Published online Jun 26, 2019. doi: 10.12998/wjcc.v7.i12.1410
Peer-review started: March 8, 2019
First decision: April 18, 2019
Revised: April 28, 2019
Accepted: May 11, 2019
Article in press: May 11, 2019
Published online: June 26, 2019
Processing time: 110 Days and 18 Hours
Transfemoral intrahepatic portosystemic shunt (TFIPS) can be performed to treat portal hypertension. However, few studies have evaluated the safety and efficacy of this technique.
To retrospectively evaluate the safety and clinical outcomes of TFIPS and compare them with those of typical transjugular intrahepatic portosystemic shunt (TIPS).
This retrospective study was approved by our hospital ethics committee. From November 2012 to November 2015, 19 patients who underwent successful TFIPS placement were included. In addition, 21 patients treated with TIPS during the same period were selected as controls. Data collected included the success rate and complications of TIPS and TFIPS. Continuous data were expressed as the mean ± SD and were compared using the Student’s t test. All categorical data were expressed as count (percentage) and were compared using the χ2 test or Fisher’s exact test. The Kaplan–Meier method was used to calculate cumulative survival rate and survival curves.
Baseline characteristics were comparable between the two groups. The success rate of TFIPS and TIPS was 95% (19/20) and 100% (21/21), respectively. Effective portal decompression and free antegrade shunt flow was completed in all patients. The portal pressure gradient prior to TIPS and TFIPS placement was 23.91 ± 4.64 mmHg and 22.61 ± 5.39 mmHg, respectively, and it was significantly decreased to 10.85 ± 3.33 mmHg and 10.84 ± 3.33 mmHg after stent placement, respectively. Time–to-event calculated rates of shunt patency at one and two years in the TFIPS and TIPS groups were not statistically different (94.7% vs 95.2% and 94.7% vs 90.5%, respectively). De nova hepatic encephalopathy was 27.5% (11/40) with five patients in the TFIPS group (26.3%) and six patients (28.6%) in the TIPS group experiencing it (P = 0.873). The cumulative survival rates were similar between the two groups: 94.7% and 94.7% at 1 and 2 years, respectively, in the TFIPS group vs 100% and 95.2% at 1 and 2 years, respectively, in the TIPS group (P = 0.942).
TFIPS may be a valuable adjunct to traditional approaches in patients with portal hypertension.
Core tip: Transjugular intrahepatic portosystemic shunt is currently an accepted therapy and has proved beneficial in the treatment of portal hypertension and their complications. However, exceptionally challenging anatomy may require unorthodox salvage techniques, such as transfemoral intrahepatic portosystemic shunt (TFIPS). Because of the rare use of TFIPS, there are few clinical trials that have assessed the safety and effectiveness of TFIPS. In this retrospective study, we describe the TFIPS procedure in detail and evaluate the safety and clinical outcomes of TFIPS and compare them with those of typical transjugular intrahepatic portosystemic shunt. The TFIPS procedure is feasible and efficacy in patients with unorthodox anatomy between the hepatic vein and the portal vein bifurcation.
- Citation: Zhang Y, Liu FQ, Yue ZD, Zhao HW, Wang L, Fan ZH, He FL. Safety and efficacy of transfemoral intrahepatic portosystemic shunt for portal hypertension: A single-center retrospective study. World J Clin Cases 2019; 7(12): 1410-1420
- URL: https://www.wjgnet.com/2307-8960/full/v7/i12/1410.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v7.i12.1410
Portal hypertension (PH) is a common clinical symptom and is mainly caused by chronic liver diseases. A recent epidemiological study suggested that in Europe, PH caused around 150000 deaths per year, and the mortality rate was equal to or greater than that due to breast cancer[1]. Transjugular intrahepatic portosystemic shunt (TIPS) is currently an accepted therapy and has proved beneficial in the treatment of compli-cations of PH, such as gastrointestinal variceal bleeding and refractory ascites[2,3]. With the wide acceptance of polytetrafluoroethylene-covered stents, there has been a significant improvement in long-term TIPS patency[4-7].
Published rates of the technical success of TIPS creation are extremely high, ranging from 90% to 100%[8]. Despite the variance in hepatic vascular anatomy, almost all patients can be successfully treated with standard access (the right jugular vein), standard equipment and standard imaging. Exceptionally challenging anatomy may need new unconventional techniques, for example left hepatic vein–to–left portal vein shunts, direct inferior vena cava (IVC)–to–portal vein (PV) shunts[9] or percutaneous mesocaval shunts[10]. Besides abnormal hepatic vascular anatomy, central venous occlusions or anatomic anomalies, such as those due to pacemaker-related instrumen-tation or previous dialysis, can also be challenging and may require the use of unorthodox alternative methods of the venous route. One study[11] reported the least common approach, through the femoral vein that involved the use of an accessory hepatic vein to create a shunt. However, because of the rare use of transfemoral intra-hepatic portosystemic shunt (TFIPS) there are few clinical trials that have assessed the safety and effectiveness of TFIPS. Thus, we report our experience on the successful creation of TFIPS in 19 patients.
The aim of this study was to compare the effectiveness and clinical outcomes of TIPS with TFIPS in the treatment of symptomatic PH and to determine the safety and efficacy of TFIPS in these patients.
This retrospective study was carried out in compliance with the Declaration of Helsinki of the World Medical Association and was approved by Shijitan Hospital Ethics Committee. Due to the retrospective nature of the study, informed consent was waived.
From a retrospectively collected database of patients who underwent successful TFIPS placement from November 2012 to November 2015, a total of 20 patients with cirrhosis were selected. One patient was excluded due to massive hemorrhage leading to the failure of TFIPS. Eventually, 19 patients who underwent successful TFIPS placement were included in this study. In addition, 21 patients with cirrhosis treated with TIPS during the same period were selected as controls from a historical cohort.
