Published online Oct 6, 2019. doi: 10.12998/wjcc.v7.i19.2930
Peer-review started: July 17, 2019
First decision: August 2, 2019
Revised: August 9, 2019
Accepted: August 27, 2019
Article in press: August 27, 2019
Published online: October 6, 2019
Processing time: 81 Days and 1.7 Hours
Melanoma is a highly malignant skin tumour, and is one of the most rapidly growing malignant tumors in recent years. According to statistics, the morbidity of cancer increases with age, accounting for 1.6% of new cancer cases and 0.6% of deaths worldwide. Melanoma has a serious impact on society and families, thus it is of great significance to find biological markers related to the diagnosis and treatment of melanoma.
To explore the expression and predictive value of mir-489 and mir-21 in melanoma metastasis.
A total of 60 patients with malignant melanoma treated at our hospital from June 2017 to December 2018 were selected as a research group, while 40 healthy subjects were selected as a control group. qRT-PCR technique was used to detect miR-489 and miR-21 in serum of the two groups. ROC curve was drawn to evaluate the predictive value and diagnostic efficiency. Spearman test was used for correlation analysis. Logistic single- and multiple-factor analyses were performed to identify the risk factors related to melanoma metastasis.
The expression of miR-489 in the research group was significantly lower than that in the control group (P < 0.001). However, the expression of miR-21 in the research group was significantly higher than that in the control group (P < 0.001). The expression of miR-489 and miR-21 was related to TNM stage and metastasis (P < 0.001). In the diagnosis of melanoma patients, the sensitivity, specificity, and AUC of miR-489 alone were 75.56%, 80.00%, and 0.852, respectively. The sensitivity, specificity, and AUC of miR-21 alone were 77.78%, 82.22%, and 0.844, respectively. MiR-489 was negatively correlated with TNM stage of melanoma (r = -0.612, P < 0.001), while miR-21 was positively correlated with TNM stage (r = 0.609, P < 0.001). Logistic single- and multiple-factor regression analyses showed that TNM stage, miR-489, and mir-21 were independent risk factors for malignant melanoma metastasis.
MiR-489 and miR-21 may participate in the process of melanoma occurrence, development, and metastasis, and can be used as potential serum biomarkers for melanoma metastasis diagnosis and disease assessment.
Core tip: Metastasis of malignant melanoma may lead to severe infection. Due to the high missed diagnosis rate of melanoma metastasis in vivo and the limited treatment options for malignant melanoma metastasis at present, the disease recurrence rate is high. Improving diagnostic efficiency and understanding risk factors are very important for the diagnosis and treatment of malignant melanoma metastasis. We aimed to investigate the expression of miR-489 and miR-21 as well as their correlation and diagnostic value in melanoma of different clinicopathological features. We also compared the predictive value of miR-489 and miR-21 in melanoma metastasis.
- Citation: Mo H, Guan J, Yuan ZC, Lin X, Wu ZJ, Liu B, He JL. Expression and predictive value of miR-489 and miR-21 in melanoma metastasis. World J Clin Cases 2019; 7(19): 2930-2941
- URL: https://www.wjgnet.com/2307-8960/full/v7/i19/2930.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v7.i19.2930
Malignant melanoma is a malignant tumor commonly found in the skin or other organs, which is produced by malignant transformation of melanocytes[1]. Although the incidence rate of malignant melanoma is relatively low, it has become one of the tumors with the fastest growth of incidence rate in the world, with a growth rate of 7% year by year, and malignant melanoma patients often suffer from metastasis of the focus and the mortality increases significantly with the metastasis of the focus[2]. Currently, the clinical treatments for melanoma are mainly surgical resection, systemic treatment, radiotherapy-based combined treatment, radiotherapy alone, and immunotherapy[3-5]. Relevant reports show that although good efficacy can be obtained by radical surgery in the early stage, it is difficult to treat malignant melanoma patients with metastasis or diffusion in the late stage, and there are still a large number of melanoma patients with recurrence or poor prognosis, which is also the reason for the high mortality of melanoma[6,7]. Analysis of the pathogenesis of human melanoma and inhibition of melanoma progression are an important part of melanoma research[8].
With the in-depth study of tumor-related molecular biology, it was found that microRNAs (miRNAs) are a class of endogenous non-coding single-stranded RNAs with tumor-promoting or tumor-inhibiting functions[9,10]. MiRNAs play a role in inhibiting or promoting the proliferation of cancer cells in different solid tumors. Current studies showed that miRNAs are abnormally expressed in melanoma[11]. Reports showed that miR-489 expression was down-regulated in malignant melanoma cells, and appropriate up-regulation of miR-489 expression could inhibit the proliferation of malignant melanoma cells[12]. On the other hand, miR-21 was proved to be much more expressed in serum of melanoma patients than in normal serum[13]. However, there is a lack of research on the expression and predictive value of miR-489 and miR-21 in melanoma metastasis. Therefore, this study aimed to provide a new theoretical basis for the molecular diagnosis and treatment of melanoma, and to conduct experimental research on the expression characteristics of miR-489 and miR-21 in melanoma and their clinical significance.
A total of 60 patients with malignant melanoma from June 2017 to December 2018 were selected as a research group, including 30 males and 30 females. The average age was 50.45 ± 15.03 years. Forty healthy people who underwent physical examination during the same period were selected as a control group, including 20 males and 20 females. The average age was 50.27 ± 16.19 years. The inclusion criteria were: (1) All melanoma patients included met the diagnostic guidelines of CSCO melanoma expert committee[14]; (2) liver and kidney functions were normal; and (3) there were no other malignant tumors. All patients who received chemotherapy, immunotherapy, and radiotherapy before operation were excluded. The research was approved by the Ethics Committee of our hospital. Patients and their families were informed in advance before the study was carried out, and informed consent was obtained.
