Published online Jun 6, 2019. doi: 10.12998/wjcc.v7.i11.1302
Peer-review started: January 23, 2019
First decision: January 27, 2019
Revised: February 15, 2019
Accepted: March 16, 2019
Article in press: March 16, 2019
Published online: June 6, 2019
Processing time: 136 Days and 6 Hours
Intertrochanteric fracture (ITF) is a common type of injury, and nearly 30% of ITF patients die in the first 12 mo, especially the elderly with limited activity. Tranexamic acid (TXA) has been widely used in reducing traumatic and surgical bleeding, however, the paucity of studies regarding its use in orthopedic trauma surgery has limited its integration into this field, which may benefit most from TXA. The safety of TXA in this group has not achieved a consensus.
This meta-analysis was designed to investigate the efficacy and safety of TXA in elderly ITF patients undergoing surgery.
Databases, including Medline and PubMed, were searched for randomized controlled trials (RCTs) that were published before October 2018 and that addressed the efficacy and safety of TXA in patients who underwent ITF surgery. The Consolidated Standards of Reporting Trials 2010 Statement Checklist was used to assess the methodological quality of each study. Trials without and with heterogeneity were compared by fixed-effects analysis and random-effects analysis, respectively. For each study, odds ratio (OR) and 95%CI and mean differences and 95%CI were calculated for dichotomous and continuous outcomes, respectively. The Power and Sample Size Program software was used to calculate power and sample size. Stability of the results was assessed via sensitivity analysis.
A total of 836 patients from eight RCTs were subjected to meta-analysis. TXA treatment compared with the control group significantly reduced postoperative blood loss (95%CI, -20.83 to -7.93 mL, P < 0.0001), hidden blood loss (95%CI, -213.67 to -64.43 mL, P = 0.0003), and total blood loss (95%CI, -332.49 to -23.18 mL, P = 0.02) by weighted mean differences of -14.38, -139.05, and -177.83 mL, respectively. However, no significant difference was observed between groups for analysis of intraoperative blood loss. The meta-analysis also proved that the usage of TXA in ITFs may not significantly increase the incidence of deep venous thrombosis. Allogeneic blood transfusion data showed that significantly fewer patients in the TXA group (42%) required transfusion than the control group (95%CI, 0.36 to 0.69; P < 0.0001).
In ITF surgery, intravenous administration of TXA reduces the risk of hidden blood loss and the need for allogeneic transfusion, without increasing thrombotic risk.
Core tip: Intertrochanteric fracture (ITF) is a common type of injury in the elderly population. Although minimally invasive surgical therapy has been routinely performed, the overall blood loss volume may still be much larger than that observed. Tranexamic acid (TXA) has been widely used in reducing surgical bleeding, however, the paucity of studies regarding its use in ITF surgery has limited its integration into this field. Thus, in order to investigate the efficacy and safety of TXA administration in elderly ITF patients, we meta-analyzed the relevant literature regarding the potential risks and benefits of TXA in ITF surgery.
- Citation: Zhou XD, Li J, Fan GM, Huang Y, Xu NW. Efficacy and safety of tranexamic acid in elderly patients with intertrochanteric fracture: An updated meta-analysis. World J Clin Cases 2019; 7(11): 1302-1314
- URL: https://www.wjgnet.com/2307-8960/full/v7/i11/1302.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v7.i11.1302
Hip fractures are a common type of injuries, but the incidence increases rapidly and will surpass 6.3 million by 2050[1,2]. In the United States, more than 250000 hip fractures occur annually and mostly in the elderly, and the 1-year mortality rates range from 14% to 36%, because of the frequently associated osteoporosis[3,4]. As one of the two subgroups of hip fractures (the other subgroup is femoral neck factures), nearly 30% of intertrochanteric fracture (ITF) patients die in the first 12 mo, especially the elderly with limited activity[5]. The major problems for the high mortality are the return to the preoperative level of activity and the dependence in daily routines[6]. Consequently, nearly half of these patients require assistance in daily living activities, and 25% need long-term care after treatments[7]. The functional outcomes and mortality are associated with several factors, especially perioperative anemia and operative blood loss[8,9]. With the improvement of surgical methods, minimally invasive surgical therapy has significantly reduced trauma with reliable efficacy. However, the overall blood loss volume may be much larger than that observed. As reported, the median total blood loss in patients with extra-capsular fracture of the hip is 2100 mL[10]. To prevent and reduce the blood loss during the perioperative period, researchers have proposed various methods, such as permissive hypotension, topical freezing saline, thromboplastic agent, auto-transfusion devices, erythropoietin administration, autologous blood transfusion, and anti-fibrinolytic agents[11-13]. Despite the effectiveness, these methods are still faced by many defects.
Tranexamic acid (TXA), a synthetic derivative of amino acid lysine, competitively inhibits the activation of plasminogen to plasmin, the serine protease, via binding to Kringle domains. TXA is also a competitive inhibitor of tissue plasminogen activator via blocking the lysine-binding sites of plasminogen[14]. Nowadays, TXA has been widely used in reducing traumatic and surgical bleeding[15]. A large trial involving 20,211 adult trauma patients reported that early administration of TXA safely and effectively reduced the risk of death in bleeding trauma patients[16]. If TXA was given to all patients with, or at risk of, traumatic bleeding, this would reduce the number of deaths by 120000 per annum worldwide[16]. However, the optimum time may be within three hours, and otherwise, the treatment would be ineffective. Because of its benefit demonstrated in several clinical trials[17,18], TXA has gained interest in orthopedic and trauma surgery recently. Since significant investigations of its use in joint replacement and spine surgery were reported[15,19], wide incorporation of TXA into the everyday practice of these surgeons has been promoted. Large prospective studies and meta-analyses demonstrate the effectiveness and safety of TXA in total knee and hip arthroplasty[15,20]. Clinical trials prove that TXA is effective in reducing blood loss in spine surgery without incremental risk or complications[21,22]. Although the use of TXA in arthroplasty and spine surgery has been verified and extensively studied, the paucity of studies regarding its use in orthopedic trauma has limited its integration into this field, which may benefit most from TXA.
Recently, more studies focus on the use of TXA in hip fractures[10,23]. Lei et al[23] reported that TXA significantly reduced postoperative hidden blood loss in ITF patients undergoing proximal femoral nail antirotation (PFNA). Tengberg et al[10] found that TXA significantly reduced total blood loss in patients with extracapsular hip fractures. However, the safety of TXA in this group has not achieved a consensus. Thus, in order to investigate and help determine the efficacy and safety of TXA administration in reducing bleeding and transfusion in elderly ITF patients, we meta-analyzed the relevant literature regarding the potential risks and benefits of TXA in ITF surgery.
This meta-analysis was performed according to the ‘Preferred Reporting Items for Systematic Reviews and Meta-Analyses’[24].
This meta-analysis evaluated randomized controlled trials (RCTs) that were published in English and investigated the efficacy and safety of TXA both in and after operation. Studies involving any type of fracture fixation were involved, while other types of surgery including arthroscopy and hemiarthroplasty were excluded. Articles with the diagnosis not being ITF were excluded. Studies providing only abstracts or protocols were excluded. The participants were adults who had undergone ITF fixation, regardless of the type or size of internal fixation material, anesthesia, postoperative care, or different methods or doses of TXA administration. We searched Medline and PubMed for the publications in English (up to October 2018), using the words of “hip fracture”, “intertrochanteric fracture”, and “tranexamic acid”, as well as their extended words, without other limits. Full-texts were obtained if the titles and abstracts did not allow us to include or exclude the studies.