Before undergoing TFIPS/TIPS for PH, all patients underwent contrast-enhanced multiphasic computed tomography (CT) and/or gadoxetic acid-enhanced liver magnetic resonance imaging. Liver cirrhosis was diagnosed by liver biopsy or unequivocal clinical, laboratory (liver function, blood coagulation, routine blood tests) and morphologic liver characteristics on CT and/or magnetic resonance imaging. Gastroscopy was performed to exclude bleeding caused by ulcers and other diseases. Coagulation function, the number of platelets and a reduction in jaundice were adjusted to adapt to the TFIPS/ TIPS procedure.
This procedure was performed after intravenous sedation, where propofol and remifentanil were infused using a target controlled infusion system. The right femoral vein access was achieved using a 10F sheath (Radifocus, Terumo, Leuven, Belgium). A snare (Amplatz Goose Neck Snare Kit, EV3, United States) with a loop diameter of 1 cm was then placed into the right hepatic vein (RHV) and left open in the lumen. Subsequently, a puncture in the abdominal wall was made via the snare loop into the PV with the appropriate position and angle obtained under CT guidance. Using a 18G puncture needle, a 0.035” guidewire (Radifocus, Terumo, Leuven, Belgium) was then advanced all the way to the PV and subsequently to the splenic vein. The puncture needle was then gently retracted, and the guidewire was pulled back into the femoral approach by retracting the snare. Consequently, an access from the right femoral vein through the RHV into the PV and splenic vein was achieved.
Additional steps were similar to the standard TIPS procedure and were performed under fluoroscopic guidance. Firstly, an 8/10 mm balloon catheter (Wanda, Boston Scientific, Galway, Ireland) was used to expand the shunt. The shunt was secured with a covered stent (8 or 10 mm, Fluency, Bard, United States) and further extended to the HV with a bare stent (8 or 10 mm, ELuminexx, Bard, United States). Portal vein pressure (PVP) was measured post-dilatation with an 8 mm balloon catheter. A pigtail catheter was used for photography, and a pressure transducer system (Combitrans, Braun Melsungen, Germany) with a multichannel monitor (Sirecust, Siemens, Germany) was used to measure hemodynamic parameters. The varicosed vein was then embolized after shunting. All measurements were performed at least three times.
TIPS stent (8 mm or 10 mm, Fluency, Bard, United States) was inserted as previously described[12]. Briefly, an intrahepatic tract was punctured in the right internal jugular vein, which was between the right or middle HV and the PV.
Conventional observations and treatment were performed after TIPS/TFIPS. Each patient was asked to remain in bed for 24 h after the procedure. Prophylactic antibio-tics were administered as previously described. Subcutaneous injection of low molecular weight heparin (5000 IU, 2 times/d) was administered for 5 d after the procedure and then changed to warfarin for at least one year. Patients receiving anticoagulant therapy were closely monitored for blood coagulation dysfunction every 2 wk, and the international normalized ratio was maintained at between 2 and 3. Intravenous injection of branched chain amino acids (250-500 mL, once/d) and oral lactulose (15-30 mL, 2-3 times/d) were routinely administered to prevent hepatic encephalopathy (HE). Oral bicyclol tablets (25 mg, 3 times/d) were routinely administered as a liver protection strategy.
Systemic examinations were performed in all patients at 1, 3, 6, 9 and 12 mo after the procedure, followed by annual re-examinations. Detailed medical history and symptoms were recorded. These examinations included liver function, coagulation, blood ammonia, routine blood tests, color ultrasonography, esophagography, CT and gastroscopy. When color ultrasonography suggested stenosis of the shunt channel, aggravation of varicosity, or gastrointestinal bleeding, refractory hydrothorax or ascites, imaging of the shunt channel was repeated, and the PVP was measured. If the blood flow in the shunt channel was normal, whereas the PVP increased or stenosis/ occlusion of the shunt channel was identified, balloon dilation of the shunt channel and re-stenting was performed.
Baseline demographic, clinical and laboratory characteristics were retrieved from clinical records. The following pre-TIPS/TFIPS clinical data were analyzed: age, sex, cause of liver cirrhosis, Child-Pugh score, MELD score, previous upper gastroin-testinal hemorrhage, portal pressure gradient (PPG) before and after TIPS creation, and the diameter of the stent-graft used. The Child-Pugh and MELD scores were also calculated on the basis of data obtained on the day of TIPS /TFIPS creation.
Quantitative data are shown as means and standard deviations and were compared using the paired t test. Qualitative variables are expressed as absolute and relative frequencies and were compared using the χ2 test or Fisher’s exact test. Statistical analysis was performed using the SPSS software (version 20.0, SPSS Inc., United States) and GraphPad Prism software (version 7.0 Graphpad Software Inc., United States). The Kaplan–Meier method was used to calculate cumulative survival rate and survival curves. P < 0.05 was considered statistically significant.
