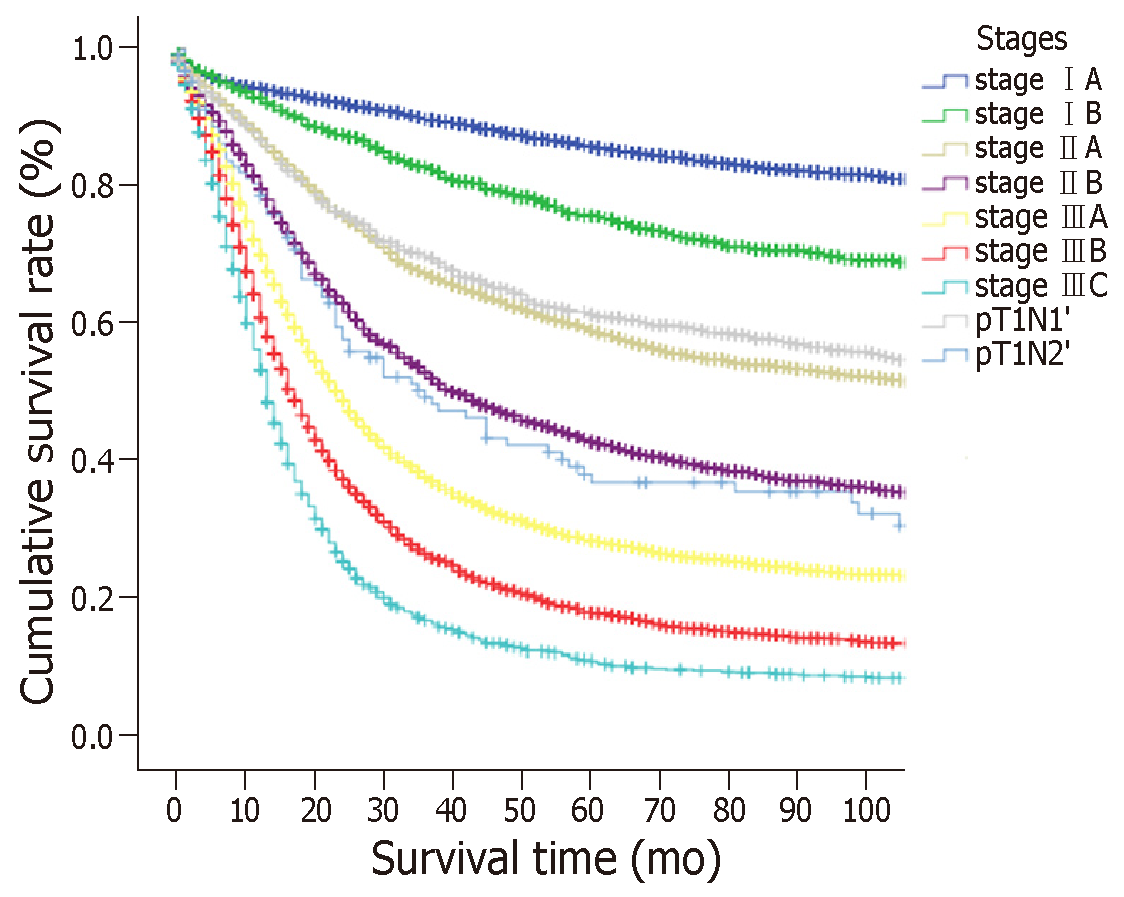
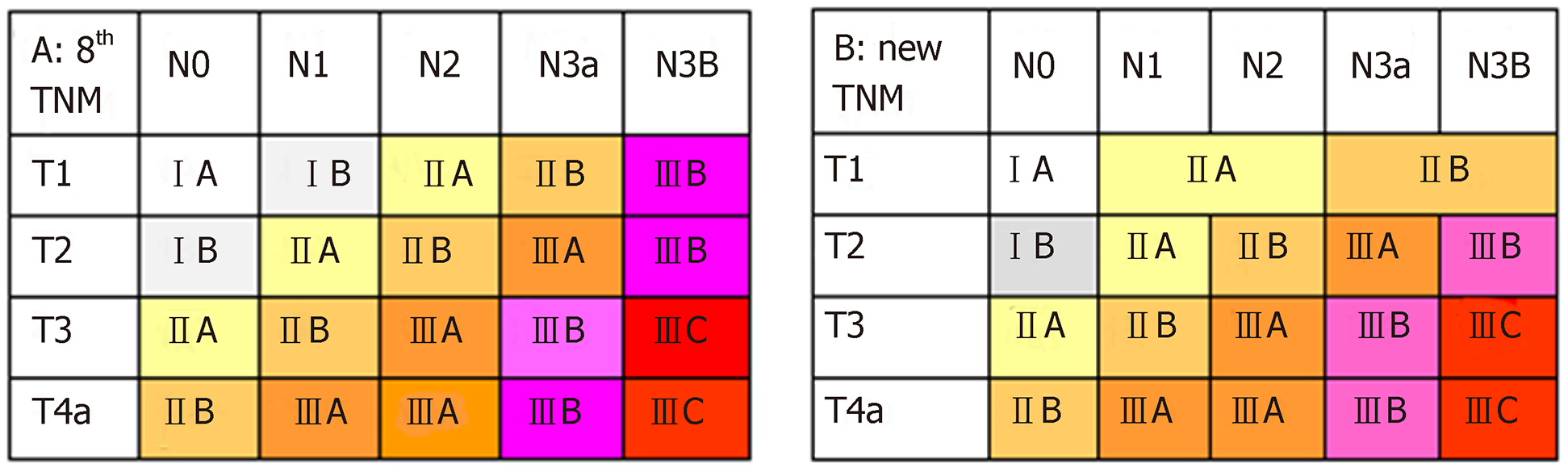
According to the eighth edition of the TNM staging system, T1N1 is stage IB, T1N2 is stage IIA, T1N3a is stage IIB, and T1N3b is stage IIIB. To identify the proposed new TNM staging system for EGC, we calculated T1N1’ and T1N2’ as new stages and compared the OS of patients with those stages to the stages according to the eighth edition of the TNM staging system which has excluded the T1N1-3 patients. The results are showed in Figure 3 and Supplemental Table 2. No significant differences were observed in patients with T1N1’ vs stage IIA or in patients with T1N2’ vs stage IIB (P > 0.05), but a significant difference was observed in patients with other stages. In addition, we found that the survival curves were comparable between T1N1’ and subgroups of stage IIA (T2N1 and T3N0), and between T1N2’ and subgroups of stage IIB (T2N2, T3N1, and T4aN0) (Supplemental Figure 1). Therefore, the proposed new stages T1N1’ and T1N2’ were considered equivalent to stage IIA and stage IIB, respectively.
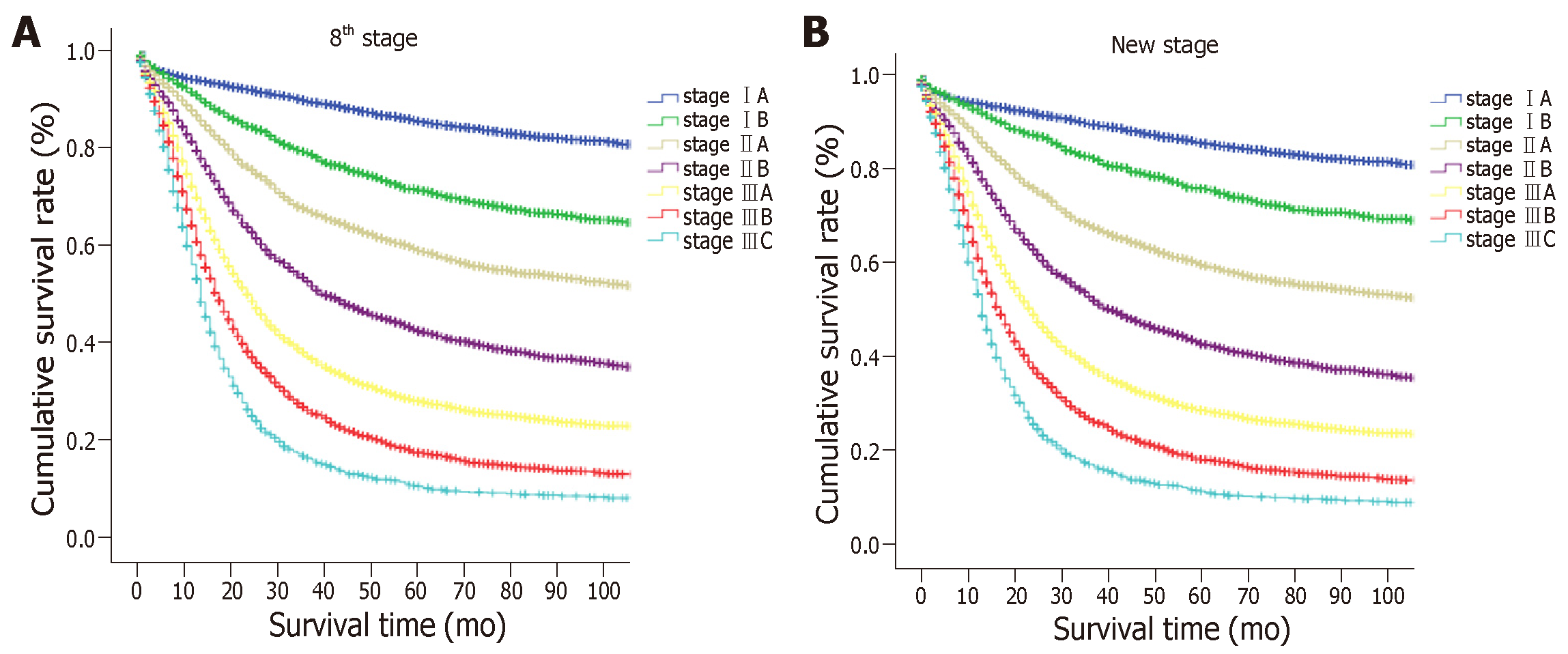
The proposed new TNM (nTNM) staging system is shown in Figure 4. We retained the same stages as in the eighth edition classification for advanced GC. The nTNM staging system revealed no crossing of the survival curves among the individual stage subgroups with