In the meta-analysis, different authors used the search strategy to independently scan the titles and abstracts for appropriate articles. Duplicates were firstly removed on Endnote X6. When any of the above vital information was uncertain, we retrieved the full article for further scrutiny, or directly contacted the authors of individual trials to acquire further information if necessary. The following data were extracted: (1) Name of first author and publication time; (2) Demographics, gender, and age of parti-cipants; (3) Methods and doses of TXA administration; (4) Anesthesia; (5) Operative methods and related information; (6) Follow-up time; (7) Blood loss; (8) Postoperative hemoglobin (Hb) and hematocrit (Hct) changes; (9) Transfusion-related information; and (10) All kinds of complications. The extracted data were then entered independently by two reviewers. Any disagreement was solved by a con-sensus through discussion with a review team. Primary outcomes including blood loss, transfusion, and complications were estimated.
Two of the authors independently assessed the methodological quality of each article according to the Consolidated Standards of Reporting Trials 2010 Statement Checklist (2010 CONSORT statement) for RCTs[25], and the scores ranged from 0 to 25. Disagreements were resolved by discussion. The quality of evidence of outcomes was judged according to the Grading of Recommendations Assessment, Development and Evaluation (GRADE) criteria, with five factors (risk of bias, inconsistency, indire-ctness, imprecision, and publication bias) which may downgrade the quality level of evidence. The recommendation level of evidence was classified into four categories: High, moderate, low, or very low. High quality meant that further research was very unlikely to change the confidence in the estimate of the effect; moderate quality indicated that further research was likely to have an important impact on our confidence in the estimate of the effect and could change the estimate; low quality implied that further research was very likely to have an important impact on our confidence in the estimate of the effect and was likely to change the estimate; and very low quality indicated that we were very uncertain about the estimate[26].
If the data is sparse, or if the test significance is repeated after adding a new trial, a meta-analysis may lead to type I and type II errors[27,28]. TSA is analogous to the interim analysis in a single trial in which the monitoring boundaries are performed to determinate whether it is sufficient for the small value of P to demonstrate the desired results and whether the study should be terminated in advance. Therefore, TSA relies on quantification of the required information size, which can be calculated according to the diversity-adjusted (D2) between trials: 5% risk of type I error, 20% risk of type II error (a power of 80%), and relative risk reduction of 20% with low risk bias (using the data of allele model).