Main reagents and instruments: Trizol reagent (American Applied Invitrogen company), qRT-PCR kit and minScript reverse transcription kit (Dalian TaKaRa company), HBS-1096A microplate reader (Nanjing Detie Experimental Equipment Co., Ltd.), and real-time quantitative PCR instrument (American BioRad company) were used. The sequences of primers, miRNA negative controls, miR-489, miR-21, and internal reference U6 were designed and synthesized by Shanghai Gemma company. More details are shown in Table 1.
| Group | Forward primer | Reverse primer |
| miR-489 | 5'-ACACTCCAGCTGGGG TGACATCACATA-3' | 5'-TGGTGTCGTGGAGTCG-3' |
| miR-21 | 5'-GCGGCGGTAGCTTATCAGACTG⁃3' | 5'-ATCCAGTGCAGGGTCCGAGG⁃3' |
| U6 | 5'-CTCGCTTCGGCAGCACA-3' | 5'-AACGCTTCACGAATTTGCGT-3' |
Detection of miR-489 and miR-21: qRT-PCR was used to detect the expression of miR-489 and miR-21 in serum in the two groups. Total RNA in serum was extracted and dissolved in 20 μL of DEPC water according to the manufacturer’s instructions. Total RNA was then reverse transcribed with a reverse transcription kit. The reaction system was as follows: M-MLV 1 μL, oligo(dT) 1 μL, RNAase inhibitor 0.5 μL, dNTPs 1 μL, and RNase free water supplemented to 15 μL. The mixture was incubated at 38 °C for 60 min and then at 85 °C for 5 s. The synthesized c DNA was used as the template for qRT-PCR amplification. A 25-μL PCR reaction system was then prepared: 10 × PCR buffer 2.5 μL, dNTPs 1 μL, upstream and downstream primers 1 μL each, Taq DNA polymerase 0.25 μL, and ddH2O supplemented to 25 μL. Reaction conditions were pre-denaturation at 95 °C for 15 min, 35 cycles of denaturation at 95 °C for 15 s and annealing at 60 °C for 30 s, and final extension at 72 °C for 15min. Each sample was provided with three multiple wells for three repeated tests. U6 was regarded as the internal reference both for miR-489 and miR-21. After the reaction was completed, the amplification curve and melting curve of real-time PCR were confirmed, and the relative amount of the target gene was calculated according to the result parameters. The relative quantification of target gene was calculated by the 2-△ CT method.
SPSS 17.0 software was used for statistical analyses. The counting data are expressed as the number of cases/percentage [n (%)], and the X2 test was used for comparison between the two groups. The measurement data are expressed as the mean ± SD, and the comparison between groups was conducted by the t-test or F-test. ROC curve was drawn, the optimal cut-off value of Jordan index was selected, and the diagnostic efficacy and predictive value of serum miR-489 and miR-21 expression were evaluated. Spearman test was used for correlation analysis. Logistic single- and multiple-factor analyses were performed on the risk factors related to melanoma metastasis. P-values < 0.05 were considered statistically significant.
The age, gender, tumor size, TNM stage, lesion site, and lymph node metastasis were compared between the research group and control group. There were no significant differences between the two group in terms of age, gender, or other clinical data (P > 0.05), as shown in Table 2.
| Variable | Research group (n = 60) | Control group (n = 40) | t | P-value |
| Age (yr) | 0.733 | 0.392 | ||
| ≤ 50 | 23 (38.33) | (30.00) | ||
| > 50 | 37 (61.67) | 28 (70.00) | ||
| Gender | 0.000 | 1.000 | ||
| Male | 30 (50.00) | 20 (50.00) | ||
| Female | 30 (50.00) | 20 (50.00) | ||
| Tumor size (cm) | ||||
| ≤ 2 | 17 (28.33) | - | - | - |
| > 2 | 43 (71.67) | - | - | - |
| Lesion site | ||||
| Non-acral | 44 (73.33) | - | - | - |
| Acral | 16 (26.67) | - | - | - |
| TNM stage | ||||
| I | 7 (11.67) | - | - | - |
| II | 8 (13.33) | - | - | - |
| III | 25 (41.67) | - | - | - |
| IV | 20 (33.33) | - | - | - |
| Lymph node metastasis | ||||
| Yes | 45 (75.00) | - | - | - |
| No | 15 (25.00) | - | - | - |
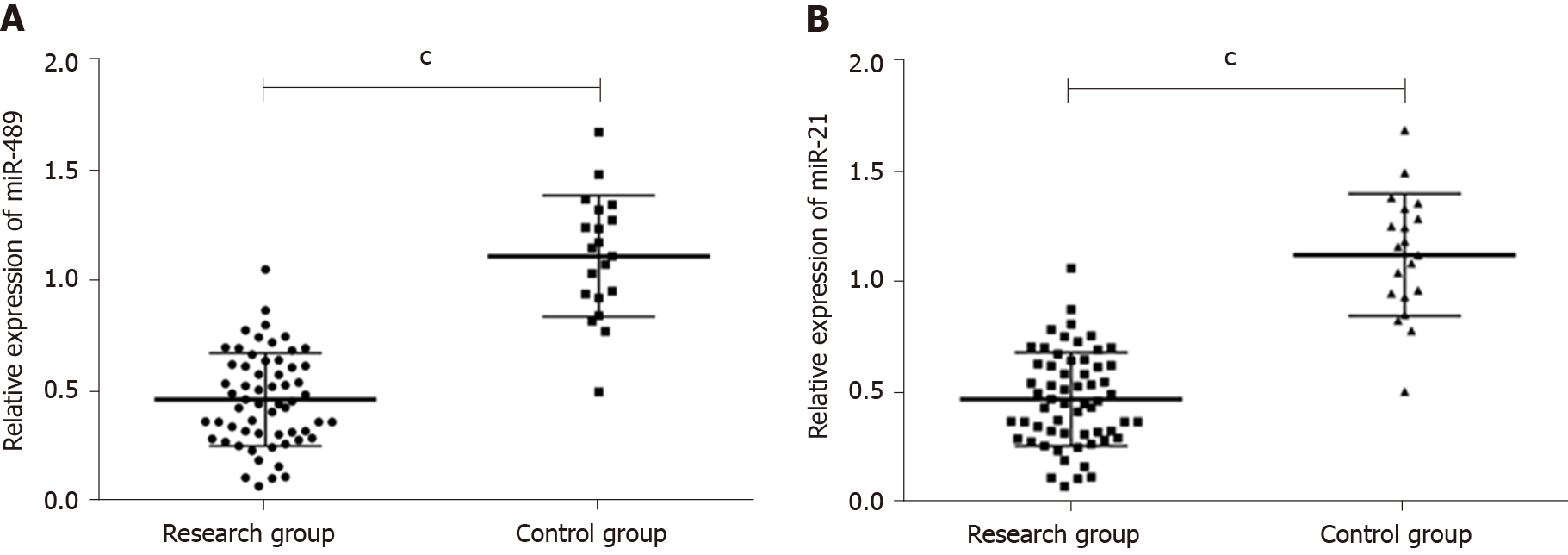
qRT-PCR results showed that the expression of miR-489 in serum of the research group and control group were, respectively, (0.47 ± 0.23) and (1.12 ± 0.26). The expression of miR-21 in serum of the research group and control group were (5.28 ± 2.10) and (0.98 ± 0.27), respectively. The expression of miR-489 in the research group was significantly lower than that in the control group (P < 0.001), while the expression of miR-21 in the research group was significantly higher than that in the control group (P < 0.001). More details are shown in Figure 1A and B.
