Published online Sep 6, 2019. doi: 10.12998/wjcc.v7.i17.2526
Peer-review started: February 26, 2019
First decision: June 19, 2019
Revised: July 10, 2019
Accepted: July 20, 2019
Article in press: July 20, 2019
Published online: September 6, 2019
Processing time: 194 Days and 10.8 Hours
Postoperative pancreatic leakage readily results in intractable pancreatic fistula and subsequent intraperitoneal abscess. This refractory complication can be fatal; therefore, intensive treatment is important. Continuous local lavage (CLL) has recently been reevaluated as effective treatment for severe infected pancreatitis, and we report three patients with postoperative intractable pancreatic fistula successfully treated by CLL. We also discuss our institutional protocol for CLL for postoperative pancreatic fistula.
The first patient underwent subtotal stomach-preserving pancreaticoduodenectomy, and pancreatic leakage was observed postoperatively. Intractable pancreatic fistula led to intraperitoneal abscess, and CLL near the pancreaticojejunostomy site was instituted from postoperative day (POD) 8. The abscess resolved after 7 d of CLL. The second patient underwent distal pancreatectomy. Pancreatic leakage was observed, and intractable pancreatic fistula led to intraperitoneal abscess near the pancreatic stump. CLL was instituted from POD 9, and the abscess resolved after 4 d of CLL. The third patient underwent aneurysmectomy and splenectomy with wide exposure of the pancreatic parenchyma. Endoscopic retrograde pancreatic drainage was performed on POD 15 to treat pancreatic fistula; however, intraperitoneal abscess was detected on POD 59. We performed CLL endoscopically via the transgastric route because the percutaneous approach was difficult. CLL was instituted from POD 63, and the abscess resolved after 1 wk of CLL.
CLL has therapeutic potential for postoperative pancreatic fistula.
Core tip: Pancreatic fistula after pancreatic surgery is a potentially fatal refractory complication. We describe the typical findings based on three patients who survived, and two patients who died of fatal complications. We also discuss the importance of intensive treatment for postoperative pancreatic fistula, with a literature review. Continuous local lavage (CLL) has been reevaluated as an effective treatment for severe infected pancreatitis, and our findings show that CLL shortened the therapeutic duration for postoperative pancreatic fistula. We suggest that CLL has therapeutic potential for postoperative pancreatic fistula. We also introduce our institutional protocol for CLL for postoperative pancreatic fistula.
- Citation: Hori T, Ogawa K, Yamamoto H, Harada H, Matsumura K, Yamamoto M, Yamada M, Yazawa T, Kuriyama K, Tani M, Yasukawa D, Kamada Y, Aisu Y, Tani R, Aoyama R, Nakayama S, Sasaki Y, Nishimoto K, Zaima M. Impact of continuous local lavage on pancreatic juice-related postoperative complications: Three case reports. World J Clin Cases 2019; 7(17): 2526-2535
- URL: https://www.wjgnet.com/2307-8960/full/v7/i17/2526.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v7.i17.2526
Postoperative complication related to pancreatic juice leakage is a major clinical problem[1]. Pancreatic surgeries are inherently accompanied by postoperative pancreatic leakage[1,2], which readily results in intractable pancreatic fistula and subsequent intraperitoneal abscess[1,2]. This refractory complication may be fatal by inducing sepsis secondary to leakage from the digestive tract and shock hemodynamics secondary to sudden rupture of a pseudoaneurysm[3,4].
In pancreatitis therapy, minimally-invasive drainage (e.g., percutaneous and transluminal approaches) is currently considered useful, especially for necrotizing and infected pancreatitis[5-7]. Recently, continuous local lavage (CLL) has been reevaluated as effective treatment for severe infected pancreatitis[8-11].
We present findings for three thought-provoking cases of postoperative intractable pancreatic fistula with bacterial infection that were successfully treated by CLL. We also discuss the typical findings of potentially fatal complications associated with intractable postoperative pancreatic fistula based on our patients' data, and we discuss the importance of intensive treatment for postoperative pancreatic fistula, with a literature review. We suggest that CLL has therapeutic potential for postoperative pancreatic juice-related complications and discuss our institutional protocol for CLL for postoperative pancreatic fistula.