As such, if the Z-curve crosses the TSA monitoring boundary before the required information size is reached, a sufficient level of evidence may have been established and further trials are not needed. Otherwise, continuous trials are necessary to identify the issue.
To perform this meta-analysis, two reviewers independently pooled data from each study for analysis using Review manager 5.0 (Cochrane Collaboration, Oxford, Eng-land). Dichotomous and continuous data were entered as number of events and mean ± SD, respectively. Numerical data and measured data were compared by the t-test and chi-squared test, respectively. Before the data were pooled, statistical hete-rogeneity for each study was assessed by the chi-squared test with significance level at P < 0.1, and quantified by I2[29]. The origin of heterogeneity, if present, was analyzed according to differences in methodological quality, characteristics of participants, and intervention. Trials without and with heterogeneity were compared by fixed-effects analysis and random-effects analysis, respectively. For each study, odds ratio (OR) and 95%CI and mean differences and 95%CI were calculated for dichotomous and continuous outcomes, respectively. The Power and Sample Size Program software was used to calculate power and sample size. The following parameters were used: α, the type Ierror probability for a two-sided test; P0, the probability of exposure in controls; N, the number of case patients; m, the ratio of control to experimental subjects; Ψ, odd ratio of exposure in cases relative to controls. Stability of the results was assessed via sensitivity analysis. If the data was enough, subgroup analysis was conducted to explore possible heterogeneity. P < 0.05 was considered as significance.
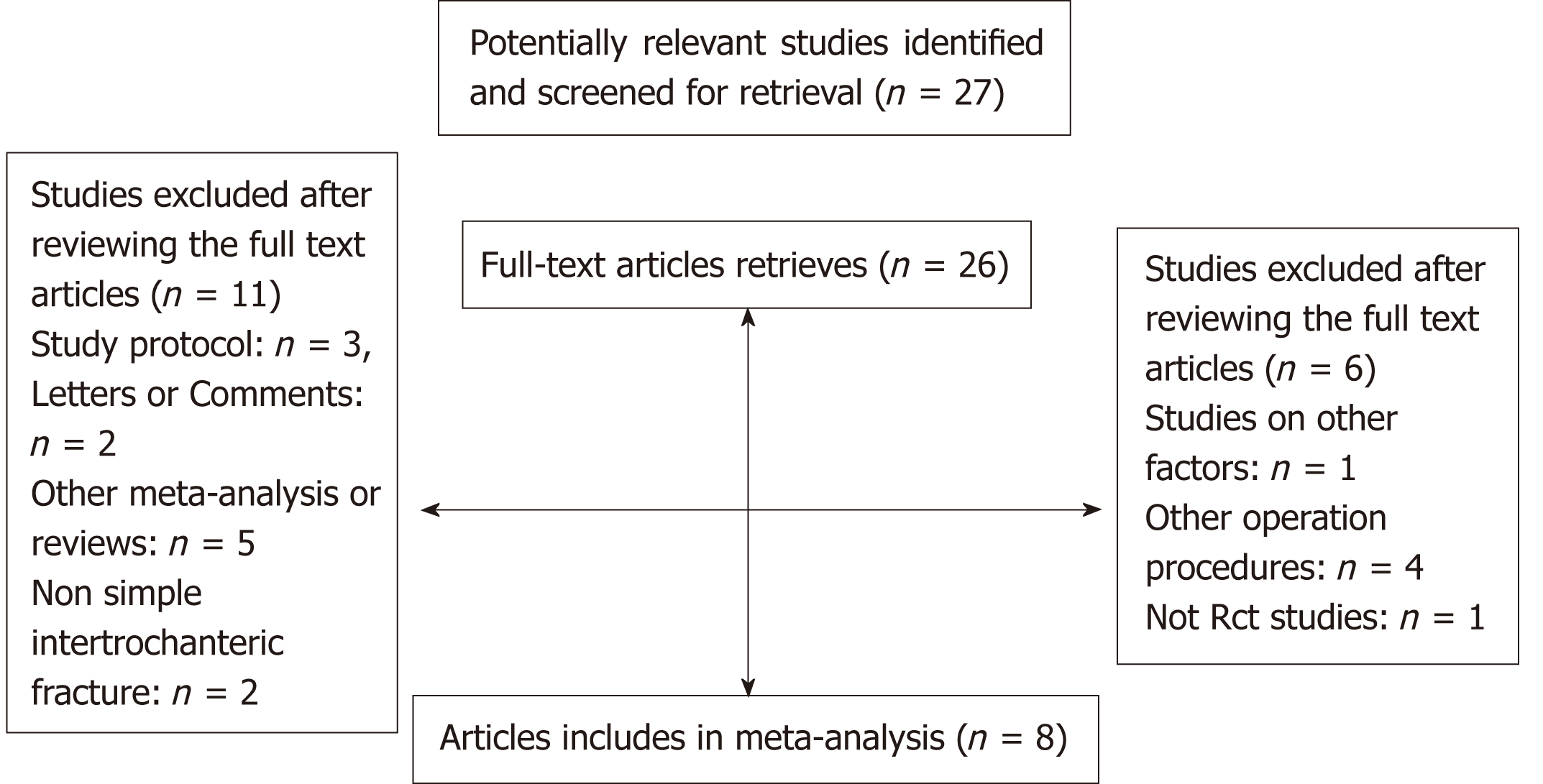
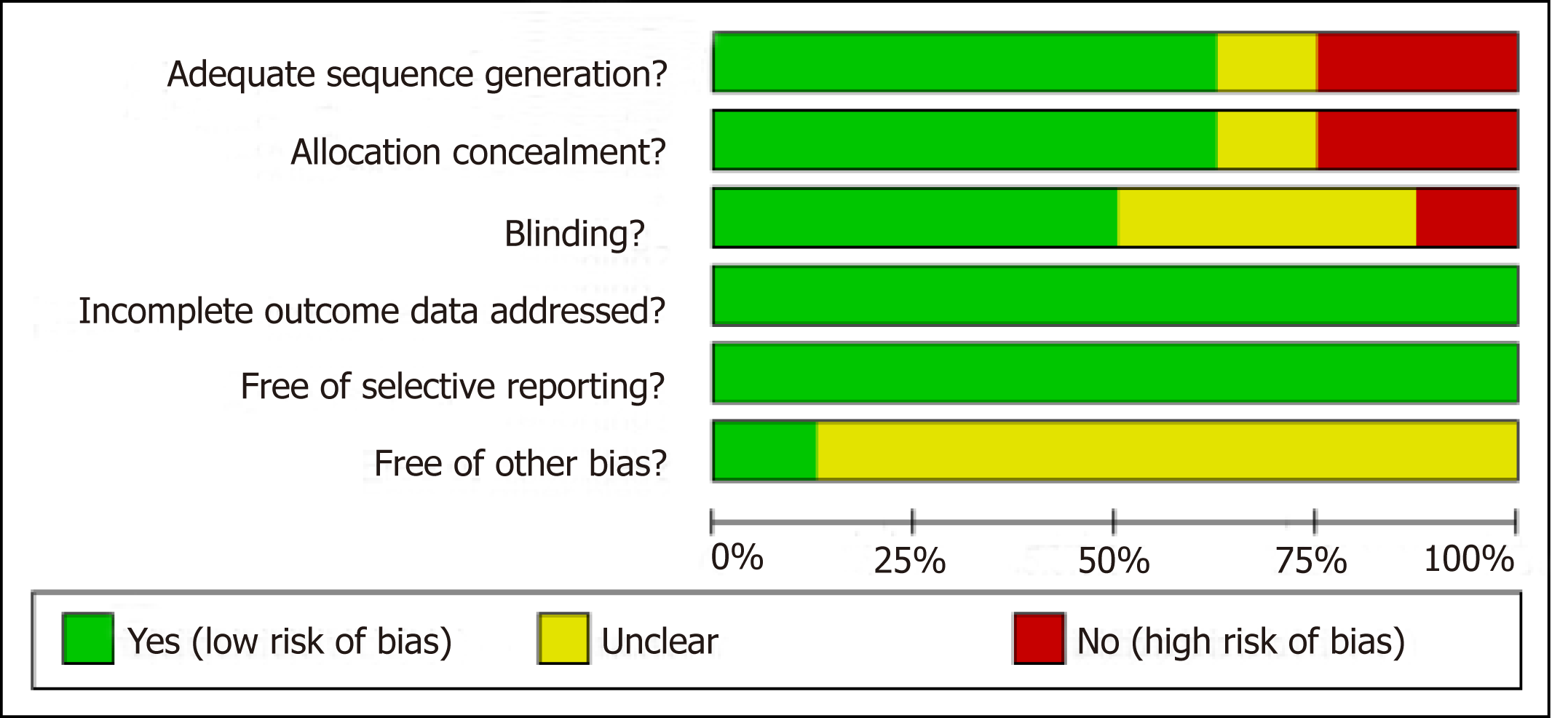
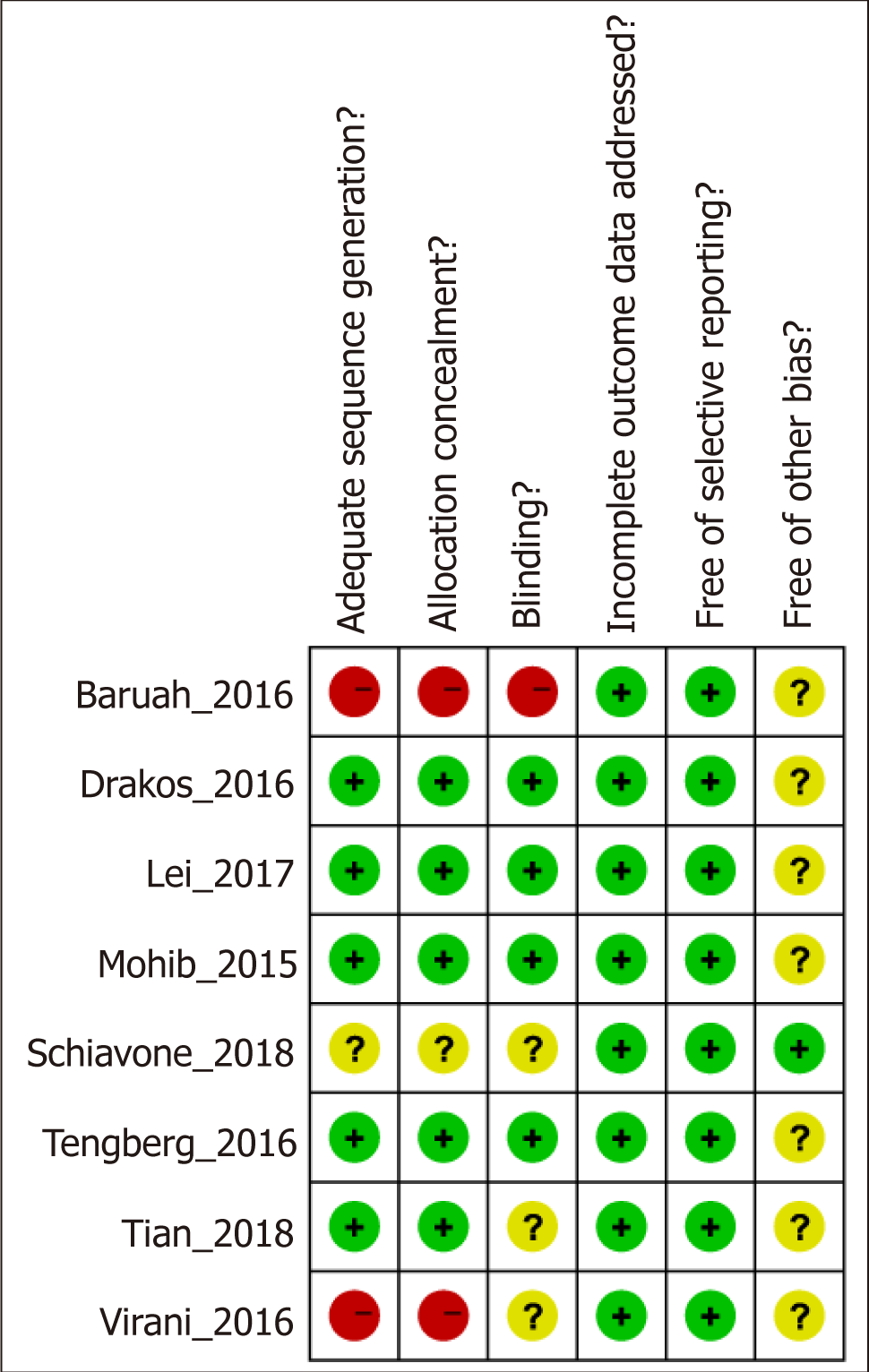
After a detailed evaluation, eight independent RCTs[10,23,30-35] with cumulatively 836 patients were included in the overall meta-analysis (Figure 1). Most of the RCTs were relatively well-designed and their CONSORT adherence scores ranged from 19 to 24, with a maximum score of 25. These eight trials were all focused on ITF patients, most of who underwent intramedullary nail. The characteristics of the included studies are summarized in Table 1, and the methodological quality is illustrated in Figure 2. Judgments about each risk of bias item are presented as percentages across all included studies (Figure 3). Six outcomes in this meta-analysis were evaluated utilizing the GRADE system, and all of them were important or critical, and the quality of the evidence was high for all of the six outcomes (Table 2).
