Published online Jun 6, 2019. doi: 10.12998/wjcc.v7.i11.1337
Peer-review started: January 25, 2019
First decision: January 30, 2019
Revised: February 19, 2019
Accepted: March 16, 2019
Article in press: March 16, 2019
Published online: June 6, 2019
Processing time: 134 Days and 8.9 Hours
Anorectal melanoma (AM) is an extremely rare malignant tumor originating from anorectal melanocytes with a poor prognosis. AM has been reported to have a much lower incidence than cutaneous or choroid melanoma, accounting for 0.4%-1.6% of all melanomas.
We report a 76-year-old female patient diagnosed with anorectal malignant melanoma by colonoscopy and biopsy. Intraoperative examination revealed two distinct anorectal tumors, one melanotic and another amelanotic, as well as two pigmented mucosal zones at the dentate line level. Abdominal perineal resection was performed. A pathological report confirmed all four lesions to be melanomas. Postoperatively, we followed an immunotherapy protocol targeting PD-1 (nivolumab). The patient had 24 mo of disease-free follow-up upon completion of nivolumab treatment.
This is the first reported case presenting coexistence of pigmented and unpigmented AMs in the same patient.
Core tip: Anorectal melanoma (AM) is an extremely rare malignant tumor. We report a 76-year-old female patient diagnosed with anorectal malignant melanoma by colonoscopy and biopsy. Intraoperative examination revealed two distinct anorectal tumors, one melanotic and another amelanotic. Two satellite melanotic implantations were also found in the near mucosal area. This is the first reported case presenting coexistence of pigmented and unpigmented AMs in the same patient and may contribute to further prognostic factor studies in the future research.
- Citation: Cai YT, Cao LC, Zhu CF, Zhao F, Tian BX, Guo SY. Multiple synchronous anorectal melanomas with different colors: A case report. World J Clin Cases 2019; 7(11): 1337-1343
- URL: https://www.wjgnet.com/2307-8960/full/v7/i11/1337.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v7.i11.1337
First reported by Moore in 1857, anorectal melanoma (AM) is an extremely rare ma-lignant tumor originating from anorectal melanocytes. AM accounts for 0.5%-4.6% of anorectal malignant tumors and 0.4%-1.6% of all melanomas[1,2]. Prognosis of AM is dismal with five-year survival rates estimated to be less than 20%[3]. About 30% of AMs appear to be amelanotic[4]. Amelanotic AM is easily misdiagnosed with other anorectal diseases, including anorectal cancer, polyps, and hemorrhoids.
In some studies, amelanotic melanoma in AM was associated with worse prognoses than melanotic melanoma[5]. To our knowledge, this is the first study to successfully demonstrate a case of multiple synchronous melanomas with different colors.
A 76-year-old female patient experiencing symptoms of hematochezia and tenesmus for one month was admitted in our institution.
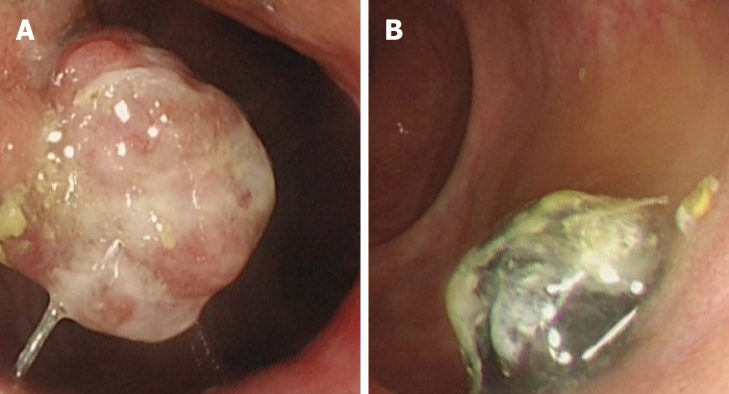
After symptom onset, the patient had previously undergone a colonoscopy at the community hospital, which revealed two masses, one located at the anal canal level (unpigmented, diameter 2.5 cm) and another 3 cm above the dentate line (pigmented, diameter 2 cm). A biopsy indicated anorectal malignant melanoma with positive expression of Melan-A and HMB-45.
No specific related past illness was found.
The patient had no specific personal or family history of cancer related disease.
When subjected to a digital rectal examination, the patient reported pain, but bleeding was not observed. Digital rectal examination revealed two firm and immobile anorectal masses, locations and size of which matched description in previous colonoscopy.