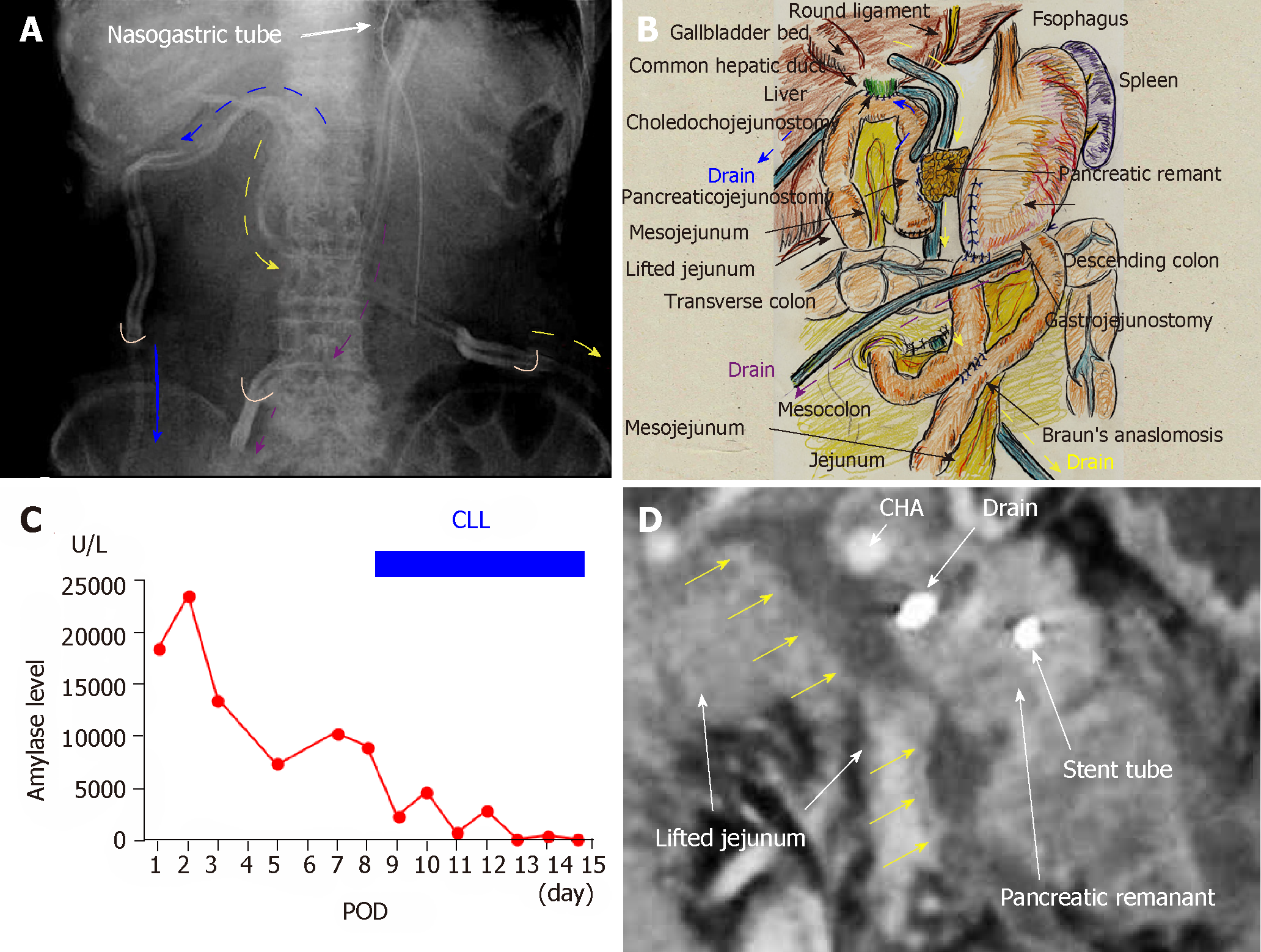
A 66-year-old man suffered from cholangitis. Contrast computed tomography (CT) revealed a hypovascular tumor in the pancreatic head and biliary dilatation. His locally-advanced pancreatic cancer was categorized as stage IIB in the tumor-node-metastasis classification[12]. Obstructive jaundice was successfully treated by endoscopic nasobiliary drainage. Thereafter, he underwent subtotal stomach-preserving pancreaticoduodenectomy with extended lymph node and nerve plexuses dissection. Operative time was 433 min, and blood loss was 950 mL. Reconstructions were performed by modified Child’s method with Braun’s anastomosis, and pancreaticojejunostomy was performed using modified Blumgart’s technique. We placed an intraductal stent (pancreatic duct tube, 7 Fr, burled; Sumitomo Bakelite Co., Ltd., Tokyo, Japan) and performed duct-to-jejunal anastomosis with 12 interrupted sutures (polydioxanone: 6-0 PDS II, violet; Ethicon, Inc., Cincinnati, OH, United States). Intentional approximation of the pancreatic stump and jejunal wall was performed using four interrupted sutures (polypropylene: 3-0 Prolene; Ethicon, Inc.). We closed all mesenteric gaps and placed three drains near the anastomoses of the pancreaticojejunostomy, choledochojejunostomy, and gastrojejunostomy sites (Figures 1 A and B). For the pancreaticojejunostomy, we ran two drains intentionally through the ventral and dorsal sides.
Pancreatic leakage was observed early postoperatively, and the peak amylase level in the drainage discharge was 23480 U/L (Figure 1C). Although drainage discharge gradually decreased to 1 wk postoperatively, high amylase levels in the drainage discharge (8980 U/L) were still observed on postoperative day (POD) 8 (Figure 1C). Contrast CT on POD 8 revealed that intractable pancreatic fistula had resulted in an intraperitoneal abscess around the pancreaticojejunostomy (Figure 1D). Fistulography via both drains revealed an abscess cavity around the pancreaticojejunostomy. Artificial fistulas along these drains had matured as accessible pathways for drain replacement, and the abscess cavity could be lavaged by injecting saline through one drain. We replaced both drains with red vulcanized rubber tubes. Fluoroscopic examination confirmed that the recovery rate of injected contrast dye was approximately 85%, and that uncollectible dye never spread, even under high-pressure administration.
Via the ventral tube to the pancreaticojejunostomy, we irrigated 1000 mL of saline per day slowly but continuously into the abscess cavity beginning on POD 8 (Figure 2). We used the dorsal tube to the pancreaticojejunostomy only to drain irrigated saline (Figure 2). Saline volume was increased on POD 12 to 1500 mL/d because the amylase level in the drainage discharge was relatively high (2880 U/L). Thereafter, amylase level in the drainage discharge decreased dramatically (Figure 1C). Fistulography on POD 15 revealed that the abscess had resolved after 7 days of CLL. The irrigation tube was removed on POD 15 because the amylase level in the discharge was 120 U/L (Figure 1C); we removed the drainage tube on POD 17. The patient's postoperative course was categorized as Clavien–Dindo grade II[13]. He was discharged on POD 22, and no recurrence was observed as of 4.3 years after surgery.