| Study and year | Country | Design | TXA group | Control group | DVT PPX | Sample size (cases/ contros) | No. of females (cases/ controls, %) | Surgical proce-dure | Anesthesia method | Draina-ge | Transfusion trigger | Follow-up | QAS |
| Mohib_2015 | Pakistan | DB-RCT | 15 mg/kg, IV, twice | Normal saline | Enoxapa-rin | 50/50 | 58/52 | Not mention-ed | Not mention-ed | Not mention-ed | < 7g/dL | Dischar-ge | 21 |
| Baruah_2016 | India | RCT | 15 mg/kg, IV | Normal saline | Not mention-ed | 30/30 | 20/16.7 | DHS | Spinal anaesthe-sia | Yes | < 8.5 g/dL | Dischar-ge | 19 |
| Drakos_2016 | Creece | DB-RCT | 3 g, local administration, at the end of surgery | None | LMWH | 100/100 | 73/79 | Short Cephalomedulla-ry Nail (GAMMA3) | Spinal anaesthe-sia | None | < 8 g/dL | 12 mo | 21 |
| Tengber-g_2016 | Denmark | DB-RCT | 1 g, IV, pre-operation; 3 g, IV, post-operation | Placebo | LMWH | 33/39 | 78.7/64.1 | Short Intramedullary Nail (IMH) | Epidural analge-sia | Not mention-ed | < 9.96 g/dL | 4 mo | 24 |
| Virani_2016 | India | RCT | 2 g, intramucsular and subfascial infiltra-tion | None | Not mention-ed | 67/70 | 62/61 | DHS | Spinal anaesthe-sia | Yes | Not mention-ed | Dischar-ge | 20 |
| Lei_2017 | China | RCT | 1 g, IV, pre-operation | Normal saline | Not mention-ed | 37/40 | 82.1/80.4 | PFNA | Not mention-ed | Yes | < 9 g/dL | 1 mo | 20 |
| Tian_2018 | China | RCT | 10 mg/kg IV, pre-operation and post-operation | None | LMWH | 50/50 | 62/72 | Intramedullary nail | Not mention-ed | Yes | < 9 g/dL | 4 mo | 21 |
| Schiavo-ne_2018 | Italy | RCT | 15 mg/kg, IV, start with surgery | Saline | Not mention-ed | 47/43 | 35/28 | Gamma nail | Loco-regional anesthe-sia, or general anesthe-sia | Not mention-ed | < 8.5 g/dL | 3 mo | 20 |
| Outcomes | Illustrative comparative risks1 (95%CI) | Relative effect (95%CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | TXA | |||||
| Intraoperative blood loss. Measurement. Scale: 0 to 5; follow-up: 0.25 to 12 mo. | See comments | See comments | 309 (4 studies) | ++++ high | ||
| Postoperative blood loss Drainage. Scale: 0 to 4; follow-up: 0.25 to 12 mo. | See comments | See comments | 214 (3 studies) | ++++ high | ||
| Hidden blood loss. Calculation. Scale: 0 to 4; follow-up: 0.25 to 12 mo. | See comments | See comments | 177 (2 studies) | ++++ high | ||
| Total blood loss. Measurement. Scale: 0 to 5; follow-up: 0.25 to 12 mo. | See comments | See comments | 309 (4 studies) | ++++ high | ||
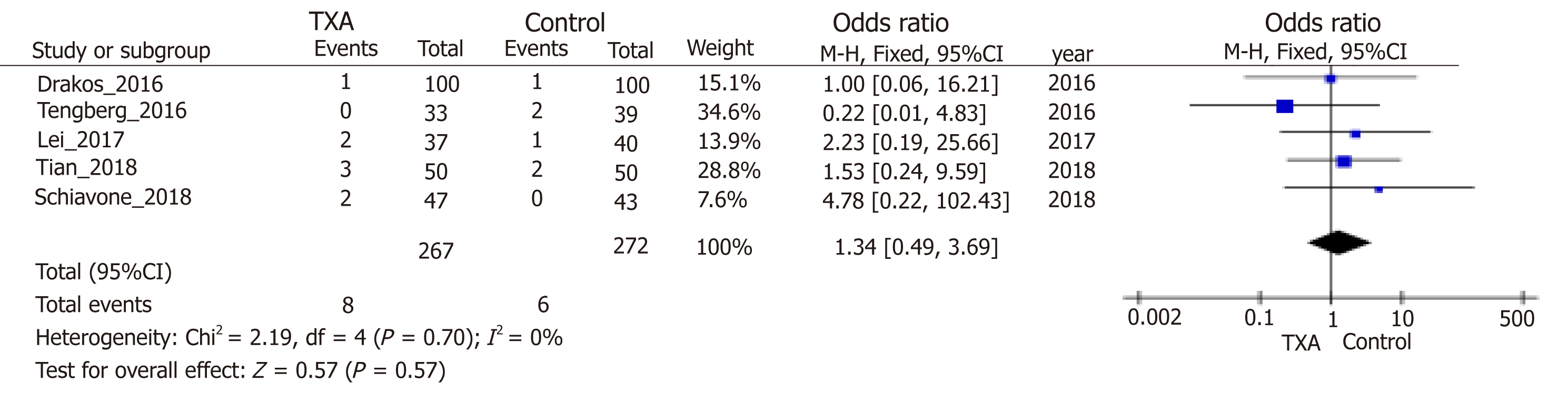
| DVT. Imageological examination; follow-up: 0.25 to 12 mo. | Study population | OR = 1.34 (0.49 to 3.69) | 539 (5 studies) | ++++ high | ||
| 22 per 1000 | 29 per 1000 (11 to 77) | |||||
| Moderate | ||||||
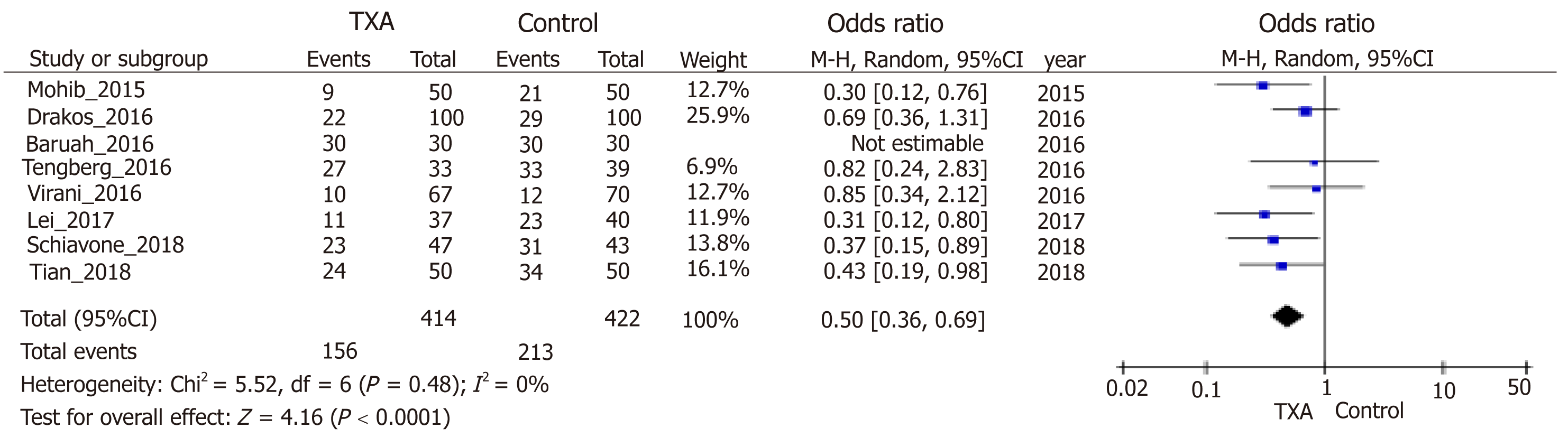
| No. of transfusion. Follow-up; follow-up: 0.25 to 12 mo. | Study population | OR = 0.50 (0.36 to 0.69) | 836 (8 studies) | ++++ high | ||
