Published online Jun 6, 2019. doi: 10.12998/wjcc.v7.i11.1351
Peer-review started: January 17, 2019
First decision: March 10, 2019
Revised: April 2, 2019
Accepted: April 9, 2019
Article in press: April 10, 2019
Published online: June 6, 2019
Processing time: 141 Days and 11.9 Hours
Basal cell adenoma (BCA) is a rare benign tumour that has unique histological characteristics and primarily arises in the parotid glands. According to published reports, nearby tissue destruction by BCA seems impossible.
We presented a case of a 54-year-old woman with a mass in the deep lobe of the right parotid gland involving the ipsilateral skull base and mastoid. The patient exhibited gradual right facial swelling but no other obvious symptoms. Combined resection of the total right parotid gland and partial skull base excision were performed. The biopsy conducted before the surgery and sections cut from intraoperatively obtained tissues were not definitive for identifying the character of the neoplasm. A final diagnosis of tubular BCA without malignant elements was established based on postoperative pathology results and immunohistochemical analysis. The tumour did not recur during the 12-mo follow-up period.
A diagnosis of BCA can only be established based on a histopathological examination after an excisional biopsy, and tubular BCA should carefully be considered as a destructive type.
Core tip: Basal cell adenoma (BCA) is a rare benign tumour that has unique histological characteristics and primarily arises in the parotid glands. Destruction of nearby tissue by BCA has not been reported. We presented a case of tubular BCA in the deep lobe of the parotid gland involving the ipsilateral skull base and mastoid. It suggests that a diagnosis of BCA can only be established after an excisional biopsy, and tubular BCA should carefully be considered as a destructive type.
- Citation: Du LY, Weng XH, Shen ZY, Cheng B. A large basal cell adenoma extending to the ipsilateral skull base and mastoid in the right parotid gland: A case report. World J Clin Cases 2019; 7(11): 1351-1357
- URL: https://www.wjgnet.com/2307-8960/full/v7/i11/1351.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v7.i11.1351
Basal cell adenoma (BCA) is an uncommon benign tumour of the salivary glands, usually the parotid gland that accounts for no more than 3% of all salivary gland tumours[1-3]. It frequently occurs in patients over 50 years of age and has a slightly higher prevalence in women[4]. With regard for clinical presentation, BCAs exhibit slow growth and are asymptomatic, movable, round or oval, normal-coloured subcutaneous masses measuring less than 3 cm in diameter[4]. Histologically, they are known to possess a prominent basal cell layer and a distinct basement membrane-like material and are classified into four subtypes according to cell arrangement[5]. Its differential diagnosis with conditions with varied prognoses, such as pleomorphic adenoma (PA) and adenoid cystic carcinoma (ACC), makes it necessary to consider this entity in cases of glandular tumours of the maxillofacial area. A nonspecific presentation and difficulty in obtaining a histology-based diagnosis characterize this benign neoplasm. Total parotidectomy is preferred for tumours in the deep portion of the parotid gland or for membranous-type BCAs, which tend to be multi-centric, have multiple recurrences, and occasionally undergo malignant transformation.
Here, we report a case in which a mass that originated from the deep lobe of the right parotid gland resulted in skull bone destruction involving the mastoid process and cerebellar dura mater. The tumour was ultimately confirmed as a tubular BCA. The results of this report emphasize the complexity and difficulty of diagnosing BCA, which must be distinguished from PA, ACC, and adenocarcinoma. It is important to obtain a histopathological confirmation in a BCA diagnosis and to explore the potential for adjacent tissue destruction in tubular BCA.
In March 2017, a 58-year-old woman attended the outpatient oral and maxillofacial surgery clinic. Her chief complaint was the presence of a painless swelling in her right parotid gland for one year.