A 45-year-old man suffered refractory pancreatitis, although he had no history of alcohol abuse, and autoimmune pancreatitis was excluded. He began suffering repeat episodes of acute pancreatitis from 21 years of age, and underwent pseudocystojejunostomy at 35 years of age. Metabolic disorders (e.g., hyperlipidemia and gout) were present, but diabetes was not observed. Pancreatic stones and peripancreatic edema were seen at 42 years of age. Although stones were previously removed endoscopically, recurrent stones and peripancreatic abscess appeared in the pancreatic body and tail at 45 years of age. Collateral vessels were well-developed secondary to venous-flow occlusion related to the chronic pancreatitis, and splenomegaly was also confirmed. Therefore, we performed distal pancreatectomy and removal of the pseudocystojejunostomy. Operative time was 272 min. Blood loss was 429 mL, and transfusion of 280 mL packed red blood cells was required. Pancreatectomy was performed using a linear stapler (Endo GIA black cartridge, Reinforced Reload with Tri-Staple technology; Medtronic, Dublin, Ireland), and we placed a drain near the staple line (Figure 3A). To prevent drain dislocation, we fixed the drain to the surrounding tissue using absorbable suture (3-0 Coated Vicryl Rapide; Ethicon, Inc.).
Pancreatic leakage occurred early postoperatively, and the peak amylase level in the drain discharge was 18846 U/L. Although drainage decreased gradually over 8 d, high amylase levels in the drain discharge (14469 U/L) were still present on POD 9. Contrast CT on POD 9 revealed that intractable pancreatic fistula led to intraperitoneal abscess near the staple line (Figure 3B). Fistulography via the drain on POD 9 identified the pancreatic fistula and abscess cavity (Figure 3C). The abscess cavity could be lavaged, and the recovery rate of injected contrast dye was approximately 90%; uncollectible dye never spread. Artificial fistulas along the drain had matured as accessible pathways for drain replacement, and we replaced the original drain with a two-way tube (Salem Sump, 14 Fr: Medtronic). To effectively lavage the pancreatic fistula and abscess cavity, the tip position of this two-way tube was appropriately adjusted during fluoroscopic examination (Figure 4).