statistically significant differences (Figure 5). A statistical assessment of the predictive performance of the two staging systems revealed that the nTNM staging system had a smaller AIC value (310672.4 vs 310674.3 for the eighth), a higher χ2 (6522.172 vs 6503.622 for the eighth), and a little higher Harrell’s C-index (0.6685 vs 0.6674 for the eighth). Although these values are not so much different between the eighth edition of the AJCC TNM staging classification and nTNM, the new N classification for EGC seems to have an optimal prognostic stratification.
An external validation of the new staging system was performed using the FMUUH data set (n = 4407). For patients in the FMUUH dataset, the 5-year OS was significantly different for patients at each stage between the eighth edition of the AJCC TNM staging system and the nTNM (P < 0.05; Supplemental Figure 2). The nTNM staging also showed a slightly smaller AIC value (24796.1 vs 24798.5 for the eighth edition), a higher χ2 (1293.584 vs 1290.275 for the eighth), and a higher Harrell’s C-index (0.7498 vs 0.7491 for the eighth).
EGC is a special type of tumor that comprises of T1 (invading the mucosa or submucosa) tumors irrespective of lymph node metastasis. Currently, given that health and endoscopic examinations are more commonly performed in clinical practice, the frequency of the diagnosis of EGC among all cases of GC has increased[25]. The 5-year survival rate of patients with EGC usually exceeds 90% in some Asian and Western countries. However, nearly 10% of patients still experience recurrence or death[26-28]. An accurate staging system for malignancy is useful to make the postoperative treatment decisions and follow-up plans[29]. Although the AJCC system is a worldwide used staging system, the N staging system is still occasionally questionable[30-32]. The eighth edition of the TNM staging system stratifies GC without distant metastasis into seven risk groups according to the pathological T and N categories. However, the classification scheme of the AJCC staging system is primarily based on the advanced GC. In EGC, lymph node metastasis is less severe than that in advanced GC[33]. Whether the AJCC TNM staging system is also appropriate for T1 cancer remains to be decided, but few studies have focused on this topic. Therefore, we investigated the survival of patients with EGC on the basis of a large sample and long-term follow-up results from the SEER database. Several highly influential studies also used the SEER database as a large-scale of multicenter database[18,34,35]. Although the SEER database may have some missing information, a part of patients have detailed primary tumor and lymph node characteristics, and survival, which were the main information we needed in our study.