| 505 per 1000 | 338 per 1000 (268 to 413) | |||||
| Moderate | ||||||
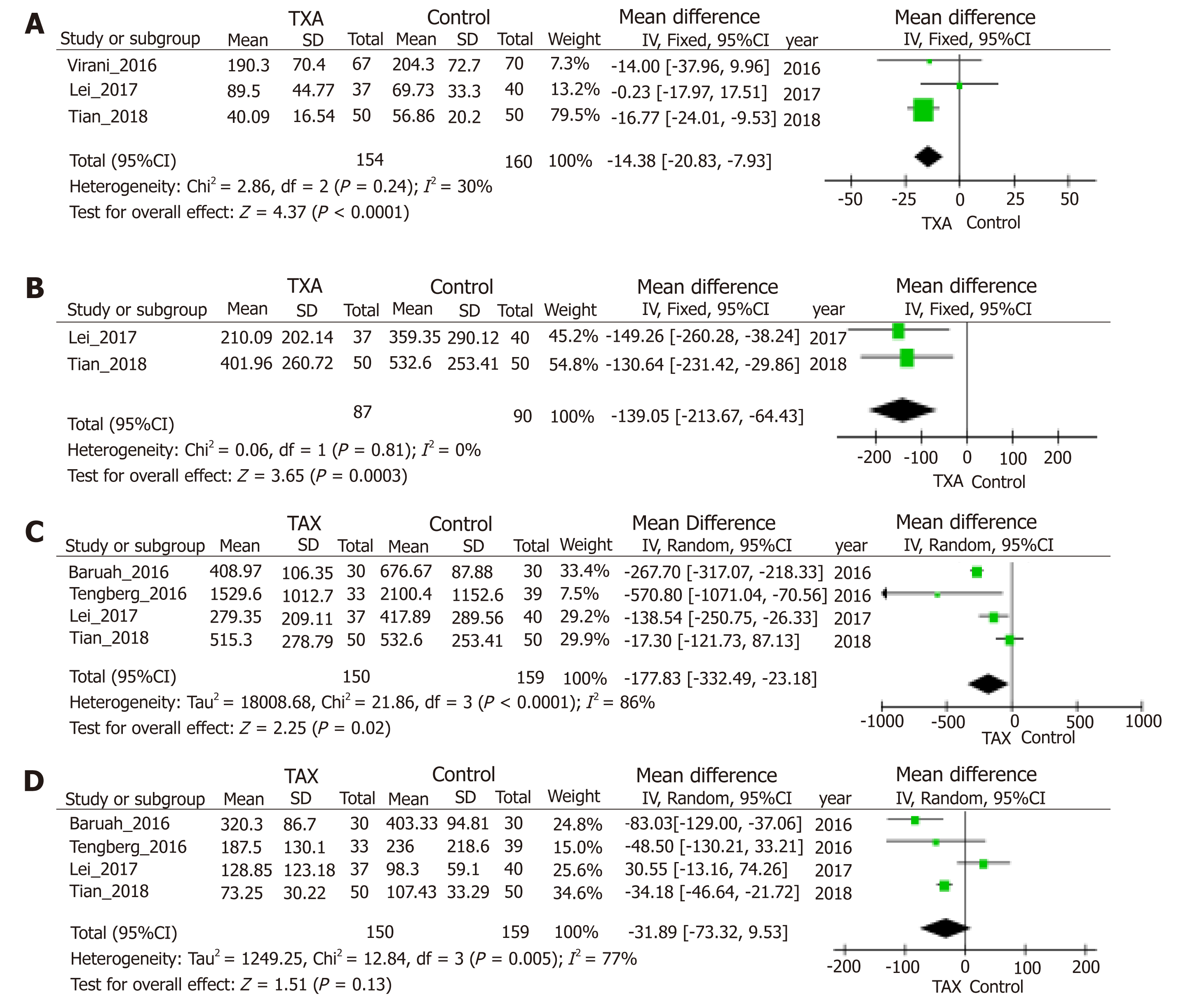
Blood loss was one of the main outcomes and reported in six included stud-ies[10,23,30-33]. TXA treatment compared with the control group significantly reduced postoperative blood loss (95%CI, -20.83 to -7.93 mL, P < 0.0001, Figure 4A), hidden blood loss (95%CI, -213.67 to -64.43 mL, P = 0.0003, Figure 4B), and total blood loss (95%CI, -332.49 to -23.18 mL, P = 0.02, Figure 4C) by weighted mean differences of -14.38, -139.05 and -177.83 mL, respectively. Four studies[10,23,30,32] including 309 patients were eligible for analysis of intraoperative blood loss, but no significant difference was observed between groups (Figure 4D). Analysis of deep venous thrombosis (DVT) from five studies[10,23,30,33,35] showed that the incidence rates of postoperative DVT in the TXA and control groups were 2.99% and 2.20%, respectively. The meta-analysis also proved that the usage of TXA in ITFs may not significantly increase the incidence of DVT (Figure 5). Due to thromboprophylaxis, only two other studies[10,23] reported four cases of pulmonary embolism (PE) during follow-up, and the incidence of PE decreased markedly, but without significant difference. Allogeneic blood transfusion data were provided by eight studies[10,23,30-35], which showed that significantly fewer patients in the TXA group (42%) required transfusion than the control group (95%CI, 0.36 to 0.69; P < 0.0001; Figure 6).
Sensitivity analysis was conducted by deleting one study from overall pooled analysis each time so as to check the influence of the removed data on the overall data set, and no significant changes were found for the outcomes of hidden blood loss, allogeneic blood transfusion data, and thrombotic events. However, with regard to intraoperative blood loss, sensitivity analysis excluding the study of Lei et al[23] resulted in statistical significance (WMD = -50.25, 95%CI, -84.02 to -16.48, P = 0.004). Moreover, concerning postoperative blood loss, sensitivity analysis excluding the study of Tian et al[30] resulted in no statistical significance (WMD = -5.1, 95%CI, -19.36 to9.15, P = 0.48). Regarding total blood loss, sensitivity analysis excluding the study of Baruah et al[32], Tengberg et al[10], or Lei et al[23] all led to no statistical significance.
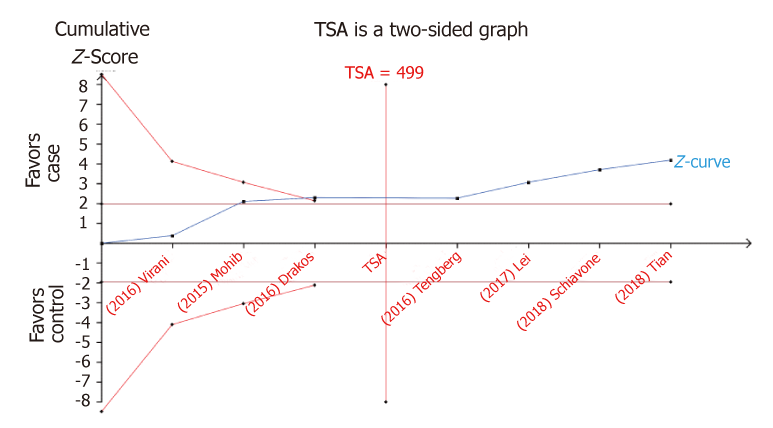
The sample size required to identify the issue using TSA is 499 subjects. Until now, the cumulative Z-curve crossed the trial sequential monitoring boundary, indicating that TXA is associated with decreased risk of ITF and further relevant trials are unnecessary (Figure 7). The TSA-adjusted 95%CI was 0.60 to 0.83. The power analysis indicated that this study had a power of 99.9% to detect the effect of TXA on the risk of transfusion of ITF patients, assuming an OR of 0.71.