Relationship between expression levels of miR-489 and clinicopathological characteristics of melanoma: The expression levels of miR-489 in serum did not differ significantly between melanoma patients aged ≤ 50 years and those aged > 50 years (0.43 ± 0.26 vs 0.51 ± 0.20 P > 0.05), between male and female melanoma patients (0.54 ± 0.18 vs 0.42 ± 0.28, P > 0.05), between melanoma patients with tumor size ≤ 2 cm and those with tumor size > 2 cm (0.45 ± 0.30 vs 0.39 ± 0.16, P > 0.05), or between patients with non-acral melanoma and those with acral melanoma (0.50 ± 0.22 vs 0.44 ± 0.24, P > 0.05). The expression levels of miR-489 in serum of patients with stages I-IV melanoma were 0.62 ± 0.29, 0.48 ± 0.24, 0.47 ± 0.22, and 0.31 ± 0.17, respectively, and there were significant differences between different groups (P < 0.05). The expression level of miR-489 in serum of patients with melanoma metastasis was significantly lower than that of patients with no metastasis (0.30 ± 0.16 vs 0.64 ± 0.30, P < 0.05). The expression of miR-489 was not related to age, gender, tumor size, or lesion site, but related to TNM stage and metastasis. More details are shown in Table 3.
| Variable | n | miR-489 | t/F | P-value |
| Age (yr) | 1.341 | 0.185 | ||
| ≤ 50 | 23 | 0.43 ± 0.26 | ||
| > 50 | 37 | 0.51 ± 0.20 | ||
| Gender | 1.975 | 0.063 | ||
| Male | 30 | 0.54 ± 0.18 | ||
| Female | 30 | 0.42 ± 0.28 | ||
| Tumor size (cm) | 0.439 | 0.779 | ||
| ≤ 2 | 17 | 0.45 ± 0.30 | ||
| > 2 | 43 | 0.39 ± 0.16 | ||
| Lesion site | 0.912 | 0.366 | ||
| Non-acral | 44 | 0.50 ± 0.22 | ||
| Acral | 16 | 0.44 ± 0.24 | ||
| TNM stage | 4.269 | 0.009 | ||
| I | 7 | 0.62 ± 0.29 | ||
| II | 8 | 0.48 ± 0.24 | ||
| III | 25 | 0.47 ± 0.22 | ||
| IV | 20 | 0.31 ± 0.17 | ||
| Metastasis | 5.622 | < 0.001 | ||
| Yes | 45 | 0.30 ± 0.16 | ||
| No | 15 | 0.64 ± 0.30 |
Relationship between expression levels of miR-21 and clinicopathological characteristics of melanoma: The expression levels of miR-21 in serum did not differ significantly between melanoma patients aged ≤ 50 years and those aged > 50 years (4.72 ± 2.00 vs 5.84 ± 2.20, P > 0.05), between male and female melanoma patients (5.12 ± 1.75 vs 5.44 ± 2.45, P > 0.05), between melanoma patients with tumor size ≤ 2 cm and those with tumor size > 2 cm (4.99 ± 1.68 vs 5.57 ± 2.52, P > 0.05), or between patients with non-acral melanoma and those with acral melanoma (5.16 ± 1.55 vs 5.40 ± 2.65, P > 0.05). The expression levels of miR-21 in serum of patients with stages I, II, III, and IV melanoma were 3.60 ± 1.79, 5.28 ± 2.13, 5.23 ± 2.01, and 7.01 ± 2.47, respectively, and there were significant differences between different groups (P < 0.05). The expression level of miR-21 in serum of patients with melanoma metastasis was significantly higher than that of patients with no metastasis (6.38 ± 2.35 vs 4.18 ± 1.85, P < 0.05). The expression of miR-21 was not related to age, gender, tumor size, or lesion site, but related to TNM stage and metastasis. More details are shown in Table 4.