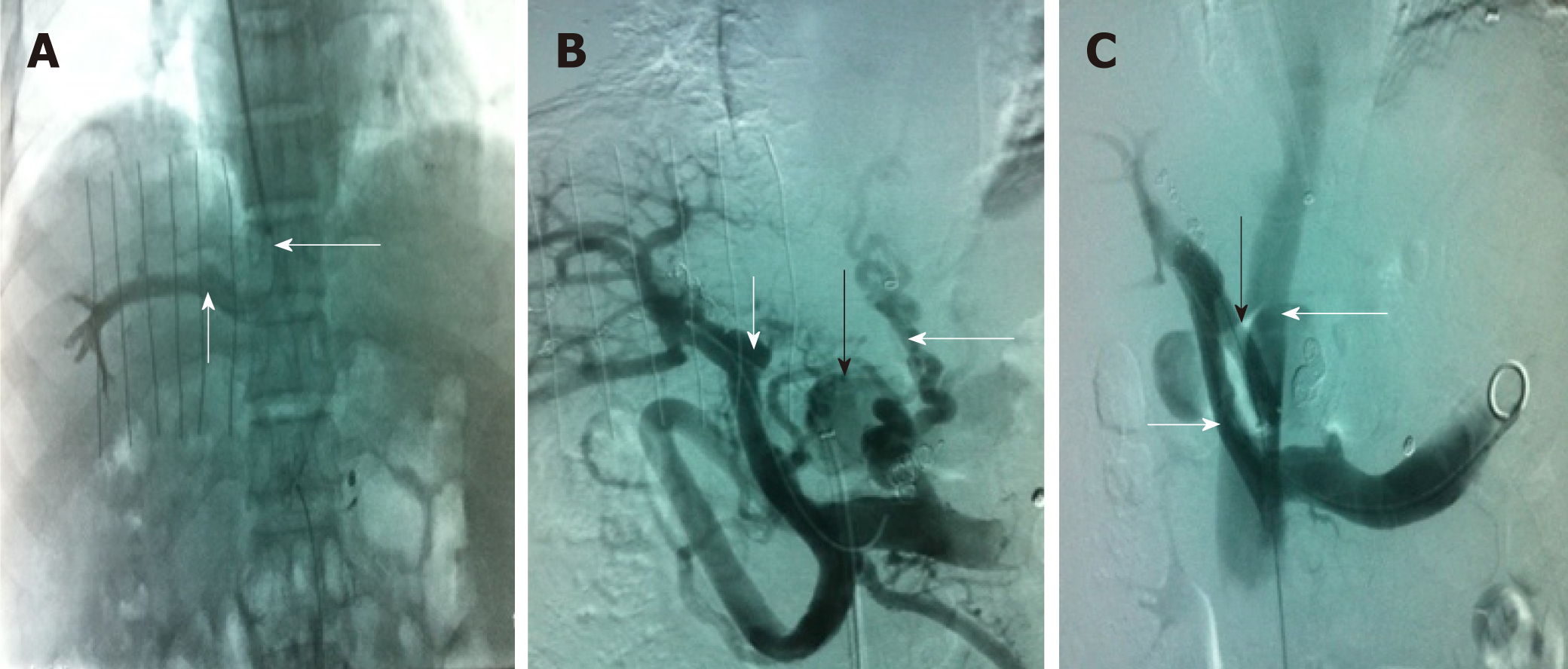
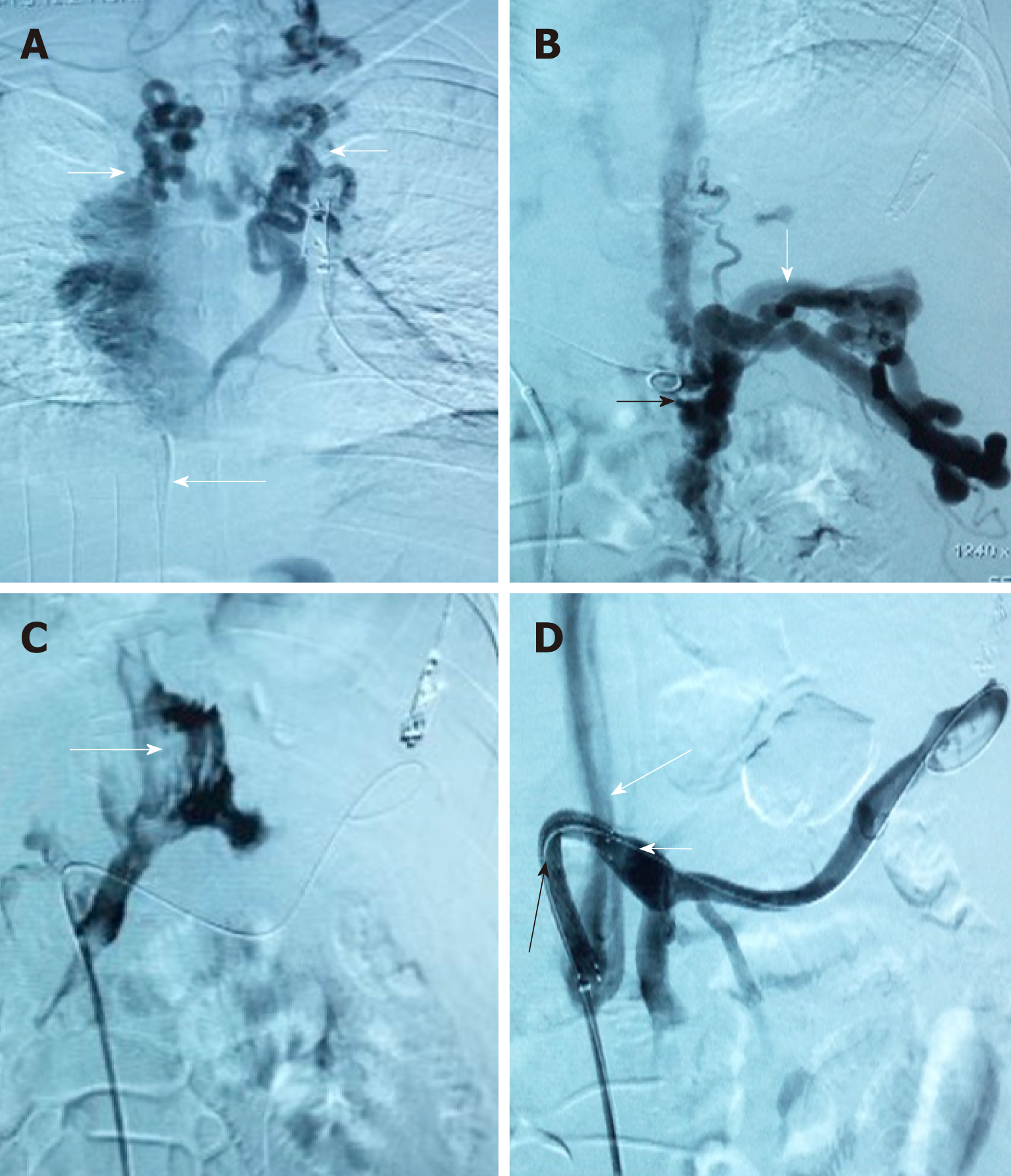
Nineteen patients (male/female, 17/2; age, 46.3 ± 13 years) were included in the TFIPS group. Because of the spatial relationship between the HV or the IVC and the main branches of the intrahepatic PV being unorthodox, TIPS could not be performed in 16 cases (Figure 1 and Figure 2). Due to complete occlusion of the bilateral jugular vein and/or superior vena cava, TIPS could not be performed in 3 cases (Figure 3). Twenty-one patients (male/female, 18/3; age, 45.7 ± 12 years) were included in the TIPS group. The mean follow-up period was 20 (range: 7-34) mo and 22 (range, 9-35) mo in the TFIPS and TIPS groups, respectively. Baseline characteristics in the two groups were comparable and are shown in Table 1.