From POD 9, saline irrigation was continuously injected via the two-way tube that followed the drainage route. A total of 1000 mL of saline per day was slowly irrigated into the abscess cavity, and amylase levels in the drainage discharge decreased immediately (767 U/L). Fistulography on POD 12 revealed that the abscess had resolved after 4 d of CLL (Figure 3D), and we removed the two-way tube on POD 13. The patient's postoperative course was categorized as Clavien–Dindo grade II[13]. He was discharged on POD 18, and 3 months after surgery, no metabolic disorders were identified.
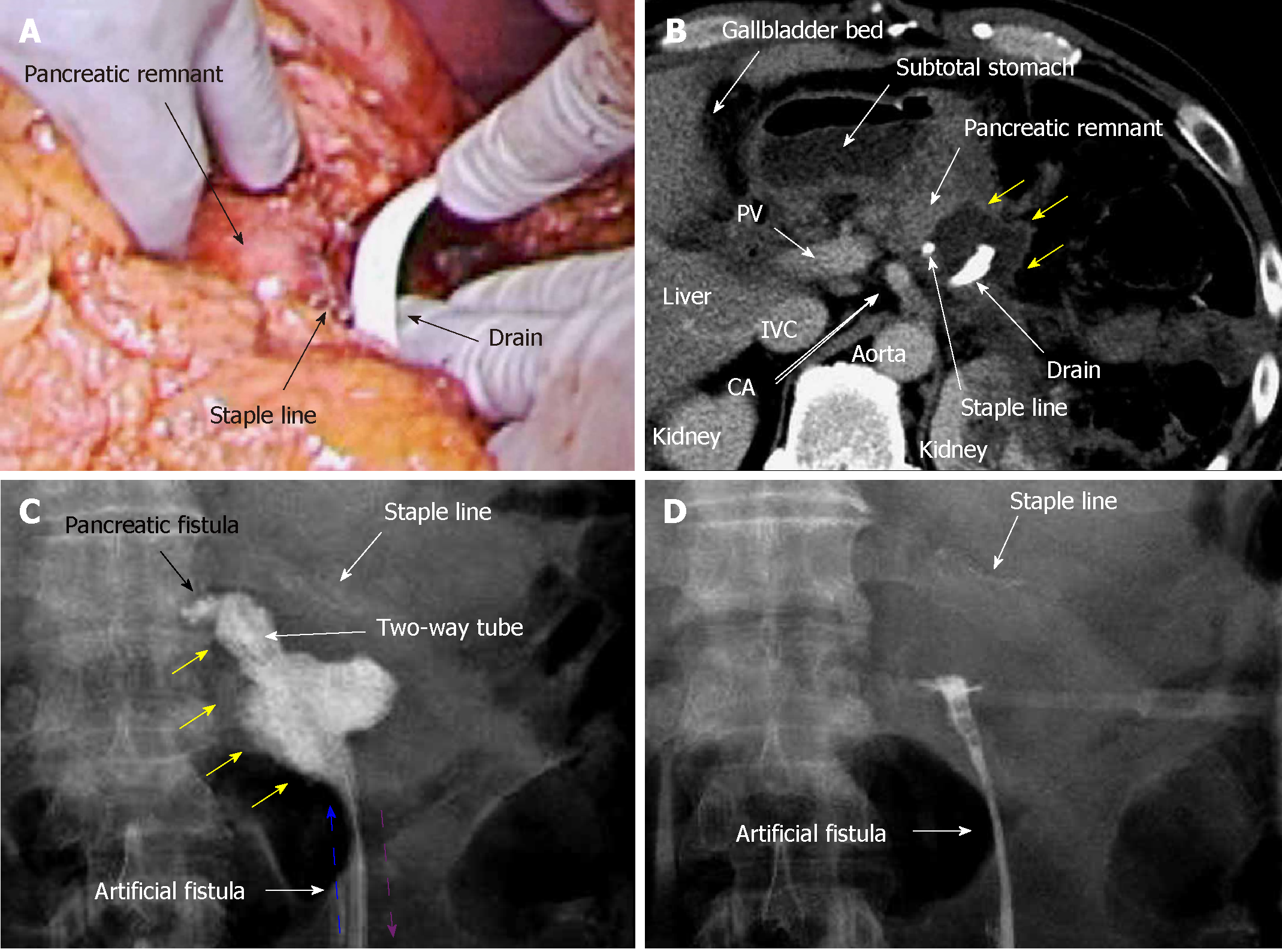
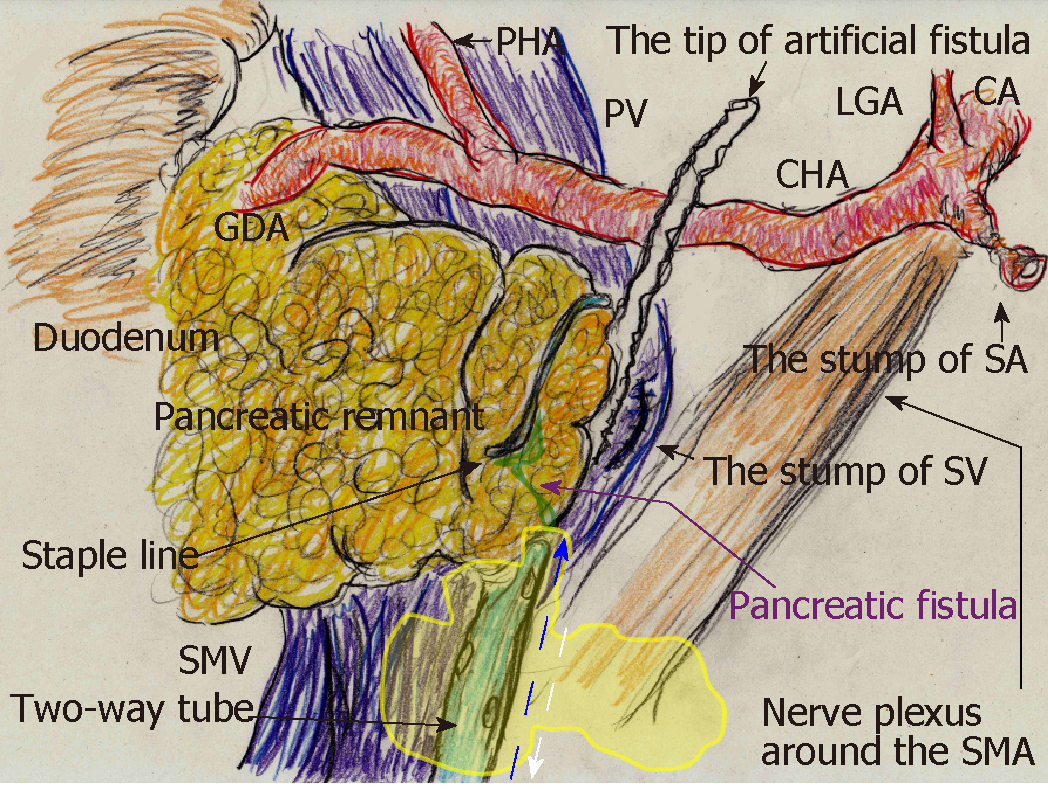
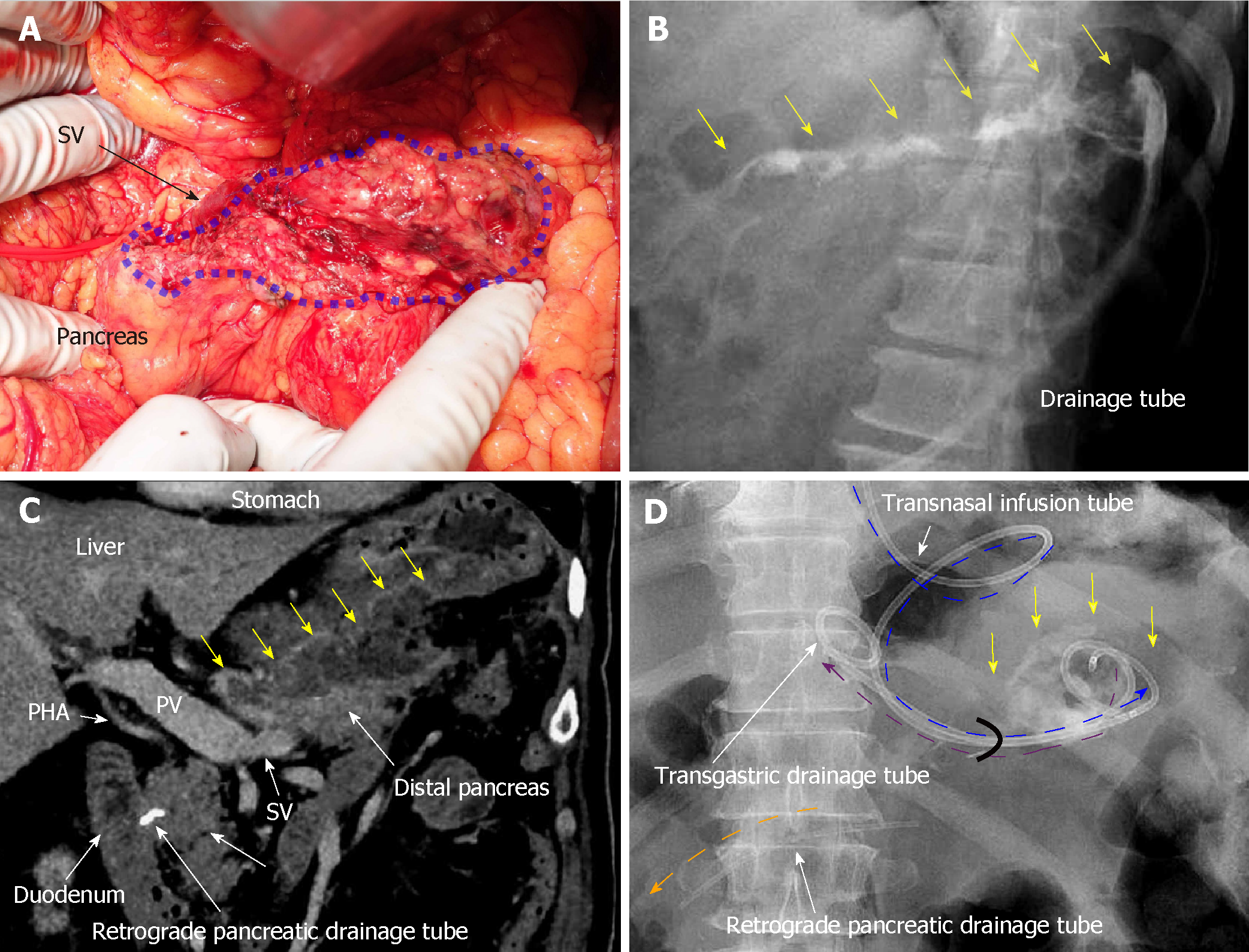
A 70-year-old man was being followed for myasthenia gravis and diabetes. Periodic CT incidentally detected a splenic arterial aneurysm that had enlarged over time. The true aneurysm extended almost the full length of the splenic artery, arising from the root to the splenic hilum. Interventional radiology was excluded from the therapeutic options because of risks associated with arterial recanalization, ischemic complications, and cost effectiveness. Therefore, we performed elective surgery with aneurysmectomy and splenectomy. The splenic artery was cut at its root without disturbing celiac arterial flow. After dissecting the pancreatic parenchyma to detect the intact portion of the dorsal pancreatic artery branching from the aneurysm, this artery was also cut. Because dense pancreatic capsule was adherent to the calcified aneurysmal wall, the aneurysm required dissection from the pancreatic parenchyma. The pancreatic parenchyma was preserved with its drainage vein, although the pancreatic parenchyma was widely exposed secondary to the aneurysmectomy (Figure 5A). Operative time was 250 min, and blood loss was 1907 ml.