TXA has been widely used to reduce traumatic and surgical bleeding, and its safety and effectiveness in total hip and knee arthroplasty[15,36] have also been proved recently. However, there are few studies about its safety and effectiveness in ITF surgery. Thus, in this meta-analysis, it was found the use of TXA could significantly reduce hidden blood losses as well as the number of patients who needed allogeneic transfusions, without increasing the risk of thromboembolism.
TXA is an antifibrinolytic agent that acts by binding to plasminogen and blocking the interaction of plasmin (ogen) with fibrin, thereby preventing the fibrin clot dissolution[37]. On this account, its significant effects of reducing perioperative blood loss have been proved in various surgical procedures, including cardiac surgery with or without cardiopulmonary bypass[38], total hip and knee replacement[15], and prostatectomy[39]. Moreover, TXA can significantly reduce all-cause mortality and death due to bleeding in trauma patients with significant bleeding, particularly when administered early after injury[40]. It is predicted that TXA use in surgery and trauma would be very cost-effective and potentially life-saving[41]. Because of the effective-ness, TXA has been increasingly used in surgical procedures. The secondary research focus is the security, especially the thromboembolism and ischemia event. In this regard, three issues that must be addressed are whether TXA influences the fibrinolytic system postoperatively, whether it also affects prothrombin time, activated partial thromboplastin time, international normalized ratio, and platelets, and whether TXA interacts with thromboprophylaxis agents. So far, the use of TXA in orthopedic surgery focuses on arthroplasties and spinal operation. Also, the outcomes are partially positive: TXA can significantly reduce blood loss and blood transfusion requirements, without intensifying the risk of thromboprophylaxis[15,36,42]. However, studies about the use of TXA in fracture surgery are still few.
Femoral ITFs are clinically one group of common fractures and especially attack the elderly. The global number of hip fractures will increase from 1.66 million in 1990 to 6.26 million in 2050[43]. Surgery is used for almost all femoral ITFs. Blood loss occurs as a consequence of both the fracture and surgery and thus RBC transfusion is frequently used. However, blood transfusions are correlated with an increased risk of bacterial infections, possibly increased mortality, and the substantial costs involved in the blood collection, preparation, transport, and administration[44,45]. The incidence of DVT is as high as 80% in femoral ITF patients[46]. Therefore, investigating the efficacy and safety of TXA in femoral ITF surgery is of great significance. As reported, TXA reduces erythrocyte transfusion but may promote a hypercoagulable state[47]. Moreover, its efficacy is lower than observed in hip or knee arthroplasty. Three studies[10,33,34] similarly confirm the safety and effectiveness of TXA administration in elderly patients undergoing ITF surgery. Blood loss, transfused blood units, and health care cost can be significantly reduced. Two other studies[23,30] and our trial verify the effectiveness of TXA in reducing hidden blood loss. Out outcomes are consistent with previous studies, although some complications, including DVT, PE, myocardial infarction, ischemic cerebral infarction, hematoma, and infection, were observed. However, we were unable to ascertain a reliable cause of these compli-cations, especially when there were no significant differences between groups, and only small dosage was used during the operation. The conclusions also were notarized by our meta-analysis. Our meta-analysis involving 836 participants from eight RCTs is an update of another meta-analysis[48] involving only four studies and 514 participants, but the findings are similar.
Despite the effectiveness of TXA in femoral ITF, there are still some problems to be solved. In our meta-analysis involving eight RCTs, heterogeneity or confounding factors still exist. Five studies[10,23,30,33,35] and two studies[31,32] reported the use of intramedullary nail and dynamic hip screw (DHS), respectively, but one study[34] referred to nothing. Subgroup analyses showed no significant difference between groups in the number of patients who needed transfusion with DHS (data not supplied). Moreover, the methods and dosages used in different studies are both inconsistent. Intravenous use was reported in six studies[10,23,30,32,34,35], and the dosages ranged from 1 to 4 g in total. We found the maximal WMD of 570.8 mL compared with the largest dosage of 4 g (Figure 4C), implying that the better effectiveness accords with the larger dose on the premise of safety. Two other studies[31,33] reported the local or intramuscular administration around the wound, and only one study[33] found the effectiveness in reducing the blood loss and need of transfusion. It was concluded that TXA did not play a significant role in reducing postoperative blood loss or blood transfusion when used locally in femoral ITF surgery[31]. At last, the outcomes of sensitivity analyses showed the heterogeneity and instability of the outcomes of blood loss, except the hidden blood loss, which might be caused by different intervention conditions, including drainage, surgical procedures, time of operation and so on. Generally, our study verifies the effectiveness of intravenous TXA in femoral ITF, but the safety still needs more experimental verification. The use of method and dosage should also be investigated in future.
This study has some limitations. First, our meta-analysis included only eight RCTs published in English, with no unpublished data, which may lead to publication bias. Second, the differences in autotransfusion protocol, surgical techniques, drainage, low-molecular-weight heparin, operative time, dosage, and mode of administration may all contribute to between-study differences in the outcomes. Third, in most of the included studies, the follow-up time only lasted to discharge, which made long-term evaluation indices (e.g., DVT and PE) unavailable.
In conclusion, our study suggests the use of TXA in ITF surgery significantly reduced the risk of hidden blood losses as well as the need for allogeneic transfusion, without increasing other complications, especially DVT, particularly for intravenous use. However, larger high-quality prospective trials are required to strengthen our conclusions, define the optimal regimen, and assess the safety and cost-effectiveness of TXA before its use is recommended in ITF surgery.
Intertrochanteric fracture is a common type of injury, and nearly 30% of intertrochanteric fracture patients die in the first 12 mo, especially the elderly with limited activity. With the improvement of surgical methods, minimal invasive surgical therapy has significantly reduced trauma with reliable efficacy. Anyway, the overall blood loss volume may be much larger than that observed. Tranexamic acid has been widely used in reducing traumatic and surgical bleeding, however, the paucity of studies regarding its use in orthopedic trauma has limited its integration into this field, which may benefit most from tranexamic acid. The safety of tranexamic acid in this group has not achieved a consensus.
Recently, the impact of tranexamic acid on intertrochanteric fracture surgery has been controversial due to several studies. For instance, some studies reported that the association between tranexamic acid and intertrochanteric fracture was significant, while others reported the opposite conclusion.
To date, although several studies focus on the use of tranexamic acid in hip fractures, the results have been controversial and limited. Thus, in order to investigate and help determine the efficacy and safety of tranexamic acid administration in reducing bleeding and transfusion in elderly intertrochanteric fracture patients, we meta-analyzed the relevant literature regarding the potential risks and benefits of tranexamic acid in intertrochanteric fracture surgery.
We searched Medline and PubMed for the publications in English (up to October 2018), that focused on the effectiveness and safety of tranexamic acid on the intertrochanteric fracture. The Consolidated Standards of Reporting Trials 2010 Statement Checklist was used to assess the methodological quality of each study. Trials without and with heterogeneity were compared by fixed-effects analysis and random-effects analysis, respectively. For each study, odds ratio (OR) and 95%CI and mean differences and 95%CI were calculated for dichotomous and continuous outcomes, respectively. The Power and Sample Size Program software was used to calculate power and sample size. Stability of the results was assessed via sensitivity analysis.