| Variable | n | miR-21 | t/F | P-value |
| Age (yr) | 1.984 | 0.052 | ||
| ≤ 50 | 23 | 4.72 ± 2.00 | ||
| > 50 | 37 | 5.84 ± 2.20 | ||
| Gender | 0.582 | 0.563 | ||
| Male | 30 | 5.12 ± 1.75 | ||
| Female | 30 | 5.44 ± 2.45 | ||
| Tumor size (cm) | 0.386 | 0.873 | ||
| ≤ 2 | 17 | 4.99 ± 1.68 | ||
| > 2 | 43 | 5.57 ± 2.52 | ||
| Lesion site | 0.386 | 0.873 | ||
| Non-acral | 44 | 5.16 ± 1.55 | ||
| Acral | 16 | 5.40 ± 2.65 | ||
| TNM stage | 5.040 | 0.004 | ||
| I | 7 | 3.60 ± 1.79 | ||
| II | 8 | 5.23 ± 2.13 | ||
| III | 25 | 5.28 ± 2.01 | ||
| IV | 20 | 7.01 ± 2.47 | ||
| Metastasis | 3.295 | 0.002 | ||
| Yes | 45 | 6.38 ± 2.35 | ||
| No | 15 | 4.18 ± 1.85 |
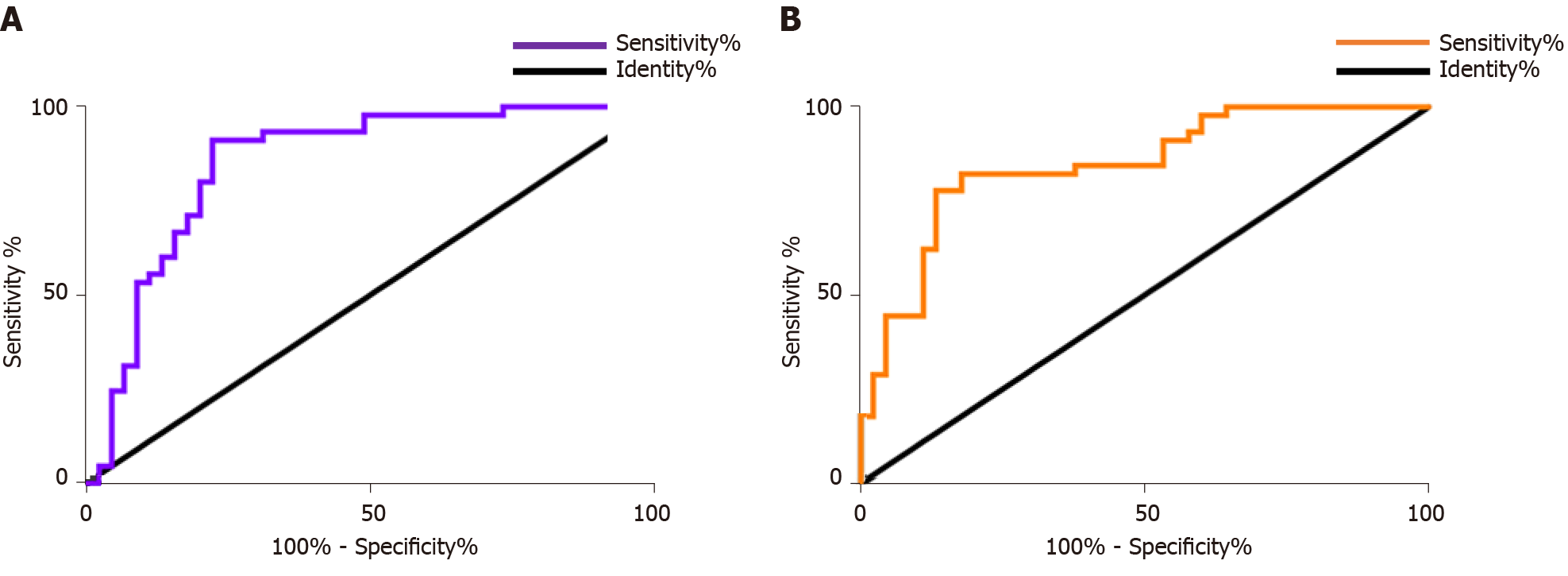
In the diagnosis of melanoma patients, the sensitivity, specificity, and AUC of miR-489 single diagnosis were 75.56%, 80.00%, and 0.8519, respectively. The sensitivity, specificity, and AUC of miR-21 single diagnosis were 77.78%, 82.22%, and 0.8444, respectively. More details are shown in Table 5 and Figure 2A and B.