The patient subsequently developed postoperative intractable pancreatic fistula, and refractory symptoms affected his postoperative course. Peak amylase level in the drainage discharge was 32197 U/L. Although intraperitoneal drainage continued, optimal tube drainage (i.e., intentional placement along the distal pancreas) was not an option because a percutaneous approach was too difficult. Fistulography via the intraperitoneal drain on POD 13 revealed intractable pancreatic fistula in the distal pancreas (Figure 5B); therefore, we performed endoscopic retrograde pancreatic drainage on POD 15. Intraperitoneal drainage with retrograde pancreatic drainage was continued for 36 days with intermittent antibiotics until POD 40. However, intractable pancreatic fistula resulted in an abscess and localized peritonitis secondary to bacterial infection. Contrast CT on POD 59 detected an intraperitoneal abscess in the distal pancreas (Figure 5C).
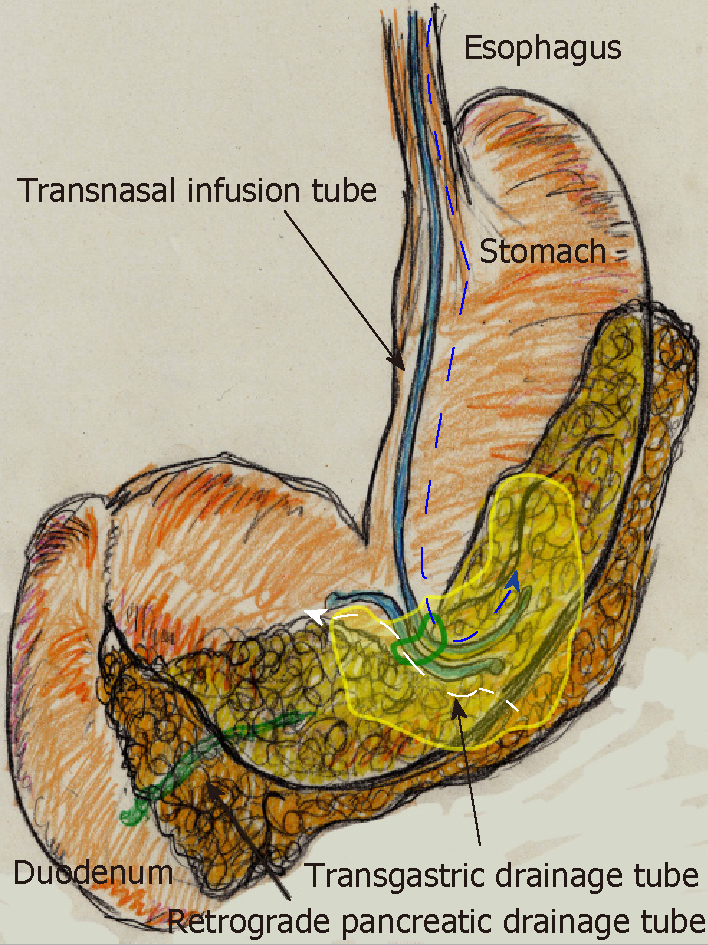
On POD 63, we initiated CLL of the abscess cavity via the transgastric route. The abscess cavity was punctured endoscopically under ultrasound guidance, and a transgastric path into the abscess cavity was made. Transnasal infusion was initiated, and transgastric drainage tubes were placed endoscopically (Figure 5D). CLL was instituted via transnasal continuous infusion and endoscopic transgastric drainage (Figure 6). The abscess cavity could be effectively lavaged, and the recovery rate of injected contrast dye was approximately 70% because of the concurrent transgastric drainage tube.
From POD 63, saline irrigation was continuously injected via the transnasal infusion tube. A total of 1000 ml of saline per day was slowly irrigated into the abscess cavity, and amylase levels in the drainage discharge decreased immediately (139 U/L). Fistulography on POD 70 revealed that the abscess resolved after 1 wk of CLL, and the refractory pancreatic fistula was no longer identifiable. Therefore, we removed the transnasal infusion and retrograde pancreatic drainage tubes. The patient was discharged on POD 72 with only the transgastric drainage tube remaining in place. His postoperative course was categorized as Clavien–Dindo grade IIIa[13], and he was in good health 9 moafter surgery.