The LN metastasis is one of the most significant prognostic factors for EGC. As reported in previous studies, LN metastasis usually occurs in less than 6% of intramucosal GCs, but when the tumor invades the sub-mucosal layer where lymphatic vessels are abundant, the rate of LN metastasis increases significantly to above 10%[36]. However, the N classification is still doubtful for EGC. A study from South Korea[37] showed that the sixth and seventh editions of the AJCC staging system were not well distributed with respect to the survival curve for patients with EGC; as a result, they developed a new N category as follows: N1 (1-5 metastatic LNs), N2 (6-10 metastatic LNs), and N3 (>10 metastatic LNs). They found that this classification system resulted in satisfactory survival curves. In the current study, we found that the OS between N1 and N2, or between N3a and N3b cancers were not significantly different in cases of EGC (P > 0.05). We used the X-tile method and the bootstrap procedure to confirm the stability of the cut off of 6 metastatic LNs, which showed a significant difference. Then, we proposed to classify EGC with LN metastasis into two subsets: T1N1’ and T1N2’ stages, which were determined to be stage IIA and stage IIB, respectively. The T1N3b classified into category IIB instead of IIIB is benefit for more actually predicting the survival. However, adjuvant chemotherapy still should be chose according to the treatment guideline. Moreover, this nTNM staging system shows a similar predictive ability (with a slightly lower AIC value and higher χ2 and c-statistic) for patients compared to the 8th edition of the AJCC TNM staging system. Although the new classification applies different N classifications between T1 and T2-4, it is meaningful of providing more accurate prognosis of N stages for EGC patients.
This study is significant, as a large cohort of patients who underwent radical gastrectomy and had a verified diagnosis based on the latest revision of the AJCC TNM classification were used to develop the nTNM staging system. An external validation is essential to ensure external applicability, although the predictive accuracy may decrease within an external validation set. It is more stringent for an external validation involving a dataset from another country than that involving using a dataset from a different institution or a validation set from the same institution based on a data-splitting method[22]. Since GC in American or European patients may differ biologically from GC in Asian patients, due to the significant variability in the extent of LN dissection and the average number of nodes retrieved[38-40], an Eastern-based population should also be used to evaluate the prognostic value of the new nTNM classification. Therefore, the additional external validation in this study was performed using an Asian dataset from the FMUUH database. Although the extent of LN dissection has changed over time, there is still without a standard extent of lymph node dissection for GC worldwide until now. In Japanese GC treatment guidelines[9], a D1 or D1 + lymphadenectomy is acceptable for EGC. Additionally, the NCCN guideline for GC indicates that gastric resection should include a systemic lymphadenectomy and remove at least 15 LNs as an adequate number of nodes for staging[8]. Therefore, in order to have a more actual estimation of N status of EGC, we only included patients with 15 or greater LNs retrieved in the current study according to the NCCN guidelines. In accordance with our results, the external validation of the nTNM classification system with the FMUUH dataset revealed similar results of AIC value, χ2, and Harrell’s C-index as the training set. Nowadays, there are no studies on how many differences between AIC, χ2, and c-statistics will be significant or clinically meaningful. Most studies mention that a smaller AIC value or a larger χ2 and c-statistics indicated a better model for predicting outcome[29,41-43]. At least, these parameters in this study were similar between the 8th edition TNM and new classification.
Although this study had a large cohort of patients from the SEER database, and had an external validation performed using the FMUUH database, there were still some limitations. First, the power of our conclusions may be somewhat limited by the retrospective nature of the study. The new classification in this study still needs more available data to confirm. Second, there are some shortcomings with the SEER data, including some incomplete information and the treatment may with some variable including quality of surgery. Third, the differences of AIC and c-index between the eighth edition and new TNM classification may be a little small. This may be because the eighth edition of the AJCC TNM staging system was revised based on the seventh edition and a large international sample size. It actually has a quite homogeneity, discriminatory, and monotonicity ability. What’s more, the data collecting periods were a little long in this study, and there were some epoch events, such as chemotherapy and endoscopic excision. Prior to 2002, adjuvant regimens were not widely used outside of Asia. In the United States now, adjuvant chemoradiotherapy is frequently employed. In Europe and Australia, adjuvant chemotherapy is usually platinum based with 5-FU or Xeloda, and S1 is more used in Asia. These variations in adjuvant therapies may affect the outcomes. However, this study focused on EGC, and most of the patients did not need chemotherapy. Additionally, we only included patients with at least 15 LNs examined in our study who might have sufficient LNs to check the LN status for EGC. As a result, there were still some meaningful findings from this study.