| Index | miR-489 | miR-21 |
| AUC | 0.852 | 0.844 |
| 95%CI | 0.7678-0.9359 | 0.7633-0.9256 |
| Std. Error | 0.0429 | 0.0414 |
| Cut-off value | 0.451 | 4.841 |
| Sensitivity (%) | 75.56 | 77.78 |
| Specificity (%) | 80.00 | 82.22 |
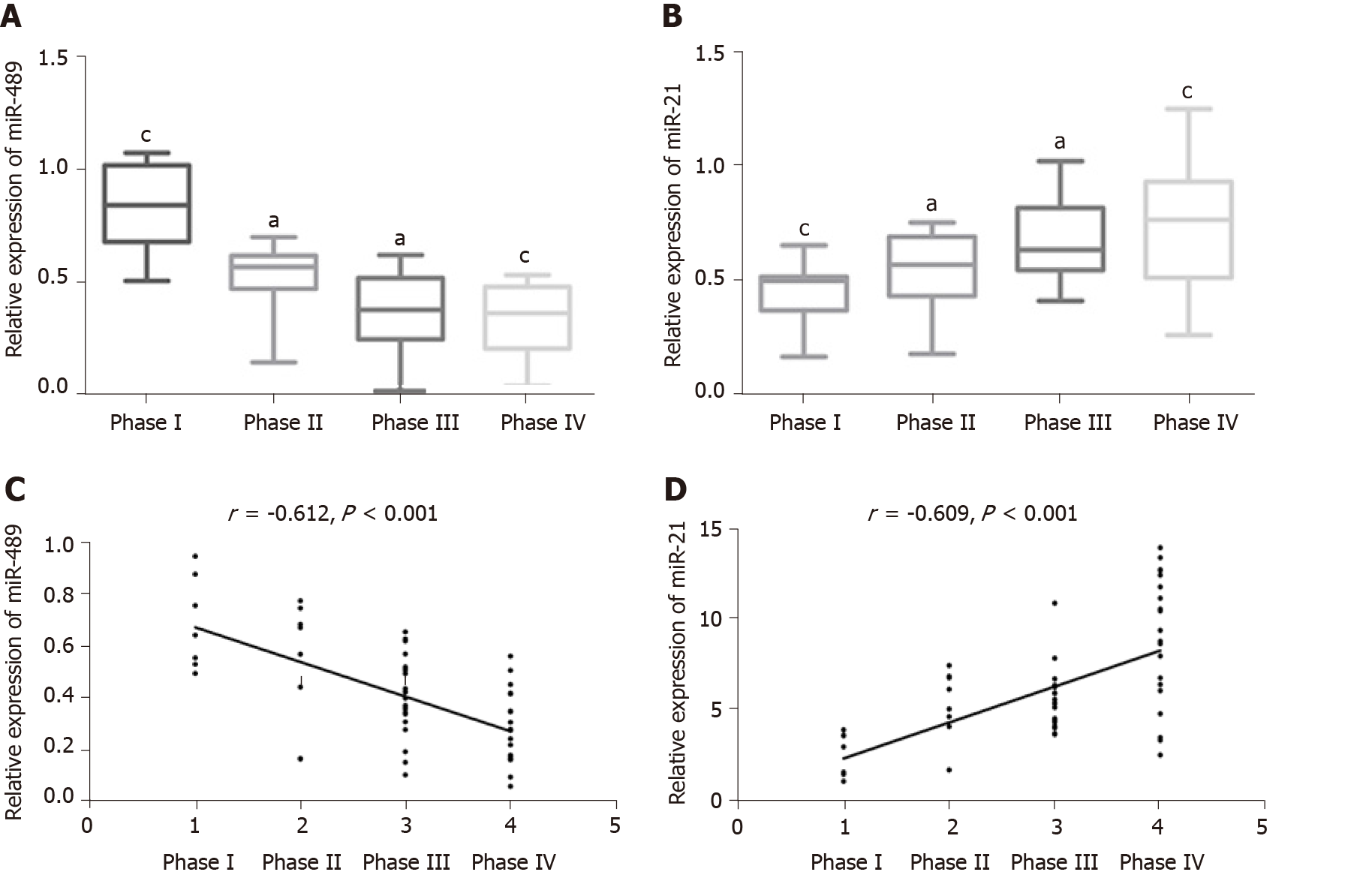
The expression levels of miR-489 in serum of patients with TNM stages I-IV melanoma were 0.62 ± 0.29, 0.48 ± 0.24, 0.47 ± 0.22, and 0.31 ± 0.17, respectively; the corresponding values for miR-21 were 3.60 ± 1.79, 5.28 ± 2.13, 5.23 ± 2.01, and 7.01 ± 2.47). Compared with patients with Hoehn-Yahr grade I, the relative expression of miR-489 and miR-21 in serum of patients with grades II and III decreased significantly (P < 0.05). With the increase of TNM stage, the relative expression of miR-489 in serum decreased, while the relative expression of miR-21 in serum increased continuously. Spearman correlation analysis of the relative expression of serum miR-489 and miR-21 in melanoma of different TNM stages showed that the relative expression of serum miR-489 was negatively correlated with melanoma TNM stage (r = -0.612, P < 0.001), while the relative expression of serum miR-21 was positively correlated with TNM stage of melanoma (r = 0.609, P < 0.001). More details are shown in Figure 3A-D.
Single-factor analysis of melanoma metastasis and related factors: Logistic single-factor analysis of risk factors related to melanoma metastasis in melanoma patients showed that there were significant differences between patients with and without melanoma metastasis in age, lesion site, TNM stage, miR-489, and miR-21 (P < 0.05). Patients’ age, lesion site, TNM stage, miR-489, and miR-21 were all related to melanoma metastasis and were risk factors for melanoma metastasis, as shown in Tables 6 and 7.
| Correlative factor | Assignment description |
| Age (yr) | < 50 = 0; ≥ 50 = 1 |
| Gender | Male = 0; female = 1 |
| Tumor size (cm) | ≤ 2 = 0; > 2 = 1 |
| Lesion site | Non-arcal = 0; arcal =1 |
| TNM stage | I-II = 0; III-IV =1 |
| miR-489 | < 0.451 = 0; > 0.451 = 1 |
| miR-21 | < 4.841= 0; > 4.841 = 1 |
| Factor | Metastasis of melanoma (n = 45) | Melanoma is non-metastatic (n = 15) | t/X2 | P-value |
| Age (yr) | 6.792 | 0.009 | ||
| ≤ 50 | 13 (28.89) | 10 (66.67) | ||
| > 50 | 32 (71.11) | 5 (33.33) | ||
| Gender | 2.222 | 0.136 | ||
| Male | 25 (55.56) | 5 (33.33) | ||
| Female | 20 (44.44) | 10 (66.67) | ||
| Tumor size (cm) | 14.470 | < 0.001 | ||
| ≤ 2 | 7 (15.56) | 10 (66.67) | ||
| > 2 | 38 (84.44) | 5 (33.33) | ||
| Lesion site | 0.000 | 1.000 | ||
| Non-acral | 33 (73.33) | 11 (73.33) | ||
| Acral | 12 (26.67) | 4 (26.67) | ||
| TNM stage | 35.920 | < 0.001 | ||
| I | 0 (0.00) | 7 (46.67) | ||
| II | 3 (6.67) | 5 (33.33) | ||
| III | 22 (48.89) | 3 (20.00) | ||
| IV | 20 (44.44) | 0 (0.00) | ||
| miR-489 | 0.64 ± 0.30 | 0.30 ± 0.16 | 4.179 | < 0.001 |
| miR-21 | 6.38 ± 2.35 | 4.18 ± 1.85 | 3.295 | 0.002 |
Multivariate analysis of melanoma metastasis and related factors: Risk factors related to malignant melanoma metastasis were analyzed by multivariate conditional Logistic regression. The results showed that TNM stage, miR-489, and miR-21 were independent risk factors for melanoma metastasis, as shown in Table 8.