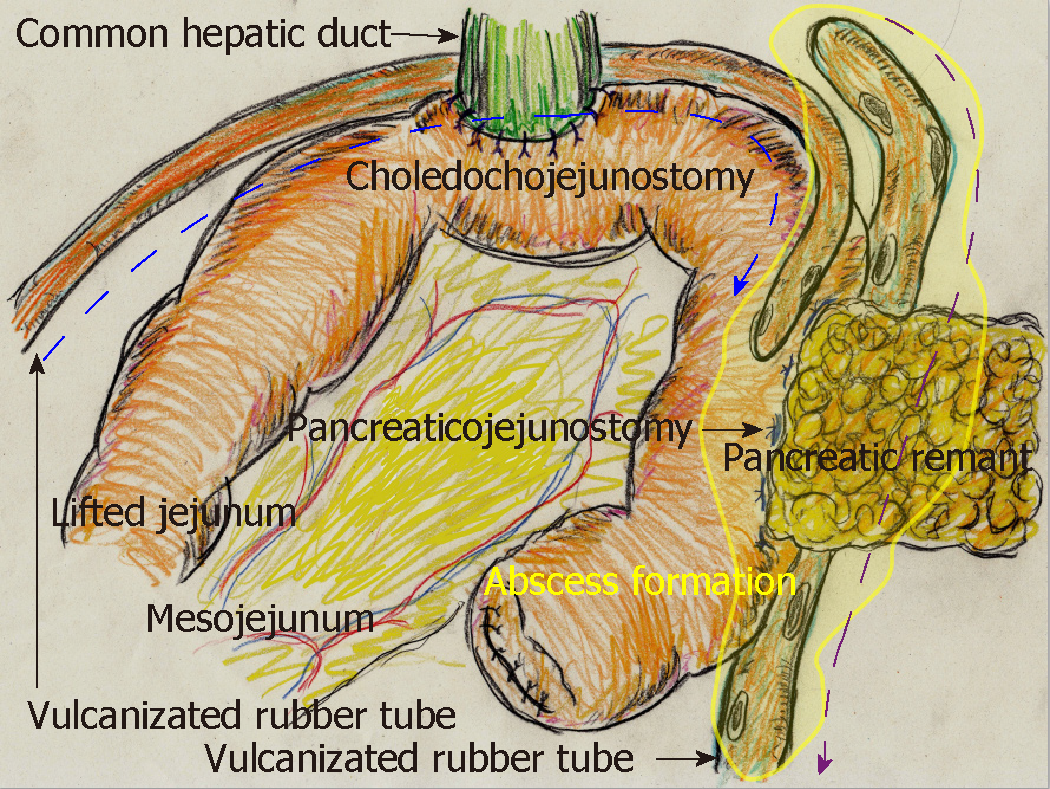
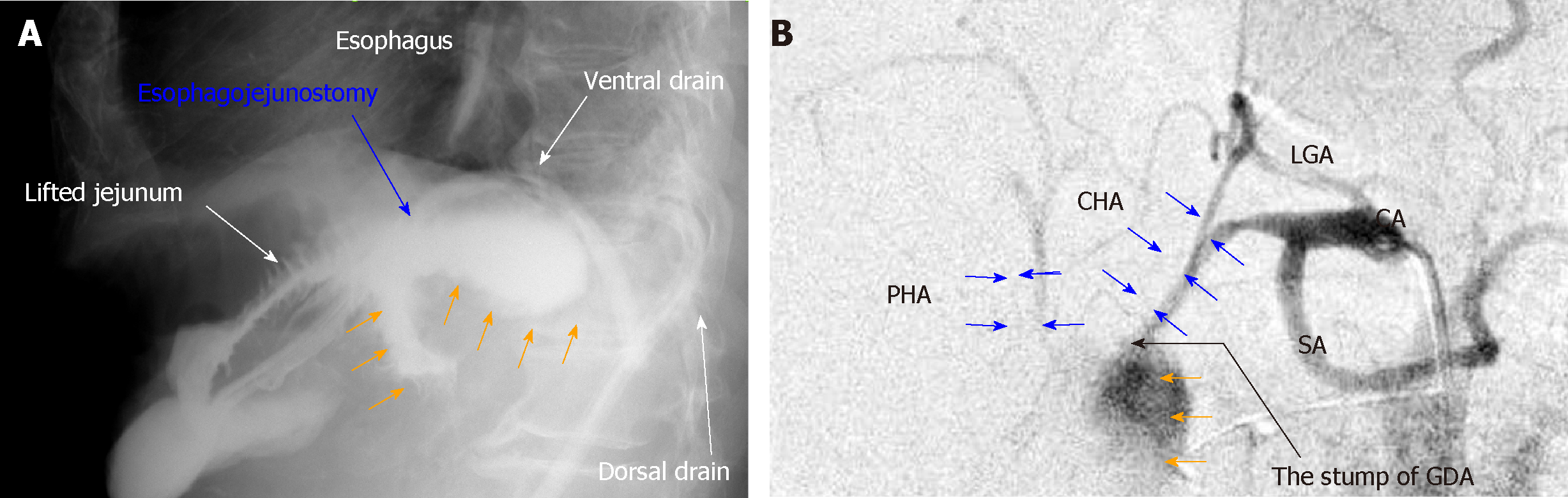
Pancreatic fistula is one of the most common complications after pancreatic surgery[1,2], and diagnostic criteria and nosological classification have been established[14]. Although pancreatic diseases may cause associated pancreatitis, pancreatic parenchyma with an intermediate or normal consistency produces more pancreatic juice and has a higher rate of pancreatic leakage[15]. Any pancreatic surgery may lead to intractable pancreatic fistula unless the pancreatic parenchyma has been destroyed secondary to the primary disease[15]. Unfortunately, pancreatic stimulation and bacterial infections trigger a vicious cycle[1,16]. Activated pancreatic juice and accelerated bacterial infection destroy digestive anastomosis sites and create arterial pseudoaneurysm; anastomotic leakage and arterial rupture can be fatal[3,4]. Based on our patient experience, Figure 7 shows the typical findings of anastomotic leakage and arterial rupture associated with intractable pancreatic fistula. These potentially fatal complications are grave concerns for hepatobiliary-pancreatic surgeons; therefore, many focus on reconstructive techniques to reduce the development of refractory pancreatic fistula[2].