Ten years before, the patient had right facial paralysis without apparent cause, and then one year before, she stumbled across a peanut-sized nodule on her right postauricular region. The mass gradually increased in size during the previous half year and was accompanied by vertigo. Six days before her visit to our hospital, she went to Jianghan Oilfield general hospital and received a fine-needle aspiration (FNA), which suggested a diagnosis of PA with cell hyperplasia. Then, she was advised to go to a higher-level hospital for further treatment. Since the onset of illness, the general condition of patients has had no significant change.
The patient underwent appendectomy in Houhu Farm Hospital 25 years ago. There is no other special trauma history, blood transfusion history, or allergy history. There was not a history of ear disease or past radiology.
Non-apparent abnormality.
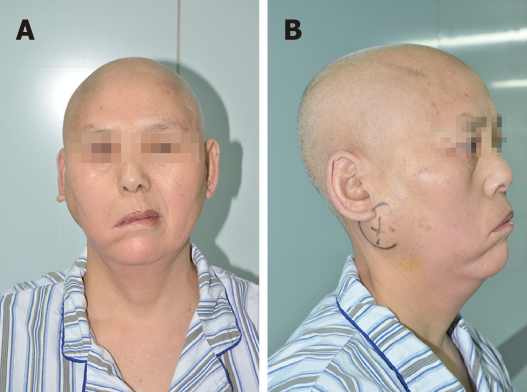
A physical examination revealed a mass measuring approximately 6 cm × 7 cm. The mass was firm, immovable, non-tender, smooth-surfaced, and well-demarcated. There was regional lymphadenopathy on the same side of the neck. She was diagnosed with right facial palsy more than ten years previously and showed clinical characteristics of peripheral facial paralysis (Figure 1), including an askew mouth, an inability to frown or to close the eye, and a smooth right nasolabial fold.
Biochemical blood tests were within normal limits. The results of a preliminary FNA biopsy suggested PA with positive immunostaining for cytokeratin, nuclear associated antigen Ki-67 (< 5%), and markers for luminal cells [epithelial membrane antigen, cluster of differentiation-17] and myoepithelial cells [soluble protein-100, transformation-related protein 63, cytokine-14, and calponin]. A possible diagnosis of ACC could not be ruled out at the time.
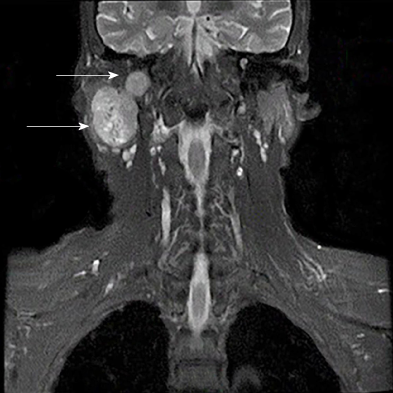
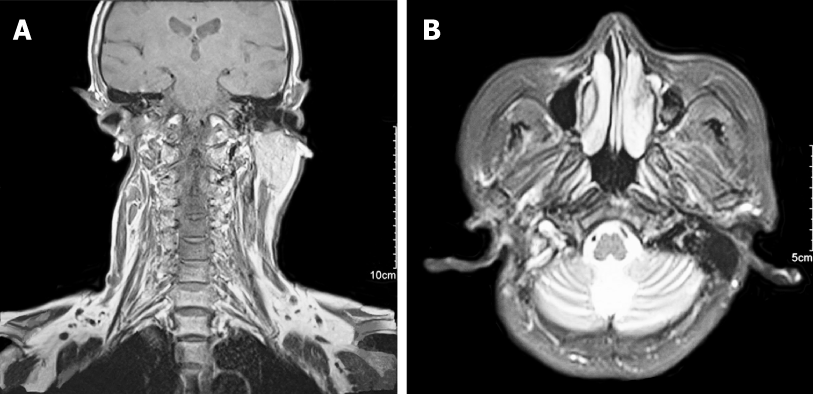
Magnetic resonance imaging was performed and revealed a well-defined, hete-rogeneously enhancing mass with a size of 3.9 cm × 2.9 cm × 5.3 cm that was mostly derived from the deep lobe of the right parotid gland. The signs showed invasion in adjacent structures (right mastoid and occipital bone) (Figure 2). It also indicated that the branches of right lateral carotid artery adjacent to the mass were pushed and squeezed.