| Index | TFIPS, n = 19 | TIPS, n = 21 | t/χ2 | P value |
| Gender, male/female | 17/2 | 18/3 | 0.019 | 0.889 |
| Age in yr, mean ± SD | 46.3 ± 13 | 45.7 ± 12 | 0.942 | 0.349 |
| Duration of follow-up in mo | 20 (7-34) | 22 (9-35) | ||
| Etiology as viral/other | 15/4 | 16/5 | 0.213 | 0.725 |
| Child-Pugh stage, n | 0.043 | 0.979 | ||
| Stage A | 5 | 5 | ||
| Stage B | 13 | 15 | ||
| Stage C | 1 | 1 | ||
| Child-Pugh score | 7.31 ± 1.60 | 6.88 ± 1.90 | 0.77 | 0.446 |
| MELD score | 11.62 ± 3.22 | 10.87 ± 2.24 | 0.862 | 0.394 |
| Gastrointestinal bleeding, yes/no | 18/1 | 20/1 | 0.005 | 0.942 |
| Refractory ascites, yes/no | 1/18 | 1/20 | 0.005 | 0.942 |
| Blood ammonia | 33.01 ± 17.89 | 34.33 ± 13.15 | 0.268 | 0.790 |
| Previous splenectomy and devascularization, yes/no | 3/16 | 4/17 | 0.049 | 0.826 |
| Previous sclerotherapy, yes/no | 17/2 | 19/2 | 0.169 | 0.681 |
The successful creation of a shunt between the HV and the intrahepatic branch of the PV was defined as a technical success[13]. The technical success rate of TFIPS and TIPS was 95% (19/20) and 100% (21/21), respectively. Polytetrafluoroethylene–covered stents were used in most TIPS creations. Seventeen patients in the TFIPS group and seventeen patients in the TIPS group were treated for variceal bleeding with adjunctive variceal embolization. One patient in the TFIPS group failed this procedure due to failure to puncture the PV. Intraperitoneal bleeding was observed following puncture of the PV in one case in the TFIPS group, and a covered stent was success-fully used to stop bleeding. The mean procedure time in the TFIPS group was 110.0 ± 12.11 min and was 74.11 ± 5.12 min in the TIPS group (P < 0.001).
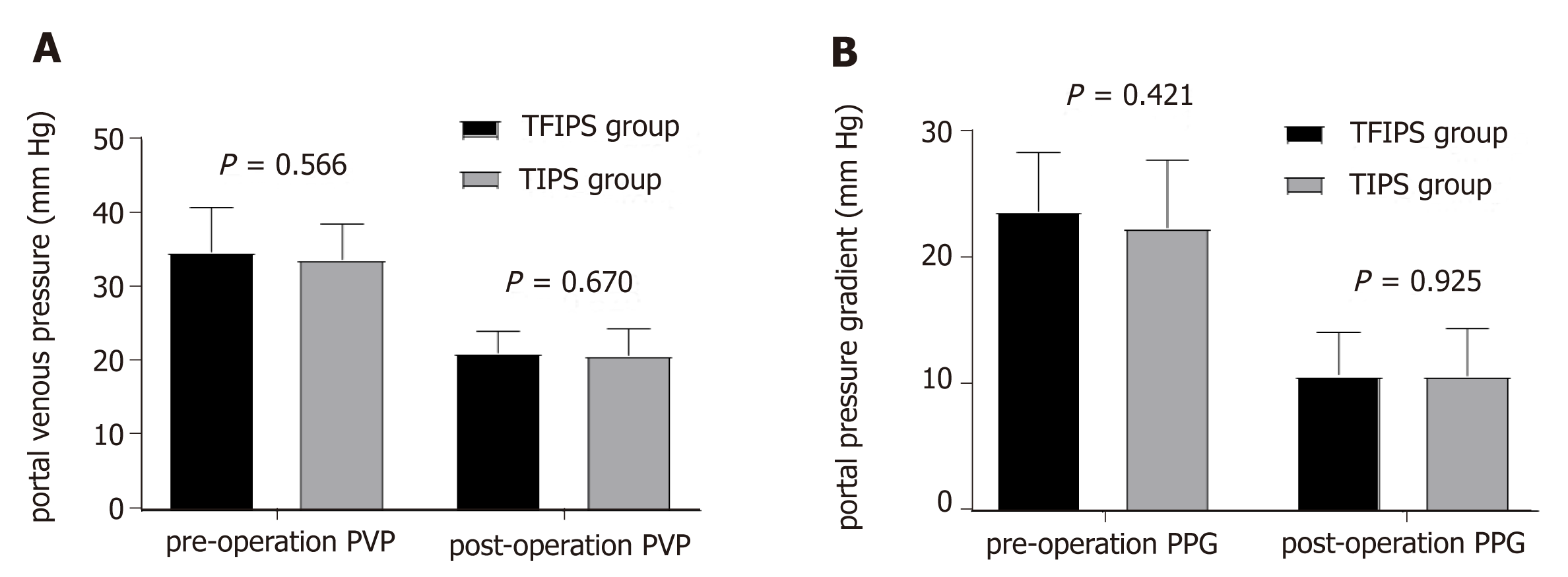
Hemodynamic success refers to the successful post-TIPS reduction of the portosy-stemic gradient below a threshold indicated for the clinical setting[14]. Effective portal decompression and free antegrade shunt flow was completed in all patients. No statistically significant differences were observed between the two groups in terms of PVP and PPG before and after surgery (Figure 4). Detailed hemodynamic changes are shown in Table 2. The PPG prior to TIPS and TFIPS placement was 23.91 ± 4.64 mmHg and 22.61 ± 5.39 mmHg, respectively. PPG decreased significantly to 10.85 ± 3.33 mmHg and 10.84 ± 3.33 mmHg after stent placement in the TIPS and TFIPS groups, respectively.