Pancreatic fistula is sometimes inevitable complication after pancreatic surgery. Soft pancreatic parenchyma with normal consistency produces more pancreatic juice[15], even though pancreatic diseases may cause associated pancreatitis. In cases with a higher risk of pancreatic leakage, intraoperative placement of two-way tube or irrigation drainage tube may be a good solution.
Minimally-invasive drainage is a standard therapeutic option for necrotizing and infected pancreatitis [5-7], although, historically, only a surgical approach was recommended[17-21]. Recently, CLL has been reevaluated as a therapeutic option for severe infected pancreatitis[8-11], and in our three patients, intractable pancreatic fistulas with bacterial infection gradually resolved after CLL. However, CLL is currently being reevaluated as an effective treatment specifically for severe infected pancreatitis[8-11]; therefore, we suggest that CLL may have therapeutic potential for postoperative pancreatic juice-related complications.
Our institutional protocol for CLL for postoperative pancreatic fistula is summarized in the Table 1. Key points and pitfalls for successful CLL are as follows: (1) Maturity of the artificial fistula(s) is important. An accessible pathway is required for drain replacement; therefore, tube replacements for CLL should be delayed until sufficient maturity of the artificial fistulas has developed along the intraperitoneal drains. Red vulcanized rubber tubes are advantageous for creating artificial fistulas. Also, injected saline should never spread outward from a mature artificial fistula because extension of the abscess cavity secondary to CLL should be avoided. Therefore, we recommend high-pressure administration of contrast dye during fistulography; (2) The optimal setting is crucial for effective CLL. The abscess cavity and pancreatic fistula should be effectively lavaged, and therefore, appropriate tube placement is required. A recovery rate of injected fluid > 80% should be guaranteed; and (3) Toxic amylase levels should decrease dramatically after CLL. Amylase levels in the drainage discharge are extremely important to evaluate pancreatic leakage after surgery[22]. Amylase levels in the draining discharge within the lower triple-digits (i.e., approximately 100–500 U/L) are ideal after CLL induction. CLL generally begins with 1000 ml of saline per day. Tubes may be dislocated during CLL, and dysfunction of the sphincter of Oddi worsens pancreatic leakage[23]. CLL should be reconsidered if a saline volume > 1500 mL per day is required to decrease amylase levels in the drainage discharge; positional change in the tube location or endoscopic pancreatic drainage may be required.
| Maturity of the artificial fistula for CLL induction |
| Wait until the fistulas along the intraperitoneal drains mature because an accessible pathway for drain replacement is needed |
| Injected saline should never spread outward from the mature fistula |
| No extension of contrast dye should be confirmed during fistulography |
| Optimal setting for effective CLL |
| The abscess cavity and pancreatic fistula should be effectively lavaged |
| Appropriate tube placement is required |
| A recovery rate of injected fluid of > 80% should be confirmed |
| Decreased toxicity after CLL |
| Amylase level in the drainage discharge within the lower triple-digits is ideal |
| CLL should be reconsidered if ≥ 1500 mL/d saline is insufficient to decrease amylase levels in the drainage discharge |
Waiting until the fistulas along the intraperitoneal drains mature is important for CLL induction (Table 1). Injected contrast dye should never spread outward from the mature fistula during fistulography. We have an impression that approximately one week after surgery is required for maturity of the artificial fistula.
In our patients, CLL shortened the therapeutic duration of postoperative pancreatic fistula and subsequently prevented fatal complications related to pancreatic juice leakage. CLL may be a powerful tool to overcome pancreatic juice-related complications with bacterial infection following pancreatic surgery.
Intensive treatment for postoperative pancreatic fistula is important to avoid fatal outcomes. CLL has therapeutic potential for postoperative pancreatic fistula. We hope that our thought-provoking cases will be informative for physicians working with patients with pancreatic disorders.