Tumor markers including CEA and CA19-9 were within normal levels. Other preoperative laboratory tests revealed normal levels.
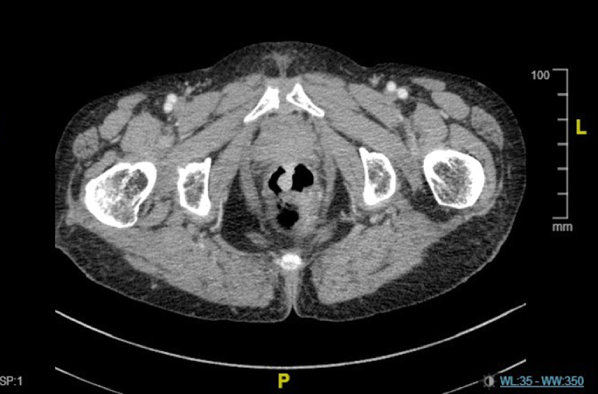
No evidence of distal metastasis or significant inguinal lymphadenopathy was suggested by B-ultrasound and enhanced computed tomography. We confirmed the location of the two anorectal masses by preoperative colonoscopy (Figure 1) and pelvic CT (Figure 2).
The final diagnosis of the presented case was anorectal malignant melanoma, according to the biopsy report.
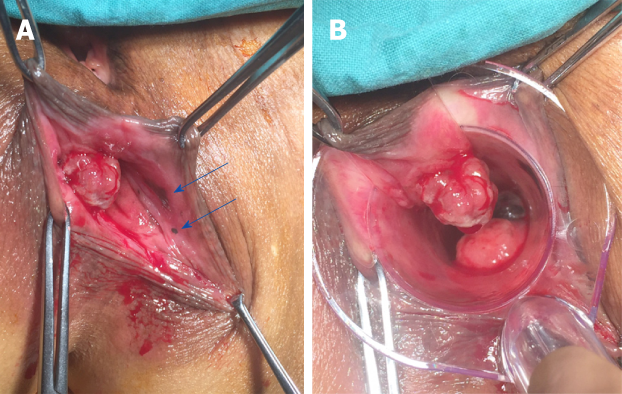
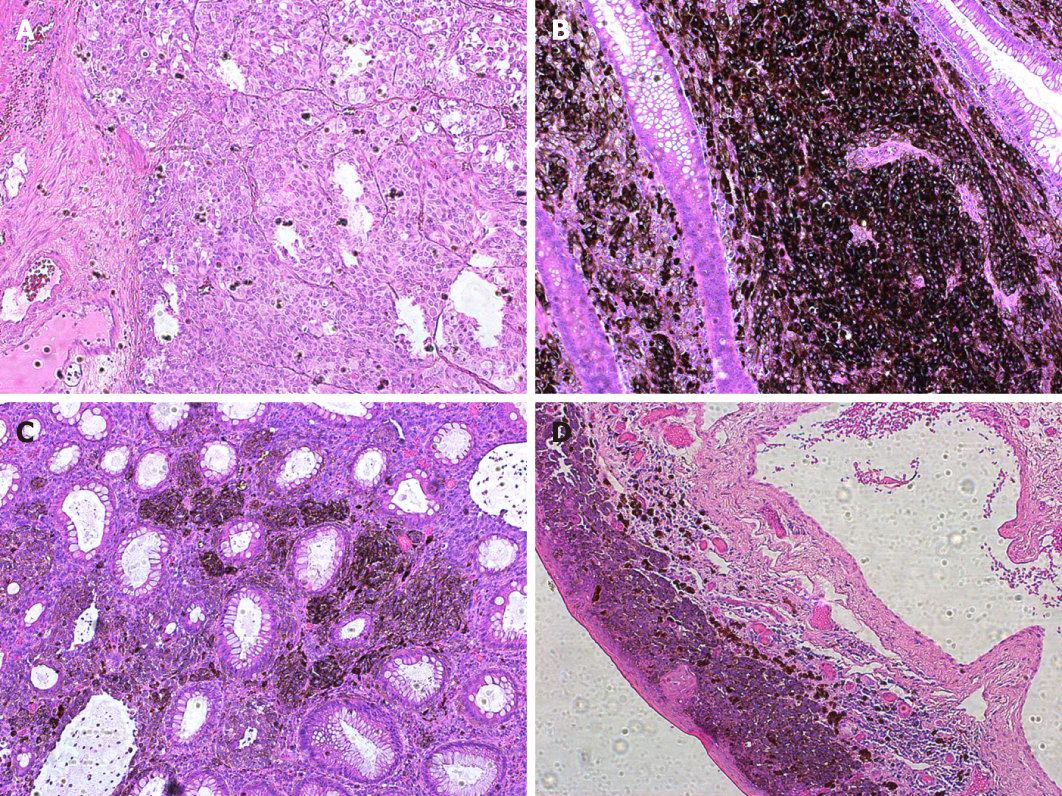
Surgery was performed under general anesthesia in the lithotomy position. During the procedure, the two anorectal tumors were observed as expected, but two additional mottled mucosal pigmented zones, 1 and 5 mm in diameter, were also observed under an anoscope (Figure 3). Abdominoperineal resection was performed eventually. Intraoperative frozen pathological report suggested a negative resection margin. The deepest invasion of the tumor extended to the muscular layer of the rectum. Histologic illustration of the four lesions were presented in Figure 4. Result of lymph nodes was 0/8. Histopathology showed S100+, HMB45+, MelanA+, CyclinD1+, CK-, EMA-, and VIM+. According to Falch’s staging classification (Table 1), the patient was grouped into stage II[6]. We followed an immunotherapy protocol involving administration of nivolumab, a monoclonal antibody targeting PD-1.
| Stage | Tumor spread | Median survival time (mo) |
| I | Local tumor spread + no infiltration of muscular layer | 29 |
| II | Local tumor spread + infiltration of the muscular layer | 11 |
| III | Regional tumor spread and/or lymph node metastasis | 9 |
| IV | Distal metastasis |
Bowel movement occurred and fluid diet was given in 48 h. Postoperative recovery was well and the patient got discharged two weeks after surgery. Upon completion of nivolumab treatment, the patient had 24 mo of disease-free follow-up. However, due to economic burden, the patient stopped nivolumab treatment 3 mo before being diagnosed with lung metastasis.