| Index | TFIPS, n = 19 | TIPS, n = 21 | t/χ2 | P value |
| Pre-operation PVP, mmHg | 35.00 ± 6.05 | 34.01 ± 4.70 | 0.578 | 0.566 |
| Post-operation PVP, mmHg | 21.43 ± 2.83 | 20.98 ± 3.65 | 0.429 | 0.670 |
| Mean decreased PVP differential, mmHg | 13.57 ± 6.70 | 13.03 ± 5.44 | 0.281 | 0.780 |
| Pre-operation PPG, mmHg | 23.91 ± 4.64 | 22.61 ± 5.39 | 0.814 | 0.421 |
| Post-operation PPG, mmHg | 10.85 ± 3.33 | 10.84 ± 3.33 | 0.095 | 0.925 |
| Mean decreased PPG differential, mmHg | 13.07 ± 5.26 | 11.87 ± 5.10 | 0.728 | 0.471 |
| PPG ≤ 12 mmHg, n (%) | 13 (68.4) | 14 (66.7) | 0.014 | 0.906 |
| PPG reduction ≥ 50%, n (%) | 19 (100) | 21 (100) | - | - |
TIPS are well described as an effective treatment for variceal bleeding and refractory ascites. The overall clinical success rate was 92.1% (35/38) for variceal bleeding and 100% (2/2) for ascites. The subgroup clinical failure rate is shown in Table 2. Rebleeding occurred due to recurrent PV thrombosis developed in one patient, and shunt dysfunction developed in two patients.
All shunts were patent on Doppler ultrasonography immediately after their creation (24-36 h). Three patients (1 in the TFIPS group vs 2 in the TIPS group) exhibited shunt dysfunction, and these patients underwent shunt revision (stent placement). Two patients developed recurrent gastrointestinal bleeding. Time-to-event calculated rates of shunt patency at one and two years in the TFIPS and TIPS groups were not statistically different (94.7% vs 95.2% and 94.7% vs 90.5%, respectively).
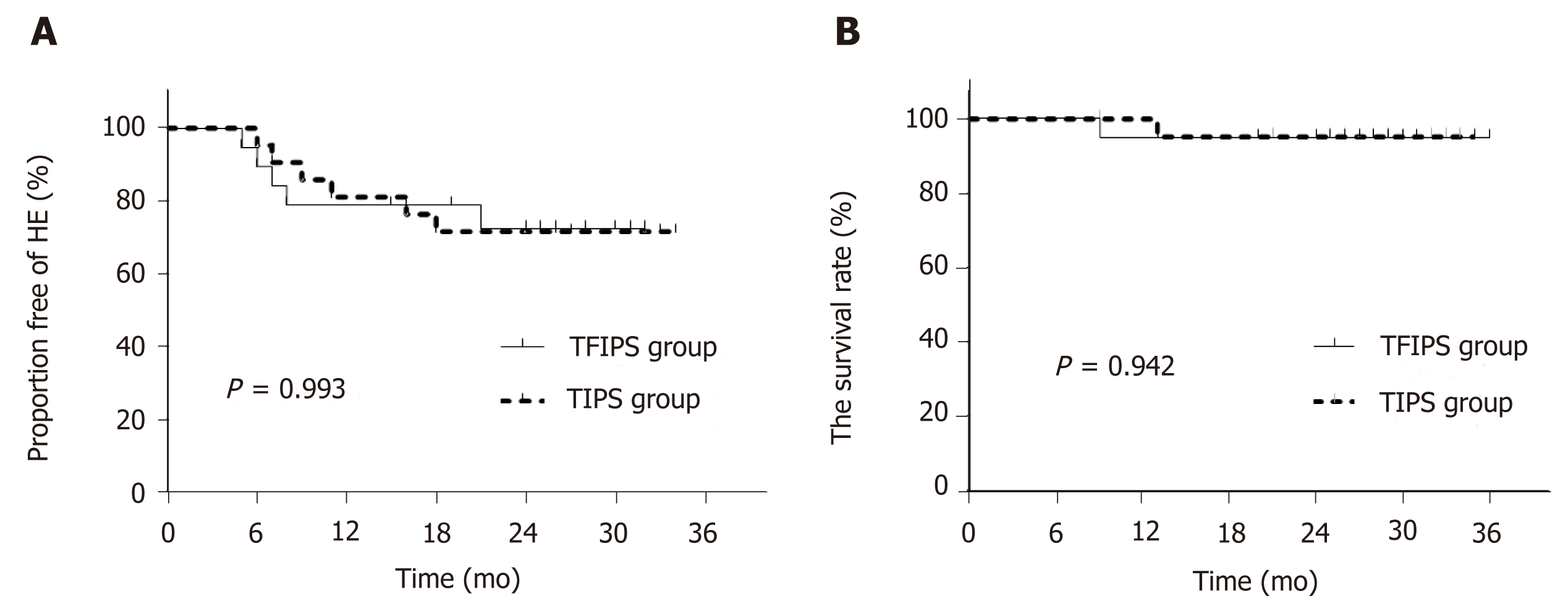
De nova HE was observed in 27.5% (11/40) of patients. Five patients in the TFIPS group (26.3%) and six patients (28.6%) in the TIPS group experienced HE (P = 0.873). Data grading the severity of HE using the West Haven criteria[15] are shown in Table 3. The percentages of severest grade HE episodes were not statistically different between the two groups. Time-to-event (Kaplan-Meier) de nova HE analysis is shown in Figure 5A and there were no significant differences between the two groups [log-rank P = 0.993, hazard ratio: 1.01, 95% confidence interval: 0.31–3.29]. No symptoms of hepatic myelopathy were observed in any of the patients.