In summary, the LN metastasis classification of the eighth edition of the AJCC TNM staging system has variation in survival for AGC patients but is still associated with some stage migration in EGC. We have developed an optional new TNM staging system that can be used to accurately predict the 5-year OS of patients with EGC. However, more prospective studies with different populations are needed in the future.
Gastric cancer (GC) is a common type of cancer with a high incidence of cancer-related death. Early gastric cancer (EGC), which comprises of T1 tumors irrespective of lymph node metastasis, is a special type of cancer. Although GC is often diagnosed at an advanced stage, the incidence of EGC is increasing. Recently, the eighth edition of the American Joint Committee on Cancer (AJCC) tumor lymph node metastasis (TNM) staging system for stomach carcinoma was published. This edition retains the same T, N, and M classifications as the seventh edition. However, several studies found that the AJCC TNM staging system was inadequate for EGC.
An accurate staging system for malignancy is useful to make the postoperative treatment decisions and follow-up plans. Although the AJCC system is a worldwide used staging system, the N staging system is still occasionally questionable. The eighth edition of the TNM staging system stratifies GC without distal metastasis into seven risk groups according to the pathological T and N categories. However, the classification scheme of the AJCC staging system is primarily based on the advanced GC. In EGC, lymph node metastasis is less severe than that in advanced GC. Whether the AJCC TNM staging system is also appropriate for T1 cancer remains to be decided, but few studies have focused on this topic.
The aim of this study was to identify a better TNM classification to improve the prognostic prediction of patients with EGC after curative surgery.
We re-evaluated the long-term survival of patients with EGC who underwent radical gastrectomy from the SEER database between 1988 and 2011 in the context of the eighth edition of the AJCC staging system, and identify a better TNM classification to improve the prognostic prediction of patients with EGC after curative surgery. We also compared the discriminatory value of the new TNM classification with that of the eighth edition of the AJCC TNM staging system. Additional external validation was performed using the dataset from Fujian Medical University Union Hospital, China with similar inclusion and exclusion criteria. Overall survival was analyzed using the Kaplan-Meier method, and log-rank tests were used for statistical comparisons of the survival curves. X-tile program (http://www.tissuearray.org/rimmlab/) was used to produce the LN cutoff points with the minimum P-values from log-rank χ2 statistics for the categorical metastatic LNs in terms of survival. The Akaike Information Criterion and the Harrell’s concordance index (c-statistic) were used to assess the relative discriminatory abilities of different TNM staging systems.
The OS between N1 and N2, or between N3a and N3b cancers did not differ significantly in cases of EGC. We identified a new metastatic lymph node classification for EGC consisting of T1N0, T1N1’ (1-6 metastatic LNs), and T1N2’ ( ≥ 7 metastatic LNs) using X tile program. The OS of patients in T1N1’ stage was similar to the 8th edition AJCC stage IIA disease, while the OS of patients in T1N2’ stage was not significantly different from that of stage IIB disease. The new TNM staging system exhibited a slightly better predictive ability of OS for EGC in the training set and an external validation set.
The LN metastasis classification of the eighth edition of the AJCC TNM staging system has variation in survival for AGC patients but is still associated with some stage migration in EGC. We have developed an optional new TNM staging system that can be used to accurately predict the 5-year OS of patients with EGC. However, more prospective studies with different populations are needed in the future.
EGC is a special type of tumor that comprises of T1 (invading the mucosa or submucosa) tumors irrespective of lymph node metastasis. The 5-year survival rate of patients with EGC usually exceeds 90% in some Asian and Western countries. However, nearly 10% of patients still experience recurrence or death. An accurate staging system for malignancy is useful for making the postoperative treatment decisions and follow-up plans. We have developed an optional new TNM staging system with a better predictive ability of overall survival for EGC. This topic requires further analysis in a larger patient subset and in randomized studies in the future.