As an extremely rare malignant disease, AM is known for its poor prognosis[1,2,7]. Systemic dissemination was recorded to occur in about 67% of patients who were diagnosed early. Misdiagnosis occurs in more than half of the AM patients, mistaken for hemorrhoids, polyps, or rectal cancer[3]. Late and incorrect diagnoses are common due to atypical symptoms and low incidence[8]. About 30% of AMs appear to be amelanotic, which also contributes to the difficulty of diagnosis[4]. But interestingly, misdiagnosis has no significant negative effect on survival time as reported by Zhang et al[9], which suggested that early diagnosis may not mean advantage in survival time because of the extreme malignancy of AM. Larger cohort of AM cases may help confirm or refute this hypothesis.
TNM classification is unsuitable for AM staging. Lymph node metastasis in AM is associated with an increased risk of metastasis and poor prognosis (5-year survival: 45% vs 0%, Ballo et al[10]). Tumor infiltration into the muscular layer has been demonstrated as an independent prognosis factor by several studies[3,11]. Falch created a 4-stage AM classification system according to retrospective analysis of total survival time (Table 1). When depth of muscular infiltration was taken into consideration, local AM was divided into two stages (stage 1 and stage 2)[6]. Median survival time was significantly worse when the tumor infiltrated into the muscular layer (29 mo in stage 1, and 11 mo in stage 2). Cases with lymph node involvement were grouped into stage 3 with a median survival time of less than 1 year. Systemic metastasis was a feature of stage 4, characterized by a very dismal prognosis.
Amelanotic melanoma type in AM was reported to have a worse prognosis than melanotic type in some studies. Reason for this phenomenon remains uncertain. Some authors believe that this is either because amelanotic melanoma is more difficult to diagnose, or it is possibly more invasive in nature[5]. Satellite lesions may have a relationship with a poor prognosis, which has been proven in cutaneous melanoma studies. Tumor size has also been proposed as another potential prognostic factor, but more subjects are needed to confirm this result[4]. AM with multiple lesions is rarely reported and currently has no sufficient evidence to be regarded as an independent prognostic factor[12].