| Index | TFIPS group, n = 19 | TIPS group, n = 21 | t/χ2 | P value | ||||
| 12 mo | 24 mo | 12 mo | 24 mo | 12 mo | 24 mo | 12 mo | 24 mo | |
| Child-Pugh score | 5.45 ± 1.60 | 6.32 ± 1.60 | 6.45 ± 1.50 | 7.11 ± 1.60 | 0.042 | 0.061 | 0.852 | 0.813 |
| MELD score | 10.31 ± 1.44 | 11.52 ± 1.43 | 11.12 ± 1.55 | 11.14 ± 1.79 | 0.487 | 0.624 | 0.794 | 0.527 |
| Blood ammonia | 83.6 ± 39.4 | 87.3 ± 33.1 | 85.7 ± 23.4 | 88.2 ± 29.6 | 0.758 | 0.518 | 0.246 | 0.238 |
| Variceal rebleeding, n (%) | 1 (5.3) | 1 (5.3) | 1 (4.8) | 2 (9.5) | 0.005 | 0.261 | 0.942 | 0.609 |
| RS, n | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 |
| Shunt patency | 18 (94.7) | 18 (94.7) | 20 (95.2) | 19 (90.5) | 0.005 | 0.261 | 0.942 | 0.609 |
| HE, n (%) | 4 (21.1) | 5 (26.3) | 4 (19.0) | 6 (28.6) | 0.025 | 0.025 | 0.874 | 0.873 |
| grade of HE, n | 0.107 | 0.166 | 0.948 | 0.573 | ||||
| I | 2 | 2 | 1 | 2 | ||||
| II | 1 | 2 | 2 | 3 | ||||
| III | 1 | 1 | 1 | 1 | ||||
| IV | 0 | 0 | 0 | 0 | ||||
| HM, n | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 |
| Survival, n (%) | 18 (94.7) | 18 (94.7) | 21(100) | 20 (95.2) | 0 | 0.001 | 1 | 0.981 |
Overall 2-year survival rate after TIPS/TFIPS creation was 95% (38/40). The subgroup survival rate was shown in Table 3. One patient in the TIPS group underwent transplant within 12 mo of TIPS creation and survived the 2-year follow-up period. One patient died in the TFIPS group due to recurrent variceal bleeding. Time-to-event (Kaplan-Meier) survival analysis was shown in Figure 5B and there were no significant differences between the two groups (log-rank P = 0.942, hazard ratio: 1.11, 95% confidence interval: 0.56–14.48).
TIPS is an effective method for decompressing PVP; thus, preventing bleeding from gastroesophageal varices and reducing the symptoms of ascites[16-18]. The standard procedure is feasible when the right or middle HV and the right PV can be aligned along a straight imaginary intrahepatic tract within the liver parenchyma[19]. In some cases, the liver may be distorted and the location of porta hepatis may be more cranially than usual. These anatomic variations are sometimes seen in cases with a small, shrunken cirrhotic liver and the standard TIPS procedure may be impossible in these cases. Despite the fact that there are many studies describing techniques to enable PV imaging and access, TIPS shunt placement may be technically difficult or impossible in certain patients for a variety of anatomic reasons[11,20-22] and may be a valuable contingency plan after an unsuccessful TIPS creation. For example, transcaval TIPS was performed in a patient with acute Budd-Chiari syndrome and a thrombosed mesocaval shunt and stenosis of the IVC[22], and a method known as the “gun-sight” approach[23] can create a portocaval shunt in patients with HVs unsuitable for conventional TIPS creation.
The right jugular vein access is the standard approach for TIPS, which usually makes it much easier to puncture the RHV. The orientation of the liver may change due to liver cirrhosis. An acute angle between the IVC and the RHV may form, and the distance between the RPV and the RHV may be decreased[20]. The TFIPS creation is an alternative technique that may successfully circumvent these anatomic constraints. Importantly, the TFIPS procedure provides a new route for the creation of an intrahepatic portosystemic shunt. The RHV is located using the right femoral vein. Furthermore, the route to the portal system is created by trans-mesenteric access. As a consequence, the liver parenchymal shunt tract was created by puncturing from the PV to RHV. Technically, it is much easier to puncture the PV under the guidance of ultrasound. After puncturing the PV, the PV can not only be visualized by direct PV angiography, but a catheter can also be inserted. By using a lateral comparison, depending on the different spatial relationship between the HV and the PV, lateral revision of the puncture needle in an arc, not across the catheter, can prevent puncture of the liver, allows better puncture accuracy and improves the success rate of TFIPS.
In the present study, the TFIPS procedure was performed as a curative treatment in 19 patients who were unable to undergo conventional TIPS. The safety and clinical outcomes of TFIPS were determined. The results showed that in patients with PH, TFIPS was an effective measure in reducing the PPG compared with TIPS. Further-more, TFIPS was not associated with a higher incidence and severity of HE or other unfavorable outcomes such as procedure-related mortality. In fact, the mean PPG before and after TFIPS was not statistically different from that seen in TIPS. The addition of the TFIPS technique for salvage cases allowed us to achieve a 100% technical success rate, regardless of venous patency.