| Factor | β | SE | Wald | P | Exp (β) | 95%CI |
| TNM stage | 1.393 | 0.752 | 4.172 | 0.041 | 4.890 | 1.038-19.304 |
| miR-489 | 1.385 | 1.625 | 0.762 | 0.529 | 3.146 | 0.182-34.021 |
| mir-21 | -2.037 | 1.232 | 6.514 | 0.038 | 0.391 | 0.059-1.449 |
Cutaneous melanoma metastasis is an important factor leading to poor efficacy and poor prognosis of melanoma patients[15]. Melanoma is an invasive skin cancer. The metastasis of melanoma is relatively hidden in the early stage. If it is detected in the early stage, it can be cured by surgical resection[16]. Clinical statistics of melanoma show that the 5-year survival rate of patients with early melanoma is 90%. However, the diagnosis of melanoma is often detected by doctors’ visual scanning currently. The diagnosis rate for malignant melanoma with metastasis and diffusion in vivo is relatively low, while the survival rate for this advanced melanoma metastasis is only 15%-20%[17]. Therefore, it is of great clinical significance to find biological indicators closely related to the diagnosis and treatment of melanoma metastasis.
In this study, we first detected the expression difference of miR-489 and miR-21 in serum of melanoma patients and healthy people by qRT-PCR technology. The results showed that the expression of miR-489 in the research group was significantly lower than that in the control group. However, the expression of miR-21 in the research group was significantly higher than that in the control group. MiRNAs can affect the biological function of tumor cells by regulating gene expression, regulating related tumor signal pathways, and thus affecting the development of tumors[18]. MiR-489 is down-regulated in ovarian cancer, gastric cancer, oral endometrial cancer cells, and other tumors. Relevant reports show that up-regulation of miR-489 can inhibit the proliferation and invasion of oral endometrial cancer cells[19,20]; researchers also showed that miR-489 expression decreased in serum of patients with melanoma, bladder cancer, and intestinal cancer. Among them, miR-489 can inhibit the proliferation and migration of melanoma A375 cells[21,22] by targeting PAK5. However, miR-21, as a potential target molecule for melanoma treatment, is abnormally elevated in various tumor tissues including melanoma, and some researchers believe that miR-21 can play a role in inducing melanoma cell apoptosis by inhibiting the expression of apoptosis protein-related protein PDCD4[23,24]. Therefore, we believe that miR-489 is down-regulated in serum of melanoma patients. On the contrary, miR-21 is up-regulated in serum of melanoma patients. Then we started with the clinical data of the patients in the research group to analyze the relationship between the expression levels of miR-489 and miR-21 and the clinicopathological characteristics of melanoma. Based on the analysis results, we speculate that the expression of miR-489 and miR-21 is related to TNM stage and metastasis of melanoma. At present, although there are no specific studies on TNM stage and metastasis of melanoma and miR-489 and miR-21, there are reports on miRNAs and melanoma, which suggest that the expression of miRNA-125b detected by qRT-PCR technology shows a decreasing trend in serum of melanoma patients. Furthermore, the expression of miRNA-125b in patients with different TNM stages and in those with and without lymph node metastasis was further detected through experiments. It was found that the higher the TNM stage, the lower the serum miRNA-125b in melanoma patients with lymph node metastasis, indicating that the expression change of miRNA-125b is related to the presence or absence of melanoma metastasis and clinicopathological stage, which extremely well supports the results of this study[25]. Then, we analyzed the correlation between miR-489 and miR-21 in melanoma of different TNM stages, and found that the relative expression of serum miR-489 decreased with the increase of TNM stage, while the relative expression of serum miR-21 increased continuously. Spearman correlation analysis showed that the relative expression of serum miR-489 was negatively correlated with TNM stage, and the relative expression of serum miR-21 was positively correlated with TNM stage. MiRNAs have been proved to be closely related to tumor stage. The expression levels of miRNAs increase or decrease significantly with tumor stage by inhibiting or promoting tumor development in different tumor types[26]. Finally, we analyzed the diagnostic and predictive value of miR-489 and miR-21 for melanoma metastasis. By drawing ROC curves, we found that in the diagnosis of melanoma patients, the single diagnosis with miR-489 or miR-21 had good sensitivity, specificity, and AUC. CT, ultrasound, MRI, and other imaging techniques are all routine clinical auxiliary examinations for the diagnosis of melanoma. There is a certain degree of misdiagnosis and missed diagnosis for metastatic melanoma in vivo. Combining with a serum tumor marker can better improve the diagnostic efficiency[27,28]. Logistic single- and multiple-factor analyses showed that TNM stage, miR-489, and miR-21 were independent risk factors for melanoma metastasis. However, the diagnostic efficacy and predictive value of miR-489 and miR-21 expression changes in serum of patients with melanoma metastasis have not been studied in the past. In this study, miR-489 and miR-21 exhibited certain predictive value for the diagnosis and prognosis of melanoma metastasis.
This study confirmed the expression of miR-489 and miR-21 in melanoma patients and the predictive value for the disease, but there are still some deficiencies in the study. If there is no more specific analysis of the regulation of miR-489 and miR-21 expression changes on melanoma cells, it is difficult to further explain their biological functions. Moreover, miR-489, miR-21, and clinical routine tumor markers should be analyzed, which have certain influence on the improvement of research design. Therefore, future studies should be performed to resolve these problems.