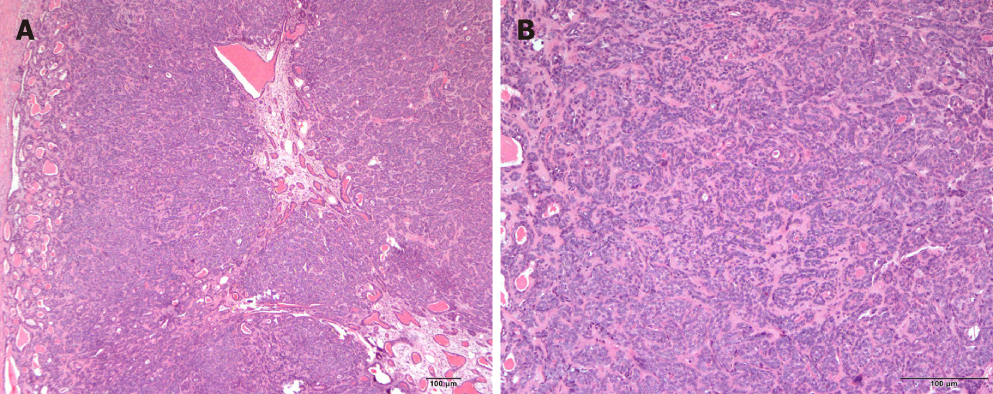
The excised specimen was sent for histopathological and immunohistochemical analysis to obtain more detailed and accurate histopathology-based diagnosis. Hematoxylin-eosin staining showed that the tumour possessed relatively uniform-appearing basaloid epithelial cells arranged in clusters around ducts, an abundant basal cell layer, and a distinctive basement membrane-like material (Figure 3). The immunohistochemical analysis showed that the tumour was positive for epithelial membrane antigen, transformation-related protein 63, calponin and Ki67 (5%). Its overall features were indicative of tubular BCA of the right parotid gland.
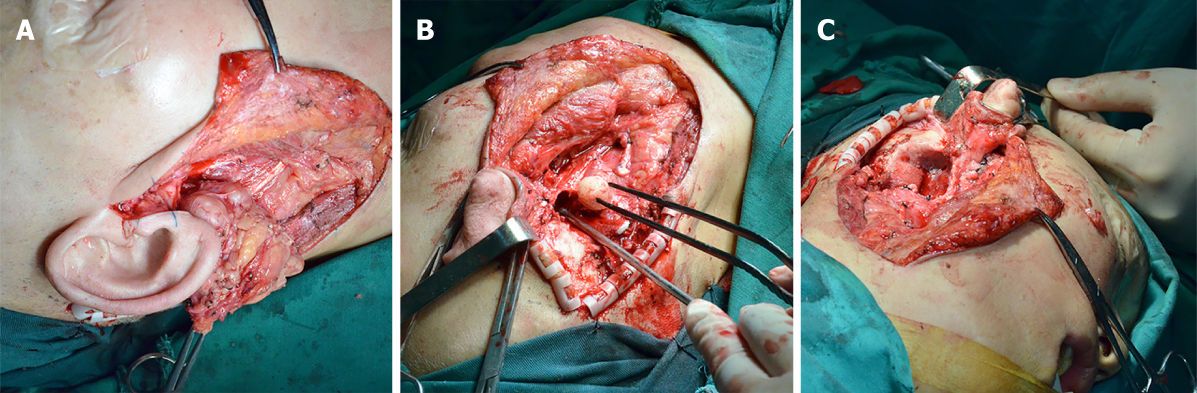
In April 2017, a total excision including the removal of the mass with the right parotid gland and involving the right mastoid process and cerebellar dura mater was planned in cooperation with a neurosurgeon. The intramastoid part of the tumour was exposed, and a partial right mastoid osteotomy followed by a total parotidectomy was performed (Figure 4). The tumour was encapsulated by fine fibrous connective tissue originating in the parotid gland and intramastoid parts. Dura mater was not involved, so we successfully departed the tumour and cerebellar dura mater. The results of an analysis of frozen sections of intraoperatively obtained tissues provided an unclear diagnosis of a benign tumour. The cut surface of the tumour was greyish-white in colour, and no necrotic and haemorrhagic changes were found in the tumour.