There are various treatment methods for variceal bleeding, which depend on liver function, patient anatomy and local expertise. Recommended societal guidelines[24] cite a 95% technical and 90% clinical success rate for TIPS creation when performed in patients with patent hepatic and portal veins. Our technical success and clinical success rates are close to those in the above report. In our institution, TFIPS creation is not the default primary intervention for PH, making the results more likely to be achievable in “real-world” practice in which the TIPS procedure is more likely to remain the default intervention. In this setting, TFIPS creation appears to be a safe, expedient and effective treatment for patients with acute variceal hemorrhage who are poor anatomic candidates for conventional TIPS creation or who have undergone an unsuccessful TIPS creation. Despite salvage circumstances, outcomes were comparable to the expected outcomes after conventional TIPS creation. However, there is a learning curve for the physician. Furthermore, increased setup time and additional equipment required in a crowded procedure room may also discourage routine use. We suggest using TFIPS for the second attempt if regular TIPS is unsucce-ssful through the right jugular access route.
The limitations of the present report include its retrospective design, a single center and small sample size. Furthermore, some patients were suggested to attend institutions for routine examination, where clinical data were available, but post-operative imaging or endoscopy may not have been routinely performed; thus, assessment for complete variceal eradication was limited. The follow-up period was 12 and 24 mo, and as such the long-term durability of salvage TFIPS is unknown.
In conclusion, our research demonstrated that TFIPS sufficiently decompressed PH and prevented variceal rebleeding compared with TIPS. The use of TFIPS did not decrease HE rates compared with TIPS and no survival benefit was observed. However, TFIPS appears to be a safe, expedient and effective treatment for patients who are poor anatomic candidates for standard TIPS creation or who have undergone an unsuccessful TIPS creation.
Transjugular intrahepatic portosystemic shunts (TIPS) have been used successfully for the treatment of portal hypertension and their complications, such as bleeding varices and refractory ascites. TIPS creation is a percutaneous image-guided procedure in which a decompressive channel is created between a hepatic vein and an intrahepatic branch of the portal vein to reduce portal vein pressure.
TIPS are currently used for the treatment of complications of portal hypertension. With advances in materials, many experimental and clinical studies have been indicated that using covered stent grafts, especially polytetrafluoroethylene covered stent graft, could improve the long-term patency of TIPS. In most situations, a shunt between the hepatic and portal veins can be successfully connected from an internal jugular vein access. Rarely, occlusion of the central veins, hepatic veins, or the vena cava precludes a conventional approach. We used an uncon-ventional procedure called transfemoral intrahepatic portosystemic shunt (TFIPS) to treat portal hypertension and compare this procedure to the traditional approach. In the future, further studies are needed to verify our results.
The main objective was to evaluate the safety and clinical outcomes of TFIPS and compare them with those of TIPS. If TFIPS procedure is as safe and effective as typical TIPS, we should use TFIPS in the patients who are not suitable for the traditional TIPS.
In this one center retrospective study,the subjects were patients diagnosed with portal hypertension who underwent TFIPS (19 patients) because of anatomic reasons and TIPS (21 patients). Patient characteristics, technical success rate, hemodynamic changes, the incidence of shunt stenosis, the incidence of hepatic encephalopathy, hepatic myelopaphy (HM) and the survival rate were compared between the two groups.
This study showed that TFIPS is as effective as TIPS in decompressing portal venous pressure. The TFIPS procedure time is obviously longer than TIPS. There was no significant difference in the incidence of shunt stenosis, hepatic encephalopathy, hepatic myelopaphy and the survival time.
We found that the TFIPS is as effective as TIPS in treating portal hypertension without increasing the complications of TIPS procedure. TFIPS may be a valuable adjunct to traditional approaches in patients with portal hypertension.
Because this study used a single-center retrospective design and included relatively few patients, further investigations, such as a multi-center randomized controlled study, are needed. In addition, due to the increased time used in TFIPS procedure, methods to reduce procedure time are needed.
| 1. | Blachier M, Leleu H, Peck-Radosavljevic M, Valla DC, Roudot-Thoraval F. The burden of liver disease in Europe: a review of available epidemiological data. J Hepatol. 2013;58:593-608. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 879] [Cited by in RCA: 919] [Article Influence: 70.7] [Reference Citation Analysis (2)] |
| 2. | Rössle M, Richter GM, Nöldge G, Palmaz JC, Wenz W, Gerok W. New non-operative treatment for variceal haemorrhage. Lancet. 1989;2:153. [PubMed] |
| 3. | Rössle M. TIPS: 25 years later. J Hepatol. 2013;59:1081-1093. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 230] [Cited by in RCA: 294] [Article Influence: 22.6] [Reference Citation Analysis (0)] |
| 4. | Bureau C, Garcia-Pagan JC, Otal P, Pomier-Layrargues G, Chabbert V, Cortez C, Perreault P, Péron JM, Abraldes JG, Bouchard L, Bilbao JI, Bosch J, Rousseau H, Vinel JP. Improved clinical outcome using polytetrafluoroethylene-coated stents for TIPS: results of a randomized study. Gastroenterology. 2004;126:469-475. [PubMed] |