Although therapy for AM has not yet been standardized, surgical resection is recognized as the primary treatment approach[1]. Patients grouped into stages 1 and 2 may benefit from radical surgery in total survival time[13]. Abdominal perineal resection (APR) and wild local excision (WLE) are the most commonly used surgical procedures. Controversy still remains regarding choice of operation method. APR showed its superiority in local control as revealed in several studies, but support for WLE is becoming more widespread as well. WLE preserves sphincter function and demonstrates less postoperative morbidity, indicating that WLE may provide superior quality of life compared to APR. Additionally, the resection margin in WLE requires no less than 10 mm to achieve R0 excision[14]. Several studies showed that WLE had lower morbidity and non-inferior prognosis compared with APR[15], but the subjects in this study were limited to early stage patients, so further work with additional subjects in later stages is needed to confirm this result. Some clinicians prefer local excision, considering that both procedures lead to very poor postoperative prognoses[16]. Most studies have indicated no difference between APR and WLE regarding postoperative prognosis[3]. On the basis of R0 resection, WLE is reco-mmended when it is technically available. APR is more commonly chosen in case of a locally advanced tumor.
Most studies do not recommend prophylactic therapy[17]. In local lymph node metastasis cases, lymph node dissection remains controversial. No strong evidence exists to demonstrate that ilioingulinal lymph node dissection prolongs total postoperative survival time[18]. Inguinal sentinel lymph node biopsy may help in assessing status of local lymph node metastasis. Lymph node metastasis usually indicates a poor prognosis and high percentage of distal metastasis. Therapeutic value of this technique remains limited. In this case, existence of the four lesions deprived the possibility for sphincter preserving surgery. APR without ilioingulinal lymph node dissection was eventually performed, because no evidence suggested inguinal lymph node metastasis.
Chemotherapy and radiotherapy might improve survival in cutaneous melanoma according to related studies, but no evidence has been found to prolong total survival time in AM cases[19]. Radiotherapy may be applied as an adjuvant or palliative intervention and may help contribute to local control. Immunotherapy is extrapolated from its research achievements and incorporated into clinical practice[20]. Because mutations are observed in most AM cases, C-Kit is regarded as a viable therapeutic target. Various inhibitors of C-kit have been tested in clinical trials. Monoclonal antibodies targeting CTLA-4, PD-1, and BRAF have also demonstrated significant impact on controlling AM[2].
In this case we report, AM was diagnosed definitely before surgery via colonoscopy and biopsy. We chose to perform APR instead of WLE, because the four lesions distributed in anorectal zone made sphincter preserving radical surgery unachievable. Pathological report suggested multiple AMs with muscular invasion without lymph node metastasis. This case should be classified as stage II with an 11-mo expected survival time according to Falch staging classification. The patient was diagnosed with lung metastasis but still alive 27 mo after surgery, which was significantly longer than expected. Whether it was nivolumab (PD-1 inhibitor) treatment or just individual difference that contributed to the prolonged survival time remains uncertain. Curative effect of nivolumab treatment and other monoclonal antibody induced targeted therapy requires further evidence from clinical trials.
In this article, we report an AM case with multiple synchronous melanomas with different colors, which has never been reported previously. Treatment of AM include R0 surgical resection (APR or WLE), chemotherapy, and immunotherapy. Further clinical research and larger cohort of patients may help to standardize treatment guidelines and improve the prognosis of AM.
| 1. | Meguerditchian AN, Meterissian SH, Dunn KB. Anorectal melanoma: diagnosis and treatment. Dis Colon Rectum. 2011;54:638-644. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 48] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 2. | Bello DM, Smyth E, Perez D, Khan S, Temple LK, Ariyan CE, Weiser MR, Carvajal RD. Anal versus rectal melanoma: does site of origin predict outcome? Dis Colon Rectum. 2013;56:150-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 48] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 3. | Che X, Zhao DB, Wu YK, Wang CF, Cai JQ, Shao YF, Zhao P. Anorectal malignant melanomas: retrospective experience with surgical management. World J Gastroenterol. 2011;17:534-539. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 34] [Cited by in RCA: 30] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 4. | Hillenbrand A, Barth TF, Henne-Bruns D, Formentini A. Anorectal amelanotic melanoma. Colorectal Dis. 2008;10:612-615. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 38] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 5. | Thomas NE, Kricker A, Waxweiler WT, Dillon PM, Busman KJ, From L, Groben PA, Armstrong BK, Anton-Culver H, Gruber SB, Marrett LD, Gallagher RP, Zanetti R, Rosso S, Dwyer T, Venn A, Kanetsky PA, Orlow I, Paine S, Ollila DW, Reiner AS, Luo L, Hao H, Frank JS, Begg CB, Berwick M; Genes, Environment, and Melanoma (GEM) Study Group. Comparison of clinicopathologic features and survival of histopathologically amelanotic and pigmented melanomas: a population-based study. JAMA Dermatol. 2014;150:1306-1314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 143] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 6. | Falch C, Stojadinovic A, Hann-von-Weyhern C, Protic M, Nissan A, Faries MB, Daumer M, Bilchik AJ, Itzhak A, Brücher BL. Anorectal malignant melanoma: extensive 45-year review and proposal for a novel staging classification. J Am Coll Surg. 2013;217:324-335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 48] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 7. | Belli F, Gallino GF, Lo Vullo S, Mariani L, Poiasina E, Leo E. Melanoma of the anorectal region: the experience of the National Cancer Institute of Milano. Eur J Surg Oncol. 2009;35:757-762. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 54] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 8. | Hicks CW, Pappou EP, Magruder JT, Gazer B, Fang S, Wick EC, Gearhart SL, Ahuja N, Efron JE. Clinicopathologic Presentation and Natural History of Anorectal Melanoma: A Case Series of 18 Patients. JAMA Surg. 2014;149:608-611. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 9. | Zhang S, Gao F, Wan D. Abdominoperineal resection or local excision? a survival analysis of anorectal malignant melanoma with surgical management. Melanoma Res. 2010;20:338-341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 10. | Ballo MT, Gershenwald JE, Zagars GK, Lee JE, Mansfield PF, Strom EA, Bedikian AY, Kim KB, Papadopoulos NE, Prieto VG, Ross MI. Sphincter-sparing local excision and adjuvant radiation for anal-rectal melanoma. J Clin Oncol. 2002;20:4555-4558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 97] [Article Influence: 4.0] [Reference Citation Analysis (1)] |
| 11. | Ren M, Lu Y, Lv J, Shen X, Kong J, Dai B, Kong Y. Prognostic factors in primary anorectal melanoma: a clinicopathological study of 60 cases in China. Hum Pathol. 2018;79:77-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 12. | Duarte P, Ramos R, Vicente C, Casteleiro Alves C. Anal melanoma with satellite implantations on the lower rectum. Rev Esp Enferm Dig. 2011;103:49-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 13. | Chen H, Cai Y, Liu Y, He J, Hu Y, Xiao Q, Hu W, Ding K. Incidence, Surgical Treatment, and Prognosis of Anorectal Melanoma From 1973 to 2011: A Population-Based SEER Analysis. Medicine (Baltimore). 2016;95:e2770. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 62] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 14. | Thibault C, Sagar P, Nivatvongs S, Ilstrup DM, Wolff BG. Anorectal melanoma--an incurable disease? Dis Colon Rectum. 1997;40:661-668. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 136] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 15. | Iddings DM, Fleisig AJ, Chen SL, Faries MB, Morton DL. Practice patterns and outcomes for anorectal melanoma in the USA, reviewing three decades of treatment: is more extensive surgical resection beneficial in all patients? Ann Surg Oncol. 2010;17:40-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 100] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 16. | Glowka TR, Keyver-Paik MD, Thiesler T, Landsberg J, Kalff JC, Pantelis D. [Anorectal malignant melanoma : Treatment recommendations]. Chirurg. 2016;87:768-774. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (1)] |
| 17. | Perez DR, Trakarnsanga A, Shia J, Nash GM, Temple LK, Paty PB, Guillem JG, Garcia-Aguilar J, Bello D, Ariyan C, Carvajal RD, Weiser MR. Locoregional lymphadenectomy in the surgical management of anorectal melanoma. Ann Surg Oncol. 2013;20:2339-2344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 41] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 18. | Latteri S, Teodoro M, Malaguarnera M, Mannino M, Currò G, La Greca G. Abdominal perineal resection or wilde local excision in primary anorectal malignant melanoma. Case report and review. Ann Med Surg (Lond). 2017;19:74-77. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 19. | Ishizone S, Koide N, Karasawa F, Akita N, Muranaka F, Uhara H, Miyagawa S. Surgical treatment for anorectal malignant melanoma: report of five cases and review of 79 Japanese cases. Int J Colorectal Dis. 2008;23:1257-1262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 54] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 20. | Miguel I, Freire J, Passos MJ, Moreira A. Anorectal malignant melanoma: retrospective analysis of management and outcome in a single Portuguese Institution. Med Oncol. 2015;32:445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See:
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Coskun A, de Moura DTH S-Editor: Ji FF L-Editor: Wang TQ E-Editor: Xing YX