In July 2017, a post-operative magnetic resonance imaging showed no mass residual and an absence of the right parotid gland (Figure 5). Except for the right facial paralysis, there was no sign of recurrence and neurosis after one year of follow-up.
BCAs are uncommon benign tumours with an appearance dominated by basaloid cells. The parotid gland, and especially its superficial lobe, is the most common site of BCA occurrence and is followed by tumour occurrence by the palate and buccal mucosa[4]. A thorough review of the literature revealed that no previous study has described a case with a BCA in the deep lobe of the parotid gland with adjacent bone destruction and intracranial invasion.
Clinically, BCA tends to be an asymptomatic, slowly enlarging, well-encapsulated tumour with a diameter of no more than 3 cm[6]. One unusual feature observed in this case was a BCA of the parotid gland that measured up to 6 cm in diameter, far exceeding the normal size of such tumours. Grossly, BCAs appear as round or oval tumours encapsulated by fine fibrous connective tissue. In many cases, cystic formations, predominantly found in tubular and trabecular BCAs, are noted in the central area of the tumour, and the lumens are usually filled with mucinous material[1]. In our case, a preliminary radiological assay seemed to show several small cysts within the tumour, leading to a suspected diagnosis of BCA.
Pathology examination is acknowledged as the most accurate method for diagnosing BCA. Histologically, BCA is divided into the following four subtypes based on morphologic patterns: solid, trabecular, tubular, and membranous. Solid variants are the most common type[7], and membranous BCAs are normally expected to be non-encapsulated, multicentric, and multilobular[8,9]. Other primary tumours, such as PA, ACC, and basal cell adenocarcinoma, can simulate its basal cell features, making this tumour difficult to diagnose and differentiate. However, the appearance of BCAs, including the lack of a chondromyxoid stroma and the presence of a distinctive basal membrane, in addition to the absence of an infiltrative growth pattern, perineural invasion, and mitotic features, and their particular pattern of immunohistochemical staining, including epithelial membrane antigen, α smooth muscle actin, and focal staining for Ki-67 (< 5%), can help to distinguish a BCA from another primary tumour, such as a PA or ACC and basal cell adenocarcinoma[2,4,10,11]. However, it is not easy to confirm a BCA diagnosis.
In our case, findings based on primary FNA and immunohistochemistry initially suggested a probable diagnosis of PA without ruling out ACC. We decided in our surgical plan to take several factors into consideration, including the patient’s clinical features, radiological examination, and histopathological findings. The results of our analysis of intraoperatively obtained sections produced an ambiguous diagnosis of a benign lesion derived from the right parotid gland. A final diagnosis of tubular BCA can be established only when a histopathological examination is performed after the tumour removal. By combining the clinical manifestations (bone destruction and local invasive behaviour) and histological features (Ki67, 5%) of the tumour, we were inclined to consider the tumour a borderline lesion, even though it was a BCA.