After a detailed evaluation, eight independent randomized controlled trials with cumulatively 836 patients were included in the overall meta-analysis. Tranexamic acid treatment compared with the control group significantly reduced postoperative blood loss (95%CI, -20.83 to -7.93 mL, P < 0.0001), hidden blood loss (95%CI, -213.67 to -64.43 mL, P = 0.0003), and total blood loss (95%CI, -332.49 to -23.18 mL, P = 0.02) by weighted mean differences of -14.38, -139.05, and -177.83 mL, respectively. But no significant difference was observed between groups for analysis of intraoperative blood loss. The meta-analysis also proved that the usage of tranexamic acid in intertrochanteric fractures may not significantly increase the incidence of deep vein thrombosis. Allogeneic blood transfusion data showed that significantly fewer patients in the tranexamic acid group (42%) required transfusion than the control group (95%CI, 0.36 to 0.69; P < 0.0001).
Our study suggests the use of tranexamic acid in intertrochanteric fracture surgery significantly reduced the risk of hidden blood losses as well as the need for allogeneic transfusion, without increasing other complications, especially deep vein thrombosis, particularly for intravenous use. However, larger high-quality prospective trials are required to strengthen our conclusions, define the optimal regimen, and assess the safety and cost-effectiveness of tranexamic acid before its use is recommended in intertrochanteric fracture surgery.
| 1. | Gullberg B, Johnell O, Kanis JA. World-wide projections for hip fracture. Osteoporos Int. 1997;7:407-413. [PubMed] |
| 2. | Miyamoto RG, Kaplan KM, Levine BR, Egol KA, Zuckerman JD. Surgical management of hip fractures: an evidence-based review of the literature. I: femoral neck fractures. J Am Acad Orthop Surg. 2008;16:596-607. [PubMed] |
| 3. | Zuckerman JD. Hip fracture. N Engl J Med. 1996;334:1519-1525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 496] [Cited by in RCA: 497] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 4. | Sheehan SE, Shyu JY, Weaver MJ, Sodickson AD, Khurana B. Proximal Femoral Fractures: What the Orthopedic Surgeon Wants to Know. Radiographics. 2015;35:1563-1584. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 80] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 5. | Panula J, Pihlajamäki H, Mattila VM, Jaatinen P, Vahlberg T, Aarnio P, Kivelä SL. Mortality and cause of death in hip fracture patients aged 65 or older: a population-based study. BMC Musculoskelet Disord. 2011;12:105. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 319] [Cited by in RCA: 420] [Article Influence: 28.0] [Reference Citation Analysis (0)] |
| 6. | Blomfeldt R, Törnkvist H, Eriksson K, Söderqvist A, Ponzer S, Tidermark J. A randomised controlled trial comparing bipolar hemiarthroplasty with total hip replacement for displaced intracapsular fractures of the femoral neck in elderly patients. J Bone Joint Surg Br. 2007;89:160-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 254] [Cited by in RCA: 263] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 7. | Lu-Yao GL, Keller RB, Littenberg B, Wennberg JE. Outcomes after displaced fractures of the femoral neck. A meta-analysis of one hundred and six published reports. J Bone Joint Surg Am. 1994;76:15-25. [PubMed] |
| 8. | Gregersen M, Borris LC, Damsgaard EM. Postoperative blood transfusion strategy in frail, anemic elderly patients with hip fracture: the TRIFE randomized controlled trial. Acta Orthop. 2015;86:363-372. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 75] [Cited by in RCA: 82] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 9. | Zhang L, Yin P, Lv H, Long A, Gao Y, Zhang L, Tang P. Anemia on Admission Is an Independent Predictor of Long-Term Mortality in Hip Fracture Population: A Prospective Study With 2-Year Follow-Up. Medicine (Baltimore). 2016;95:e2469. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 25] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 10. | Tengberg PT, Foss NB, Palm H, Kallemose T, Troelsen A. Tranexamic acid reduces blood loss in patients with extracapsular fractures of the hip: results of a randomised controlled trial. Bone Joint J. 2016;98-B:747-753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 71] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 11. | Hughes NT, Burd RS, Teach SJ. Damage control resuscitation: permissive hypotension and massive transfusion protocols. Pediatr Emerg Care. 2014;30:651-6; quiz 657-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 19] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 12. | Rüegger CM, Hagmann CF, Bührer C, Held L, Bucher HU, Wellmann S; EpoRepair Investigators. Erythropoietin for the Repair of Cerebral Injury in Very Preterm Infants (EpoRepair). Neonatology. 2015;108:198-204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 13. | Scully C, Robinson NA. Anti-thrombotic agents. Br Dent J. 2015;219:515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 14. | Astedt B. Clinical pharmacology of tranexamic acid. Scand J Gastroenterol Suppl. 1987;137:22-25. [PubMed] |
| 15. | Zhou XD, Tao LJ, Li J, Wu LD. Do we really need tranexamic acid in total hip arthroplasty? A meta-analysis of nineteen randomized controlled trials. Arch Orthop Trauma Surg. 2013;133:1017-1027. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 107] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 16. | Roberts I, Shakur H, Coats T, Hunt B, Balogun E, Barnetson L, Cook L, Kawahara T, Perel P, Prieto-Merino D, Ramos M, Cairns J, Guerriero C. The CRASH-2 trial: a randomised controlled trial and economic evaluation of the effects of tranexamic acid on death, vascular occlusive events and transfusion requirement in bleeding trauma patients. Health Technol Assess. 2013;17:1-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 306] [Cited by in RCA: 439] [Article Influence: 33.8] [Reference Citation Analysis (0)] |
| 17. | Chen TT, Jiandong-Liu, Wang G, Jiang SL, Li LB, Gao CQ. Combined treatment of ulinastatin and tranexamic acid provides beneficial effects by inhibiting inflammatory and fibrinolytic response in patients undergoing heart valve replacement surgery. Heart Surg Forum. 2013;16:E38-E47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 22] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 18. | Hasegawa T, Oshima Y, Maruo A, Matsuhisa H, Tanaka A, Noda R, Yokoyama S, Iwasaki K. Intraoperative tranexamic acid in pediatric bloodless cardiac surgery. Asian Cardiovasc Thorac Ann. 2014;22:1039-1045. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 19. | Wang W, Duan K, Ma M, Jiang Y, Liu T, Liu J, Hao D. Tranexamic Acid Decreases Visible and Hidden Blood Loss Without Affecting Prethrombotic State Molecular Markers in Transforaminal Thoracic Interbody Fusion for Treatment of Thoracolumbar Fracture-Dislocation. Spine (Phila Pa 1976). 2018;43:E734-E739. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 40] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 20. | Poeran J, Rasul R, Suzuki S, Danninger T, Mazumdar M, Opperer M, Boettner F, Memtsoudis SG. Tranexamic acid use and postoperative outcomes in patients undergoing total hip or knee arthroplasty in the United States: retrospective analysis of effectiveness and safety. BMJ. 2014;349:g4829. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 286] [Cited by in RCA: 341] [Article Influence: 28.4] [Reference Citation Analysis (0)] |
| 21. | Cheriyan T, Maier SP 2nd, Bianco K, Slobodyanyuk K, Rattenni RN, Lafage V, Schwab FJ, Lonner BS, Errico TJ. Efficacy of tranexamic acid on surgical bleeding in spine surgery: a meta-analysis. Spine J. 2015;15:752-761. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 201] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 22. | Raksakietisak M, Sathitkarnmanee B, Srisaen P, Duangrat T, Chinachoti T, Rushatamukayanunt P, Sakulpacharoen N. Two Doses of Tranexamic Acid Reduce Blood Transfusion in Complex Spine Surgery: A Prospective Randomized Study. Spine (Phila Pa 1976). 2015;40:E1257-E1263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 57] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 23. | Lei J, Zhang B, Cong Y, Zhuang Y, Wei X, Fu Y, Wei W, Wang P, Wen S, Huang H, Wang H, Han S, Liu S, Zhang K. Tranexamic acid reduces hidden blood loss in the treatment of intertrochanteric fractures with PFNA: a single-center randomized controlled trial. J Orthop Surg Res. 2017;12:124. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 69] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 24. | Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13930] [Cited by in RCA: 13810] [Article Influence: 812.4] [Reference Citation Analysis (3)] |