| 5. | Riggio O, Ridola L, Angeloni S, Cerini F, Pasquale C, Attili AF, Fanelli F, Merli M, Salvatori FM. Clinical efficacy of transjugular intrahepatic portosystemic shunt created with covered stents with different diameters: results of a randomized controlled trial. J Hepatol. 2010;53:267-272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 113] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 6. | Weber CN, Nadolski GJ, White SB, Clark TW, Mondschein JI, Stavropoulos SW, Shlansky-Goldberg RD, Trerotola SO, Soulen MC. Long-Term Patency and Clinical Analysis of Expanded Polytetrafluoroethylene-Covered Transjugular Intrahepatic Portosystemic Shunt Stent Grafts. J Vasc Interv Radiol. 2015;26:1257-65; quiz 1265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 53] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 7. | Wang L, Xiao Z, Yue Z, Zhao H, Fan Z, Zhao M, He F, Dai S, Qiu B, Yao J, Lin Q, Dong X, Liu F. Efficacy of covered and bare stent in TIPS for cirrhotic portal hypertension: A single-center randomized trial. Sci Rep. 2016;6:21011. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 33] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 8. | Boyer TD, Haskal ZJ; American Association for the Study of Liver Diseases. The role of transjugular intrahepatic portosystemic shunt in the management of portal hypertension. Hepatology. 2005;41:386-400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 315] [Cited by in RCA: 303] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 9. | Petersen B, Binkert C. Intravascular ultrasound-guided direct intrahepatic portacaval shunt: midterm follow-up. J Vasc Interv Radiol. 2004;15:927-938. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 38] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 10. | Nyman UR, Semba CP, Chang H, Hoffman C, Dake MD. Percutaneous creation of a mesocaval shunt. J Vasc Interv Radiol. 1996;7:769-773. [PubMed] |
| 11. | LaBerge JM, Ring EJ, Gordon RL. Percutaneous intrahepatic portosystemic shunt created via a femoral vein approach. Radiology. 1991;181:679-681. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 32] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 12. | Yao J, Zuo L, An G, Yue Z, Zhao H, Wang L, Liu F. Risk Factors for Hepatic Encephalopathy after Transjugular Intrahepatic Portosystemic Shunt in Patients with Hepatocellular Carcinoma and Portal Hypertension. J Gastrointestin Liver Dis. 2015;24:301-307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 26] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 13. | Haskal ZJ, Rees CR, Ring EJ, Saxon R, Sacks D. Reporting standards for transjugular intrahepatic portosystemic shunts. Technology Assessment Committee of the SCVIR. J Vasc Interv Radiol. 1997;8:289-297. [PubMed] |
| 14. | Dariushnia SR, Haskal ZJ, Midia M, Martin LG, Walker TG, Kalva SP, Clark TW, Ganguli S, Krishnamurthy V, Saiter CK, Nikolic B; Society of Interventional Radiology Standards of Practice Committee. Quality Improvement Guidelines for Transjugular Intrahepatic Portosystemic Shunts. J Vasc Interv Radiol. 2016;27:1-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 132] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 15. | Wijdicks EF. Hepatic Encephalopathy. N Engl J Med. 2016;375:1660-1670. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 230] [Cited by in RCA: 309] [Article Influence: 30.9] [Reference Citation Analysis (0)] |
| 16. | Heinzow HS, Lenz P, Köhler M, Reinecke F, Ullerich H, Domschke W, Domagk D, Meister T. Clinical outcome and predictors of survival after TIPS insertion in patients with liver cirrhosis. World J Gastroenterol. 2012;18:5211-5218. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 17. | Merli M, Salerno F, Riggio O, de Franchis R, Fiaccadori F, Meddi P, Primignani M, Pedretti G, Maggi A, Capocaccia L, Lovaria A, Ugolotti U, Salvatori F, Bezzi M, Rossi P. Transjugular intrahepatic portosystemic shunt versus endoscopic sclerotherapy for the prevention of variceal bleeding in cirrhosis: a randomized multicenter trial. Gruppo Italiano Studio TIPS (G.I.S.T.). Hepatology. 1998;27:48-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 120] [Article Influence: 4.3] [Reference Citation Analysis (1)] |
| 18. | Narahara Y, Kanazawa H, Fukuda T, Matsushita Y, Harimoto H, Kidokoro H, Katakura T, Atsukawa M, Taki Y, Kimura Y, Nakatsuka K, Sakamoto C. Transjugular intrahepatic portosystemic shunt versus paracentesis plus albumin in patients with refractory ascites who have good hepatic and renal function: a prospective randomized trial. J Gastroenterol. 2011;46:78-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 130] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 19. | Liang HL, Liu WC, Huang JS, Chen MC, Lai KH, Pan HB, Chen CK. TIPS in patients with cranial porta hepatis: ultrasound-guided transhepatic portohepatic-portocaval puncture in single needle pass. AJR Am J Roentgenol. 2011;196:914-918. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 20. | Hausegger KA, Tauss J, Karaic K, Klein GE, Uggowitzer M. Use of the left internal jugular vein approach for transjugular portosystemic shunt. AJR Am J Roentgenol. 1998;171:1637-1639. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 21. | Sze DY, Magsamen KE, Frisoli JK. Successful transfemoral creation of an intrahepatic portosystemic shunt with use of the Viatorr device. J Vasc Interv Radiol. 2006;17:569-572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 22. | Lee KH, Lee DY, Won JY, Park SJ, Kim JK, Yoon W. Transcaval transjugular intrahepatic portosystemic shunt: preliminary clinical results. Korean J Radiol. 2003;4:35-41. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 23. | Haskal ZJ, Duszak R, Furth EE. Transjugular intrahepatic transcaval portosystemic shunt: the gun-sight approach. J Vasc Interv Radiol. 1996;7:139-142. [PubMed] |
| 24. | Haskal ZJ, Martin L, Cardella JF, Cole PE, Drooz A, Grassi CJ, McCowan TC, Meranze SG, Neithamer CD, Oglevie SB, Roberts AC, Sacks D, Silverstein MI, Swan TL, Towbin RB, Lewis CA; Society of Cardiovascular & Interventional Radiology, Standards of Practice Committee. Quality improvement guidelines for transjugular intrahepatic portosystemic shunts. SCVIR Standards of Practice Committee. J Vasc Interv Radiol. 2001;12:131-136. [PubMed] |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See:
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Alexopoulou A, Garbuzenko DV S-Editor: Wang JL L-Editor: Filipodia E-Editor: Wang J