| 1. | Vin Y, Sima CS, Getrajdman GI, Brown KT, Covey A, Brennan MF, Allen PJ. Management and outcomes of postpancreatectomy fistula, leak, and abscess: results of 908 patients resected at a single institution between 2000 and 2005. J Am Coll Surg. 2008;207:490-498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 152] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 2. | Hirono S, Kawai M, Okada KI, Miyazawa M, Kitahata Y, Hayami S, Ueno M, Yamaue H. Modified Blumgart Mattress Suture Versus Conventional Interrupted Suture in Pancreaticojejunostomy During Pancreaticoduodenectomy: Randomized Controlled Trial. Ann Surg. 2019;269:243-251. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 100] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 3. | Roulin D, Cerantola Y, Demartines N, Schäfer M. Systematic review of delayed postoperative hemorrhage after pancreatic resection. J Gastrointest Surg. 2011;15:1055-1062. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 122] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 4. | Zhong X, Wang X, Pan J, Zhu H, Gu L, Shi Z. Mesh-reinforced pancreaticojejunostomy versus conventional pancreaticojejunostomy after pancreaticoduodenectomy: a retrospective study of 126 patients. World J Surg Oncol. 2018;16:68. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 5. | Bang JY, Arnoletti JP, Holt BA, Sutton B, Hasan MK, Navaneethan U, Feranec N, Wilcox CM, Tharian B, Hawes RH, Varadarajulu S. An Endoscopic Transluminal Approach, Compared With Minimally Invasive Surgery, Reduces Complications and Costs for Patients With Necrotizing Pancreatitis. Gastroenterology. 2019;156:1027-1040.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 255] [Cited by in RCA: 239] [Article Influence: 34.1] [Reference Citation Analysis (1)] |
| 6. | Zhang ZH, Ding YX, Wu YD, Gao CC, Li F. A meta-analysis and systematic review of percutaneous catheter drainage in treating infected pancreatitis necrosis. Medicine (Baltimore). 2018;97:e12999. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 7. | Darrivere L, Lapidus N, Colignon N, Chafai N, Chaput U, Verdonk F, Paye F, Lescot T. Minimally invasive drainage in critically ill patients with severe necrotizing pancreatitis is associated with better outcomes: an observational study. Crit Care. 2018;22:321. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 8. | Liu P, Song J, Ke HJ, Lv NH, Zhu Y, Zeng H, Zhu Y, Xia L, He WH, Li J, Huang X, Lei YP. Double-catheter lavage combined with percutaneous flexible endoscopic debridement for infected pancreatic necrosis failed to percutaneous catheter drainage. BMC Gastroenterol. 2017;17:155. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 22] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 9. | González-López J, Macías-García F, Lariño-Noia J, Domínguez-Muñoz JE. Theoretical approach to local infusion of antibiotics for infected pancreatic necrosis. Pancreatology. 2016;16:719-725. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 10. | Hackert T, Büchler MW. Decision Making in Necrotizing Pancreatitis. Dig Dis. 2016;34:517-524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 11. | Matsumoto K, Miyake Y, Nakatsu M, Toyokawa T, Ando M, Hirohata M, Kato H, Yamamoto K. Usefulness of early-phase peritoneal lavage for treating severe acute pancreatitis. Intern Med. 2014;53:1-6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 12. | Union for International Cancer Control. TNM classification of malignant tumors, 8th edition. New York: Wiley Blackwell; 2017; . |
| 13. | Clavien PA, Sanabria JR, Strasberg SM. Proposed classification of complications of surgery with examples of utility in cholecystectomy. Surgery. 1992;111:518-526. [PubMed] |
| 14. | Bassi C, Dervenis C, Butturini G, Fingerhut A, Yeo C, Izbicki J, Neoptolemos J, Sarr M, Traverso W, Buchler M; International Study Group on Pancreatic Fistula Definition. Postoperative pancreatic fistula: an international study group (ISGPF) definition. Surgery. 2005;138:8-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3282] [Cited by in RCA: 3553] [Article Influence: 169.2] [Reference Citation Analysis (35)] |
| 15. | Hamanaka Y, Nishihara K, Hamasaki T, Kawabata A, Yamamoto S, Tsurumi M, Ueno T, Suzuki T. Pancreatic juice output after pancreatoduodenectomy in relation to pancreatic consistency, duct size, and leakage. Surgery. 1996;119:281-287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 103] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 16. | Yamashita K, Sasaki T, Itoh R, Kato D, Hatano N, Soejima T, Ishii K, Takenawa T, Hiromatsu K, Yamashita Y. Pancreatic fistulae secondary to trypsinogen activation by Pseudomonas aeruginosa infection after pancreatoduodenectomy. J Hepatobiliary Pancreat Sci. 2015;22:454-462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 33] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 17. | Yokoe M, Takada T, Mayumi T, Yoshida M, Isaji S, Wada K, Itoi T, Sata N, Gabata T, Igarashi H, Kataoka K, Hirota M, Kadoya M, Kitamura N, Kimura Y, Kiriyama S, Shirai K, Hattori T, Takeda K, Takeyama Y, Hirota M, Sekimoto M, Shikata S, Arata S, Hirata K. Japanese guidelines for the management of acute pancreatitis: Japanese Guidelines 2015. J Hepatobiliary Pancreat Sci. 2015;22:405-432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 327] [Cited by in RCA: 296] [Article Influence: 26.9] [Reference Citation Analysis (0)] |
| 18. | Sakorafas GH, Sampanis D, Lappas C, Kokoropoulos P, Mastoraki A, Smyrniotis V. Necrotizing acute pancreatitis current status - emerging new strategies in surgical management. Infect Disord Drug Targets. 2012;12:138-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 19. | Hwang TL, Chiu CT, Chen HM, Chen SC, Jeng LB, Jan YY, Wang CS, Chen MF. Surgical results for severe acute pancreatitis--comparison of the different surgical procedures. Hepatogastroenterology. 1995;42:1026-1029. [PubMed] |
| 20. | Werner J. [Fulminant pancreatitis--surgical point of view]. Praxis (Bern 1994). 2006;95:1887-1892. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 21. | Driedger M, Zyromski NJ, Visser BC, Jester A, Sutherland FR, Nakeeb A, Dixon E, Dua MM, House MG, Worhunsky DJ, Munene G, Ball CG. Surgical Transgastric Necrosectomy for Necrotizing Pancreatitis: A Single-stage Procedure for Walled-off Pancreatic Necrosis. Ann Surg. 2018;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 45] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 22. | Serene TEL, G SV, Padmakumar JS, Terence HCW, Keem LJ, Bei W, Winston WWL. Predictive value of post-operative drain amylase levels for post-operative pancreatic fistula. Ann Hepatobiliary Pancreat Surg. 2018;22:397-404. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 23. | Hyun JJ, Kozarek RA. Sphincter of Oddi dysfunction: sphincter of Oddi dysfunction or discordance? What is the state of the art in 2018? Curr Opin Gastroenterol. 2018;34:282-287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See:
Manuscript source: Invited Manuscript
Specialty type: Medicine, research and experimental
Country of origin: Japan
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ochiai T S-Editor: Cui LJ L-Editor: A E-Editor: Zhou BX