| 1. | Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23762] [Cited by in RCA: 25604] [Article Influence: 1706.9] [Reference Citation Analysis (10)] |
| 2. | Hartgrink HH, Jansen EP, van Grieken NC, van de Velde CJ. Gastric cancer. Lancet. 2009;374:477-490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 692] [Cited by in RCA: 798] [Article Influence: 46.9] [Reference Citation Analysis (1)] |
| 3. | Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9172] [Cited by in RCA: 9978] [Article Influence: 907.1] [Reference Citation Analysis (15)] |
| 4. | Songun I, Putter H, Kranenbarg EM, Sasako M, van de Velde CJ. Surgical treatment of gastric cancer: 15-year follow-up results of the randomised nationwide Dutch D1D2 trial. Lancet Oncol. 2010;11:439-449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1140] [Cited by in RCA: 1335] [Article Influence: 83.4] [Reference Citation Analysis (1)] |
| 5. | Isobe Y, Nashimoto A, Akazawa K, Oda I, Hayashi K, Miyashiro I, Katai H, Tsujitani S, Kodera Y, Seto Y, Kaminishi M. Gastric cancer treatment in Japan: 2008 annual report of the JGCA nationwide registry. Gastric Cancer. 2011;14:301-316. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 194] [Cited by in RCA: 249] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 6. | Baiocchi GL, Tiberio GA, Minicozzi AM, Morgagni P, Marrelli D, Bruno L, Rosa F, Marchet A, Coniglio A, Saragoni L, Veltri M, Pacelli F, Roviello F, Nitti D, Giulini SM, De Manzoni G. A multicentric Western analysis of prognostic factors in advanced, node-negative gastric cancer patients. Ann Surg. 2010;252:70-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 107] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 7. | Amin MB, Greene FL, Edge SB, Compton CC, Gershenwald JE, Brookland RK, Meyer L, Gress DM, Byrd DR, Winchester DP. The Eighth Edition AJCC Cancer Staging Manual: Continuing to build a bridge from a population-based to a more "personalized" approach to cancer staging. CA Cancer J Clin. 2017;67:93-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2341] [Cited by in RCA: 4679] [Article Influence: 519.9] [Reference Citation Analysis (4)] |
| 8. | Amin MB, Greene FL, Edge SB, Compton CC, Gershenwald JE, Brookland RK, Meyer L, Gress DM, Byrd DR, Winchester DP. AJCC Cancer Staging Manual. 8th ed. New York: Springer 2016; . |
| 9. | Japanese Gastric Cancer Association. Japanese classification of gastric carcinoma: 3rd English edition. Gastric Cancer. 2011;14:101-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2390] [Cited by in RCA: 2945] [Article Influence: 196.3] [Reference Citation Analysis (1)] |
| 10. | Lee JH, Chang KK, Yoon C, Tang LH, Strong VE, Yoon SS. Lauren Histologic Type Is the Most Important Factor Associated With Pattern of Recurrence Following Resection of Gastric Adenocarcinoma. Ann Surg. 2018;267:105-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 129] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 11. | Pyo JH, Lee H, Min BH, Lee JH, Choi MG, Lee JH, Sohn TS, Bae JM, Kim KM, Ahn JH, Carriere KC, Kim JJ, Kim S. Long-Term Outcome of Endoscopic Resection vs. Surgery for Early Gastric Cancer: A Non-inferiority-Matched Cohort Study. Am J Gastroenterol. 2016;111:240-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 159] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 12. | Wu B, Wu D, Wang M, Wang G. Recurrence in patients following curative resection of early gastric carcinoma. J Surg Oncol. 2008;98:411-414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 39] [Article Influence: 2.2] [Reference Citation Analysis (1)] |
| 13. | Yamamoto M, Yamanaka T, Baba H, Kakeji Y, Maehara Y. The postoperative recurrence and the occurrence of second primary carcinomas in patients with early gastric carcinoma. J Surg Oncol. 2008;97:231-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 26] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 14. | Takizawa K, Ono H, Kakushima N, Tanaka M, Hasuike N, Matsubayashi H, Yamagichi Y, Bando E, Terashima M, Kusafuka K, Nakajima T. Risk of lymph node metastases from intramucosal gastric cancer in relation to histological types: how to manage the mixed histological type for endoscopic submucosal dissection. Gastric Cancer. 2013;16:531-536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 92] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 15. | Al-Refaie WB, Tseng JF, Gay G, Patel-Parekh L, Mansfield PF, Pisters PW, Yao JC, Feig BW. The impact of ethnicity on the presentation and prognosis of patients with gastric adenocarcinoma. Results from the National Cancer Data Base. Cancer. 