| 25. | Schulz KF, Altman DG, Moher D; CONSORT Group. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. BMJ. 2010;340:c332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5694] [Cited by in RCA: 6982] [Article Influence: 436.4] [Reference Citation Analysis (108)] |
| 26. | Atkins D, Best D, Briss PA, Eccles M, Falck-Ytter Y, Flottorp S, Guyatt GH, Harbour RT, Haugh MC, Henry D, Hill S, Jaeschke R, Leng G, Liberati A, Magrini N, Mason J, Middleton P, Mrukowicz J, O'Connell D, Oxman AD, Phillips B, Schünemann HJ, Edejer T, Varonen H, Vist GE, Williams JW, Zaza S; GRADE Working Group. Grading quality of evidence and strength of recommendations. BMJ. 2004;328:1490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5096] [Cited by in RCA: 6201] [Article Influence: 281.9] [Reference Citation Analysis (1)] |
| 27. | Wetterslev J, Thorlund K, Brok J, Gluud C. Trial sequential analysis may establish when firm evidence is reached in cumulative meta-analysis. J Clin Epidemiol. 2008;61:64-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1232] [Cited by in RCA: 1490] [Article Influence: 78.4] [Reference Citation Analysis (0)] |
| 28. | Brok J, Thorlund K, Wetterslev J, Gluud C. Apparently conclusive meta-analyses may be inconclusive--Trial sequential analysis adjustment of random error risk due to repetitive testing of accumulating data in apparently conclusive neonatal meta-analyses. Int J Epidemiol. 2009;38:287-298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 657] [Cited by in RCA: 762] [Article Influence: 42.3] [Reference Citation Analysis (0)] |
| 29. | Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539-1558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21630] [Cited by in RCA: 27073] [Article Influence: 1128.0] [Reference Citation Analysis (0)] |
| 30. | Tian S, Shen Z, Liu Y, Zhang Y, Peng A. The effect of tranexamic acid on hidden bleeding in older intertrochanteric fracture patients treated with PFNA. Injury. 2018;49:680-684. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 60] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 31. | Virani SR, Dahapute AA, Panda I, Bava SS. Role of Local Infiltration of Tranexamic Acid in Reducing Blood Loss in Peritrochanteric Fracture Surgery in the Elderly Population. Malays Orthop J. 2016;10:26-30. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 32] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 32. | Baruah RK, Borah PJ, Haque R. Use of tranexamic acid in dynamic hip screw plate fixation for trochanteric fractures. J Orthop Surg (Hong Kong). 2016;24:379-382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 36] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 33. | Drakos A, Raoulis V, Karatzios K, Doxariotis N, Kontogeorgakos V, Malizos K, Varitimidis SE. Efficacy of Local Administration of Tranexamic Acid for Blood Salvage in Patients Undergoing Intertrochanteric Fracture Surgery. J Orthop Trauma. 2016;30:409-414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 56] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 34. | Mohib Y, Rashid RH, Ali M, Zubairi AJ, Umer M. Does tranexamic acid reduce blood transfusion following surgery for inter-trochanteric fracture? A randomized control trial. J Pak Med Assoc. 2015;65:S17-S20. [PubMed] |
| 35. | Schiavone A, Bisaccia M, Inkov I, Rinonapoli G, Manni M, Rollo G, Meccariello L, Vicente CI, Ceccarini P, Ruggiero C, Caraffa A. Tranexamic Acid in Pertrochanteric Femoral Fracture: Is it a Safe Drug or Not? Folia Med (Plovdiv). 2018;60:67-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 28] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 36. | Yang ZG, Chen WP, Wu LD. Effectiveness and safety of tranexamic acid in reducing blood loss in total knee arthroplasty: a meta-analysis. J Bone Joint Surg Am. 2012;94:1153-1159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 270] [Cited by in RCA: 310] [Article Influence: 22.1] [Reference Citation Analysis (0)] |
| 37. | McCormack PL. Tranexamic acid: a review of its use in the treatment of hyperfibrinolysis. Drugs. 2012;72:585-617. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 360] [Cited by in RCA: 474] [Article Influence: 33.9] [Reference Citation Analysis (0)] |
| 38. | Fiechtner BK, Nuttall GA, Johnson ME, Dong Y, Sujirattanawimol N, Oliver WC, Sarpal RS, Oyen LJ, Ereth MH. Plasma tranexamic acid concentrations during cardiopulmonary bypass. Anesth Analg. 2001;92:1131-1136. [PubMed] |
| 39. | Crescenti A, Borghi G, Bignami E, Bertarelli G, Landoni G, Casiraghi GM, Briganti A, Montorsi F, Rigatti P, Zangrillo A. Intraoperative use of tranexamic acid to reduce transfusion rate in patients undergoing radical retropubic prostatectomy: double blind, randomised, placebo controlled trial. BMJ. 2011;343:d5701. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 83] [Cited by in RCA: 99] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 40. | Williams-Johnson JA, McDonald AH, Strachan GG, Williams EW. Effects of tranexamic acid on death, vascular occlusive events, and blood transfusion in trauma patients with significant haemorrhage (CRASH-2) A randomised, placebo-controlled trial. West Indian Med J. 2010;59:612-624. [PubMed] |
| 41. | Guerriero C, Cairns J, Perel P, Shakur H, Roberts I; CRASH 2 trial collaborators. Cost-effectiveness analysis of administering tranexamic acid to bleeding trauma patients using evidence from the CRASH-2 trial. PLoS One. 2011;6:e18987. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 144] [Cited by in RCA: 144] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 42. | Elwatidy S, Jamjoom Z, Elgamal E, Zakaria A, Turkistani A, El-Dawlatly A. Efficacy and safety of prophylactic large dose of tranexamic acid in spine surgery: a prospective, randomized, double-blind, placebo-controlled study. Spine (Phila Pa 1976). 2008;33:2577-2580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 166] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 43. | Dennison E, Mohamed MA, Cooper C. Epidemiology of osteoporosis. Rheum Dis Clin North Am. 2006;32:617-629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 133] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 44. | Carson JL, Altman DG, Duff A, Noveck H, Weinstein MP, Sonnenberg FA, Hudson JI, Provenzano G. Risk of bacterial infection associated with allogeneic blood transfusion among patients undergoing hip fracture repair. Transfusion. 1999;39:694-700. [PubMed] |
| 45. | Vincent JL, Baron JF, Reinhart K, Gattinoni L, Thijs L, Webb A, Meier-Hellmann A, Nollet G, Peres-Bota D; ABC (Anemia and Blood Transfusion in Critical Care) Investigators. Anemia and blood transfusion in critically ill patients. JAMA. 2002;288:1499-1507. [PubMed] |
| 46. | Geerts WH, Code KI, Jay RM, Chen E, Szalai JP. A prospective study of venous thromboembolism after major trauma. N Engl J Med. 1994;331:1601-1606. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1113] [Cited by in RCA: 1091] [Article Influence: 34.1] [Reference Citation Analysis (0)] |
| 47. | Zufferey PJ, Miquet M, Quenet S, Martin P, Adam P, Albaladejo P, Mismetti P, Molliex S; tranexamic acid in hip-fracture surgery (THIF) study. Tranexamic acid in hip fracture surgery: a randomized controlled trial. Br J Anaesth. 2010;104:23-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 166] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 48. | Wang W, Yu J. Tranexamic acid reduces blood loss in intertrochanteric fractures: A meta-analysis from randomized controlled trials. Medicine (Baltimore). 2017;96:e9396. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 14] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See:
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Anand A, Emara KM, Tangtrakulwanich B S-Editor: Gong ZM L-Editor: Wang TQ E-Editor: Wang J