To sum up, miR-489 is down-regulated in serum of melanoma patients. On the contrary, miR-21 is up-regulated in serum of melanoma patients. MiR-489 and miR-21 may be involved in melanoma occurrence, development, and metastasis, and can be used as potential serum biomarkers for melanoma metastasis diagnosis and disease assessment.
Malignant melanoma is a malignant neoplasm common in the skin or other organs. Patients with malignant melanoma often have metastasis due to the lesion, and the mortality increases significantly with the metastasis of the lesion, which has a serious impact on society and families. Therefore, it is of great significance to analyze the pathogenesis of human melanoma and to search for biological markers related to the diagnosis and treatment of melanoma.
At present, the diagnosis and clinical evaluation of melanoma are influenced by subjective factors to a certain extent. It takes a long time to monitor the metastasis of melanoma. Therefore, this study aimed to provide a new theoretical basis for the diagnosis and treatment of melanoma, and to study the expression characteristics of miR-489 and miR-21 in serum of patients with melanoma and their clinical significance.
To study the value of miR-489 and miR-21 in peripheral blood in the diagnosis and treatment of melanoma, and explore the application of serological analysis in the diagnosis and treatment of melanoma, so as to provide reference for clinical diagnosis and treatment of this malignancy.
Sixty patients with malignant melanoma treated at our hospital from June 2017 to December 2018 were selected as a study group, and 40 healthy people in the same period were selected as a control group. The levels of miR-489 and miR-21 in serum were detected by qRT-PCR. The ROC curves were drawn to evaluate their predictive value and diagnostic efficiency. Spearman test was used for correlation analysis. The risk factors for melanoma metastasis were analyzed by Logistic single- and multiple-factor analyses. The value of miR-489 and miR-21 in the diagnosis and treatment of melanoma was verified.
The expression of miR-489 in the research group was significantly lower than that in the control group, while the expression of miR-21 in the research group was significantly higher than that in the control group. The expression of miR-489 and miR-21 was related to TNM stage and metastasis of melanoma. The relative expression of serum miR-489 decreased with the increase of TNM stage, while the relative expression of serum miR-21 increased. The relative expression of serum miR-489 was negatively correlated with TNM stage, and the relative expression of serum miR-21 was positively correlated with TNM stage. The single diagnosis with mir-489 or miR-21 had good sensitivity, specificity, and AUC in the diagnosis of melanoma patients. Logistic single-factor analysis showed that TNM stage, miR-489, and miR-21 were independent risk factors for melanoma metastasis. MiR-489 and miR-21 had certain predictive value in the diagnosis and prognosis of melanoma metastasis.
MiR-489 and miR-21 have potential value in the diagnosis and treatment of melanoma, and they are expected to become potential indicators for the diagnosis and evaluation of melanoma in the future.
Future studies need to further confirm the timing of blood collection for detection of miR-489 and miR-21 so that they can be better used in clinical practice. What’s more, whether mir-489 and miR-21 can be targets for melanoma metastasis needs further analysis.
| 1. | Kunte C, Letulé V, Gehl J, Dahlstroem K, Curatolo P, Rotunno R, Muir T, Occhini A, Bertino G, Powell B, Saxinger W, Lechner G, Liew SH, Pritchard-Jones R, Rutkowski P, Zdzienicki M, Mowatt D, Sykes AJ, Orlando A, Mitsala G, Rossi CR, Campana L, Brizio M, de Terlizzi F, Quaglino P, Odili J; InspECT (the International Network for Sharing Practices on Electrochemotherapy). Electrochemotherapy in the treatment of metastatic malignant melanoma: a prospective cohort study by InspECT. Br J Dermatol. 2017;176:1475-1485. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 85] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 2. | Vardabasso C, Gaspar-Maia A, Hasson D, Pünzeler S, Valle-Garcia D, Straub T, Keilhauer EC, Strub T, Dong J, Panda T, Chung CY, Yao JL, Singh R, Segura MF, Fontanals-Cirera B, Verma A, Mann M, Hernando E, Hake SB, Bernstein E. Histone Variant H2A.Z.2 Mediates Proliferation and Drug Sensitivity of Malignant Melanoma. Mol Cell. 2015;59:75-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 165] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 3. | Reyes E, Uribe C, de Vries E. Population-based incidence and melanoma-specific survival of cutaneous malignant melanoma in a Colombian population 2000-2009. Int J Dermatol. 2018;57:21-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 4. | Helgadottir H, Rocha Trocoli Drakensjö I, Girnita A. Personalized Medicine in Malignant Melanoma: Towards Patient Tailored Treatment. Front Oncol. 2018;8:202. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 34] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 5. | Ito K, Schöder H, Teng R, Humm JL, Ni A, Wolchok JD, Weber WA. Prognostic value of baseline metabolic tumor volume measured on 18F-fluorodeoxyglucose positron emission tomography/computed tomography in melanoma patients treated with ipilimumab therapy. Eur J Nucl Med Mol Imaging. 2019;46:930-939. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 75] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 6. | Ikeda T, Yamaguchi H, Dotsu Y, Taniguchi H, Gyoutoku H, Senju H, Sakamoto N, Iwanaga S, Kuwatsuka Y, Fukuda M, Mukae H. Diffuse alveolar hemorrhage with pseudoprogression during nivolumab therapy in a patient with malignant melanoma. Thorac Cancer. 2018;9:1522-1524. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 7. | Santos CAD, Souza DLB. Melanoma mortality in Brazil: trends and projections (1998-2032). Cien Saude Colet. 2019;24:1551-1561. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 8. | Chhabra G, Wojdyla L, Frakes M, Schrank Z, Leviskas B, Ivancich M, Vinay P, Ganapathy R, Ramirez BE, Puri N. Mechanism of Action of G-Quadruplex-Forming Oligonucleotide Homologous to the Telomere Overhang in Melanoma. J Invest Dermatol. 2018;138:903-910. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 19] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 9. | Gaudet AD, Fonken LK, Watkins LR, Nelson RJ, Popovich PG. MicroRNAs: Roles in Regulating Neuroinflammation. Neuroscientist. 2018;24:221-245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 214] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 10. | Motti ML, D Angelo S, Meccariello R. MicroRNAs, Cancer and Diet: Facts and New Exciting Perspectives. Curr Mol Pharmacol. 2018;11:90-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 24] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 11. | Huber V, Vallacchi V, Fleming V, Hu X, Cova A, Dugo M, Shahaj E, Sulsenti R, Vergani E, Filipazzi P, De Laurentiis A, Lalli L, Di Guardo L, Patuzzo R, Vergani B, Casiraghi E, Cossa M, Gualeni A, Bollati V, Arienti F, De Braud F, Mariani L, Villa A, Altevogt P, Umansky V, Rodolfo M, Rivoltini L. Tumor-derived microRNAs induce myeloid suppressor cells and predict immunotherapy resistance in melanoma. J Clin Invest. 2018;128:5505-5516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 198] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 12. | Li Y, Ma X, Wang Y, Li G. miR-489 inhibits proliferation, cell cycle progression and induces apoptosis of glioma cells via targeting SPIN1-mediated PI3K/AKT pathway. Biomed Pharmacother. 2017;93:435-443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 51] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 13. | Martin del Campo SE, Latchana N, Levine KM, Grignol VP, Fairchild ET, Jaime-Ramirez AC, Dao TV, Karpa VI, Carson M, Ganju A, Chan AN, Carson WE. MiR-21 enhances melanoma invasiveness via inhibition of tissue inhibitor of metalloproteinases 3 expression: in vivo effects of MiR-21 inhibitor. PLoS One. 2015;10:e0115919. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 81] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 14. | Yang T, Peng S, Hu P, Huang L. Pigmented skin lesion segmentation based on random forest and full convolutional neural networks. International Society for Optics and Photonics. 2018;10820:108203M. [DOI] [Full Text] |
| 15. | Jiang H, Gebhardt C, Umansky L, Beckhove P, Schulze TJ, Utikal J, Umansky V. Elevated chronic inflammatory factors and myeloid-derived suppressor cells indicate poor prognosis in advanced melanoma patients. Int J Cancer. 2015;136:2352-2360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 150] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 16. | Valentín-Nogueras SM, Brodland DG, Zitelli JA, González-Sepúlveda L, Nazario CM. Mohs Micrographic Surgery Using MART-1 Immunostain in the Treatment of Invasive Melanoma and Melanoma In Situ. Dermatol Surg. 2016;42:733-744. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 94] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 17. | Wu Y, Wang L, Ma X, Guo W, Ren G. The existence of early stage oral mucosal melanoma: A 10-year retrospective analysis of 170 patients in a single institute. Oral Oncol. 2018;87:70-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 18. | Xu P, Wang J, Sun B, Xiao Z. Integrated analysis of miRNA and mRNA expression data identifies multiple miRNAs regulatory networks for the tumorigenesis of colorectal cancer. Gene. 2018;659:44-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 22] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 19. | Chen Z, Yu T, Cabay RJ, Jin Y, Mahjabeen I, Luan X, Huang L, Dai Y, Zhou X. miR-486-3p, miR-139-5p, and miR-21 as Biomarkers for the Detection of Oral Tongue Squamous Cell Carcinoma. Biomark Cancer. 2017;9:1-8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 21] [Reference Citation Analysis (0)] |
| 20. | Piletič K, Kunej T. MicroRNA epigenetic signatures in human disease. Arch Toxicol. 2016;90:2405-2419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 200] [Cited by in RCA: 223] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 21. | Hidaka H, Seki N, Yoshino H, Yamasaki T, Yamada Y, Nohata N, Fuse M, Nakagawa M, Enokida H. Tumor suppressive microRNA-1285 regulates novel molecular targets: aberrant expression and functional significance in renal cell carcinoma. Oncotarget. 2012;3:44-57. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 137] [Cited by in RCA: 162] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 22. | Mou K, Liu B, Ding M, Mu X, Han D, Zhou Y, Wang LJ. lncRNA-ATB functions as a competing endogenous RNA to promote YAP1 by sponging miR-590-5p in malignant melanoma. Int J Oncol. 2018;53:1094-1104. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 27] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 23. | Yan F, Wang C, Li T, Cai W, Sun J. Role of miR-21 in the growth and metastasis of human salivary adenoid cystic carcinoma. Mol Med Rep. 2018;17:4237-4244. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 24. | Liu KH, Lee WR, Hsieh YJ, Chou CL, Jiang MC, Shen SC. Differential expression of PTEN and PDCD4 Tumor suppressors in melanoma and microRNA-21-positive melanoma cells and squamous carcinoma cells. Dermatol Sin. 2019;37:19-27. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 25. | Latchana N, Ganju A, Howard JH, Carson WE. MicroRNA dysregulation in melanoma. Surg Oncol. 2016;25:184-189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 45] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 26. | Van Laar R, Lincoln M, Van Laar B. Development and validation of a plasma-based melanoma biomarker suitable for clinical use. Br J Cancer. 2018;118:857-866. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 48] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 27. | Jacobsen BH, Ricks C, Harrie RP. Ocular ultrasound versus MRI in the detection of extrascleral extension in a patient with choroidal melanoma. BMC Ophthalmol. 2018;18:320. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 28. | Song G, Xiong GY, Fan Y, Huang C, Kang YM, Ji GJ, Chen JC, Xin ZC, Zhou LQ. The role of tumor size, ultrasonographic findings, and serum tumor markers in predicting the likelihood of malignant testicular histology. Asian J Androl. 2019;21:196-200. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See:
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Fujiwara T, Mohamed SY S-Editor: Gong ZM L-Editor: Wang TQ E-Editor: Li X