2008;113:461-469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 70] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 16. | Nogueira C, Silva AS, Santos JN, Silva AG, Ferreira J, Matos E, Vilaça H. Early gastric cancer: ten years of experience. World J Surg. 2002;26:330-334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 17. | Zhao X, Cai A, Xi H, Chen L, Peng Z, Li P, Liu N, Cui J, Li H. Predictive Factors for Lymph Node Metastasis in Undifferentiated Early Gastric Cancer: a Systematic Review and Meta-analysis. J Gastrointest Surg. 2017;21:700-711. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 28] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 18. | Wang J, Dang P, Raut CP, Pandalai PK, Maduekwe UN, Rattner DW, Lauwers GY, Yoon SS. Comparison of a lymph node ratio-based staging system with the 7th AJCC system for gastric cancer: analysis of 18,043 patients from the SEER database. Ann Surg. 2012;255:478-485. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 178] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 19. | Washington K. 7th edition of the AJCC cancer staging manual: stomach. Ann Surg Oncol. 2010;17:3077-3079. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 702] [Cited by in RCA: 820] [Article Influence: 54.7] [Reference Citation Analysis (0)] |
| 20. | Sasako M, Sakuramoto S, Katai H, Kinoshita T, Furukawa H, Yamaguchi T, Nashimoto A, Fujii M, Nakajima T, Ohashi Y. Five-year outcomes of a randomized phase III trial comparing adjuvant chemotherapy with S-1 versus surgery alone in stage II or III gastric cancer. J Clin Oncol. 2011;29:4387-4393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 869] [Cited by in RCA: 1119] [Article Influence: 74.6] [Reference Citation Analysis (0)] |
| 21. | Bang YJ, Kim YW, Yang HK, Chung HC, Park YK, Lee KH, Lee KW, Kim YH, Noh SI, Cho JY, Mok YJ, Kim YH, Ji J, Yeh TS, Button P, Sirzén F, Noh SH; CLASSIC trial investigators. Adjuvant capecitabine and oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): a phase 3 open-label, randomised controlled trial. Lancet. 2012;379:315-321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1267] [Cited by in RCA: 1331] [Article Influence: 95.1] [Reference Citation Analysis (0)] |
| 22. | Han DS, Suh YS, Kong SH, Lee HJ, Choi Y, Aikou S, Sano T, Park BJ, Kim WH, Yang HK. Nomogram predicting long-term survival after d2 gastrectomy for gastric cancer. J Clin Oncol. 2012;30:3834-3840. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 220] [Cited by in RCA: 281] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 23. | Harrell FE. Regression Modeling Strategies with Application to Linear Models, Logistic Regression, and Survival Analysis. New York, NY, Springer Verlag. 2001;. |
| 24. | Harrell FE, Lee KL, Mark DB. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med. 1996;15:361-387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 25. | Hwang JJ, Lee DH, Yoon H, Shin CM, Park YS, Kim N. Clinicopathological characteristics of patients who underwent additional gastrectomy after incomplete endoscopic resection for early gastric cancer. Medicine (Baltimore). 2017;96:e6172. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 26. | Hanyu T, Matsuki A, Kosugi S, Ishikawa T, Nashimoto A, Yabusaki H, Aizawa M, Ichikawa H, Shimada Y, Hirose Y, Wakai T. Prognostic analysis of submucosa-invasive gastric cancer with lymph node metastasis. Surgery. 2015;157:716-722. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 27. | Kubota H, Kotoh T, Masunaga R, Dhar DK, Shibakita M, Tachibana M, Kohno H, Nagasue N. Impact of screening survey of gastric cancer on clinicopathological features and survival: retrospective study at a single institution. Surgery. 2000;128:41-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 64] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 28. | Lee JH, Choi IJ, Han HS, Kim YW, Ryu KW, Yoon HM, Eom BW, Kim CG, Lee JY, Cho SJ, Kim YI, Nam BH, Kook MC. Risk of lymph node metastasis in differentiated type mucosal early gastric cancer mixed with minor undifferentiated type histology. Ann Surg Oncol. 2015;22:1813-1819. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 39] [Article Influence: 3.3] [Reference Citation Analysis (1)] |