BCA can be managed by conservative resection resulting in a negative surgical margin. Additionally, the results of an accurate surgical treatment can differ based on the preoperative and intraoperative diagnoses. While superficial or total parotidectomy is generally considered the primary treatment for BCA, the membranous subtype requires complete resection of the entire gland[8]. In contrast to the high recurrence rate for this specific BCA type (24%), the recurrence rates for the other three types of BCA is nearly zero. We observed no recurrence after a follow-up of one year. Malignant transformation is also more common in membranous BCA, although it remains extremely rare[8]. However, signs of invasion into adjacent structures were evident in our case, which was a tubular BCA, and these tend to occur in membranous type BCAs or malignant neoplasms. Further studies aimed at increasing our understanding of BCA should be performed in the future. Overall, when treatment achieves a wide excision exhibiting a negative surgical margin and regular follow-up is performed, the prognosis of BCA is good.
It is rare for a BCA in the parotid gland to extend into the mastoid and ear canal, and this is the first such case to be reported in the literature. A diagnosis of BCA can only be established based on a histopathological examination after an excisional biopsy, and FNA, immunohistochemistry, and magnetic resonance imaging scans should not be considered conclusive. Both the membranous and tubular types of BCA should prompt attention to the possibility of local tissue invasion. The goal of the present paper was to add our case to the literature related to BCAs that arise from the parotid gland and extend into the skull base and to prompt clinicians to carefully consider tubular BCA with parotid gland swelling that shows adjacent tissue destruction. Long-term follow-up and more research are indeed necessary to prevent recurrence and improve the prognosis in this group of patients.
| 1. | Jang M, Park D, Lee SR, Hahm CK, Kim Y, Kim Y, Park CK, Tae K, Park MH, Park YW. Basal cell adenoma in the parotid gland: CT and MR findings. AJNR Am J Neuroradiol. 2004;25:631-635. [PubMed] |
| 2. | González-García R, Nam-Cha SH, Muñoz-Guerra MF, Gamallo-Amat C. Basal cell adenoma of the parotid gland. Case report and review of the literature. Med Oral Patol Oral Cir Bucal. 2006;11:E206-E209. [PubMed] [DOI] [Full Text] |
| 3. | Chawla AJ, Tan TY, Tan GJ. Basal cell adenomas of the parotid gland: CT scan features. Eur J Radiol. 2006;58:260-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 4. | Yadav AB, Narwal A, Devi A, Kumar S, Yadav SK. Basal Cell Adenoma of Palate, a Rare Occurrence with Review of Literature. J Dent (Shiraz). 2015;16:291-295. [PubMed] |
| 5. | El-Naggar, Adel K. Diagnostic Surgical Pathology of the Head and Neck. American Journal of Surgical Pathology. 2001;25:976. |
| 6. | Chung WY, Kim CH. Basal cell adenoma in the deep portion of the parotid gland: a case report. J Korean Assoc Oral Maxillofac Surg. 2015;41:352-356. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 7. | Machado de Sousa SO, Soares de Araújo N, Corrêa L, Pires Soubhia AM, Cavalcanti de Araújo V. Immunohistochemical aspects of basal cell adenoma and canalicular adenoma of salivary glands. Oral Oncol. 2001;37:365-368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 42] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 8. | Lambade PN, Rajkhokar D, Lambade D. Basal Cell Adenoma of Submandibular Salivary Gland: A Case Report and Literature Review. J Maxillofac Oral Surg. 2015;14:999-1003. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 9. | Junquera L, Gallego L, de Vicente JC, Fresno MF. Bilateral parotid basal cell adenoma: an unusual case report and review of the literature. J Oral Maxillofac Surg. 2010;68:179-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 10. | Jeddy N, Prasannamoorthy L, Thavarajah R, Radhika T, Ramachandran A. Membranous Basal Cell Adenoma - A Rare Entity in an Unusual Location. J Clin Diagn Res. 2017;11:ZD21-ZD22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 11. | Veeresh M, Bavle RM, Vinay KN, Nandakumar H. Basal cell adenoma of the submandibular gland. J Maxillofac Oral Surg. 2010;9:289-291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See:
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, Research and Experimental
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Vissink A, Mogulkoc R, Ciuman RR S-Editor: Dou Y L-Editor: Filipodia E-Editor: Xing YX