| 29. | Spolverato G, Ejaz A, Kim Y, Squires MH, Poultsides G, Fields RC, Bloomston M, Weber SM, Votanopoulos K, Acher AW, Jin LX, Hawkins WG, Schmidt C, Kooby DA, Worhunsky D, Saunders N, Cho CS, Levine EA, Maithel SK, Pawlik TM. Prognostic Performance of Different Lymph Node Staging Systems After Curative Intent Resection for Gastric Adenocarcinoma. Ann Surg. 2015;262:991-998. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 90] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 30. | Wang W, Sun XW, Li CF, Lv L, Li YF, Chen YB, Xu DZ, Kesari R, Huang CY, Li W, Zhan YQ, Zhou ZW. Comparison of the 6th and 7th editions of the UICC TNM staging system for gastric cancer: results of a Chinese single-institution study of 1,503 patients. Ann Surg Oncol. 2011;18:1060-1067. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 70] [Cited by in RCA: 84] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 31. | Chae S, Lee A, Lee JH. The effectiveness of the new (7th) UICC N classification in the prognosis evaluation of gastric cancer patients: a comparative study between the 5th/6th and 7th UICC N classification. Gastric Cancer. 2011;14:166-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 75] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 32. | Warneke VS, Behrens HM, Hartmann JT, Held H, Becker T, Schwarz NT, Röcken C. Cohort study based on the seventh edition of the TNM classification for gastric cancer: proposal of a new staging system. J Clin Oncol. 2011;29:2364-2371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 85] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 33. | Sekiguchi M, Oda I, Taniguchi H, Suzuki H, Morita S, Fukagawa T, Sekine S, Kushima R, Katai H. Risk stratification and predictive risk-scoring model for lymph node metastasis in early gastric cancer. J Gastroenterol. 2016;51:961-970. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 89] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 34. | Anderson WF, Camargo MC, Fraumeni JF, Correa P, Rosenberg PS, Rabkin CS. Age-specific trends in incidence of noncardia gastric cancer in US adults. JAMA. 2010;303:1723-1728. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 354] [Cited by in RCA: 360] [Article Influence: 22.5] [Reference Citation Analysis (0)] |
| 35. | Wang J, Sun Y, Bertagnolli MM. Comparison of gastric cancer survival between Caucasian and Asian patients treated in the United States: results from the Surveillance Epidemiology and End Results (SEER) database. Ann Surg Oncol. 2015;22:2965-2971. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 83] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 36. | Namieno T, Koito K, Higashi T, Sato N, Uchino J. General pattern of lymph node metastasis in early gastric carcinoma. World J Surg. 1996;20:996-1000. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 18] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 37. | Choi KH, Kim BS, Oh ST, Yook JH, Kim BS. Comparison the sixth and seventh editions of the AJCC staging system for T1 gastric cancer: a long-term follow-up study of 2124 patients. Gastric Cancer. 2017;20:43-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 38. | Woo Y, Son T, Song K, Okumura N, Hu Y, Cho GS, Kim JW, Choi SH, Noh SH, Hyung WJ. A Novel Prediction Model of Prognosis After Gastrectomy for Gastric Carcinoma: Development and Validation Using Asian Databases. Ann Surg. 2016;264:114-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 38] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 39. | Naylor GM, Gotoda T, Dixon M, Shimoda T, Gatta L, Owen R, Tompkins D, Axon A. Why does Japan have a high incidence of gastric cancer? Comparison of gastritis between UK and Japanese patients. Gut. 2006;55:1545-1552. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 130] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 40. | Theuer CP, Campbell BS, Peel DJ, Lin F, Carpenter P, Ziogas A, Butler JA. Microsatellite instability in Japanese vs European American patients with gastric cancer. Arch Surg. 2002;137:960-965-965-966. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 32] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 41. | Lu J, Wang W, Zheng CH, Fang C, Li P, Xie JW, Wang JB, Lin JX, Chen QY, Cao LL, Lin M, Huang CM, Zhou ZW. Influence of Total Lymph Node Count on Staging and Survival After Gastrectomy for Gastric Cancer: An Analysis From a Two-Institution Database in China. Ann Surg Oncol. 2017;24:486-493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 68] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 42. | Wang X, Appleby DH, Zhang X, Gan L, Wang JJ, Wan F. Comparison of three lymph node staging schemes for predicting outcome in patients with gastric cancer. Br J Surg. 2013;100:505-514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 64] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 43. | Hsu PK, Wu YC, Chou TY, Huang CS, Hsu WH. Comparison of the 6th and 7th editions of the American Joint Committee on Cancer tumor-node-metastasis staging system in patients with resected esophageal carcinoma. Ann Thorac Surg. 2010;89:1024-1031. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 96] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See:
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): E
P- Reviewer: Chiu CC, Muguruma N, Noshiro H, Tsunoda S S- Editor: Ji FF L- Editor: Wang TQ E- Editor: Wu YXJ