Published online Dec 6, 2018. doi: 10.12998/wjcc.v6.i15.1067
Peer-review started: August 14, 2018
First decision: October 5, 2018
Revised: November 5, 2018
Accepted: November 7, 2018
Article in press: November 7, 2018
Published online: December 6, 2018
Processing time: 118 Days and 2.8 Hours
Plexiform fibromyxoma is a rare, special type of mesenchymal tumor. The most common presenting symptoms are anemia, hematemesis, and hematochezia, without sex or age predilection. The reported cases have mainly occurred in the gastric antrum and pylorus region, with some cases in the duodenum.
We report here a case of plexiform fibromyxoma in the upper segment of the jejunum, which was continuously followed up for 3 years after surgical removal. Plexiform fibromyxoma showed multinodular or plexiform growth. The cells in the tumor node were spindle-shaped but few in number and mitotic figures. Small blood vessels and mucous matrix were found among the tumor cells. Immunohistochemistry revealed that the plexiform fibromyxoma cells were positive for smooth muscle actin, focally positive for CD10, and negative for cytokeratin, CD117, DOG-1 (discovered on GIST-1) desmin, S-100, epithelial membrane antigen, and CD34. Ki-67 labeling index was < 5%. Plexiform fibromyxoma showed benign biological behavior. After 3 years of consecutive postoperative follow-up, no obvious signs of metastasis or recurrence were found by imaging examination.
Plexiform fibromyxoma is a rare type of mesenchymal tumor. The diagnosis mainly depends on pathological examination, and it should be distinguished from other gastrointestinal mesenchymal tumors.
Core tip: Plexiform fibromyxoma is a rare, special type of mesenchymal tumor. It is reported to occur mainly in the gastric antrum and pylorus region, but it may also occur in the duodenum. We here report a case of plexiform fibromyxoma in the upper segment of the jejunum, which was continuously followed up for 3 years after surgical removal. No obvious signs of metastasis or recurrence were found by imaging examination.
- Citation: Zhang WG, Xu LB, Xiang YN, Duan CH. Plexiform fibromyxoma of the small bowel: A case report. World J Clin Cases 2018; 6(15): 1067-1072
- URL: https://www.wjgnet.com/2307-8960/full/v6/i15/1067.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v6.i15.1067
Plexiform fibromyxoma is a rare type of mesenchymal tumor. It was known as plexiform angiomyxoid myofibroblastic tumor (PAMT), which was first described by Takahashi et al[1] in 2007. In 2009, Miettinen et al[2] reported benign gastric antral fibromyxoid tumors and designated them as plexiform fibromyxoma. It was classified as a gastrointestinal mesenchymal tumor in the 2010 World Health Organization Classification of Digestive System Neoplasms[3] and was termed plexiform fibromyxoma. Many scholars, however, still prefer using PAMT rather than plexiform fibromyxoma[4,5].
Plexiform fibromyxoma has a wide range of onset age, without sex or age predilection. So far, > 60 cases of PAMT or plexiform fibromyxoma have been reported worldwide[5]. The most common presenting symptoms are anemia, hematemesis, and hematochezia. The reported cases have mainly occurred in the gastric antrum and pylorus region, with some cases in the duodenum[6]. We here report a case of plexiform fibromyxoma in the upper segment of the jejunum, which was continuously followed up for 3 years after surgical removal.
A 31-year-old woman with repeated hematochezia and syncope without obvious cause for 20 d presented to a local hospital for treatment in 2013. No discomfort, such as hematemesis or abdominal pain, was found at disease onset. Gastroscopic examinations performed in the local hospital showed no evidence of upper gastrointestinal hemorrhage. However, her condition was not improved after inpatient care, so she was transferred to our hospital after 2 d. Physical examination on admission showed no abnormal signs except for pale appearance. Routine blood examination indicated anemia (red blood cell count 2.18 T/L, hemoglobin 51.0 g/L, and mean cell hemoglobin 22.90 pg). One gastroscopic examination and one colonoscopic examination performed on the same day failed to find the hemorrhagic focus. In order to avoid more severe gastrointestinal bleeding, the patient was not prepared for intestinal cleaning.
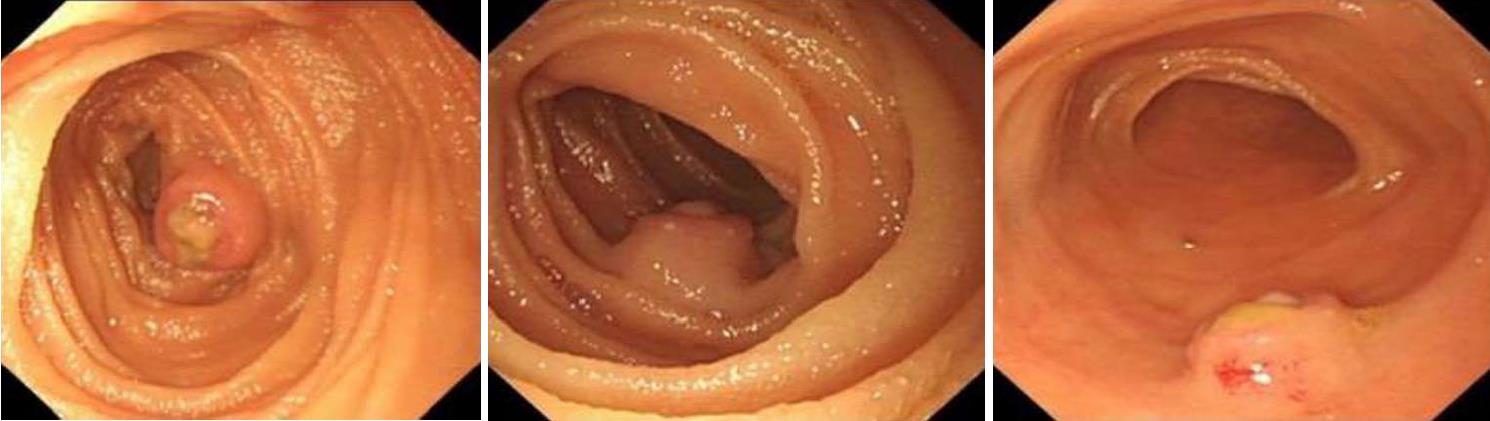
No abnormalities were found by B-ultrasound examination of the upper abdomen, enhanced computed tomography (CT) of the abdomen, and CT angiography (CTA) of the small intestine. The patient stopped bleeding after 3 d in the hospital. We suspected that the hemorrhage was caused by small intestinal disease. Therefore, we performed capsule endoscopic examination after cleaning the intestinal tract after 2 d. Capsule endoscopic examination revealed one protuberant lesion about 1.2 cm × 1.0 cm in the upper segment of the jejunum; the margin of which was unclear. Ulceration was found at the top, which was covered with uneven white necrotic substance (Figure 1). Single balloon enteroscopy was performed to determine further the position of the lesion. This indicated that the protuberant lesion was located about 100 cm away from the duodenal papilla, and its size and morphology were consistent with the findings of capsule endoscopic examination (Figure 2).
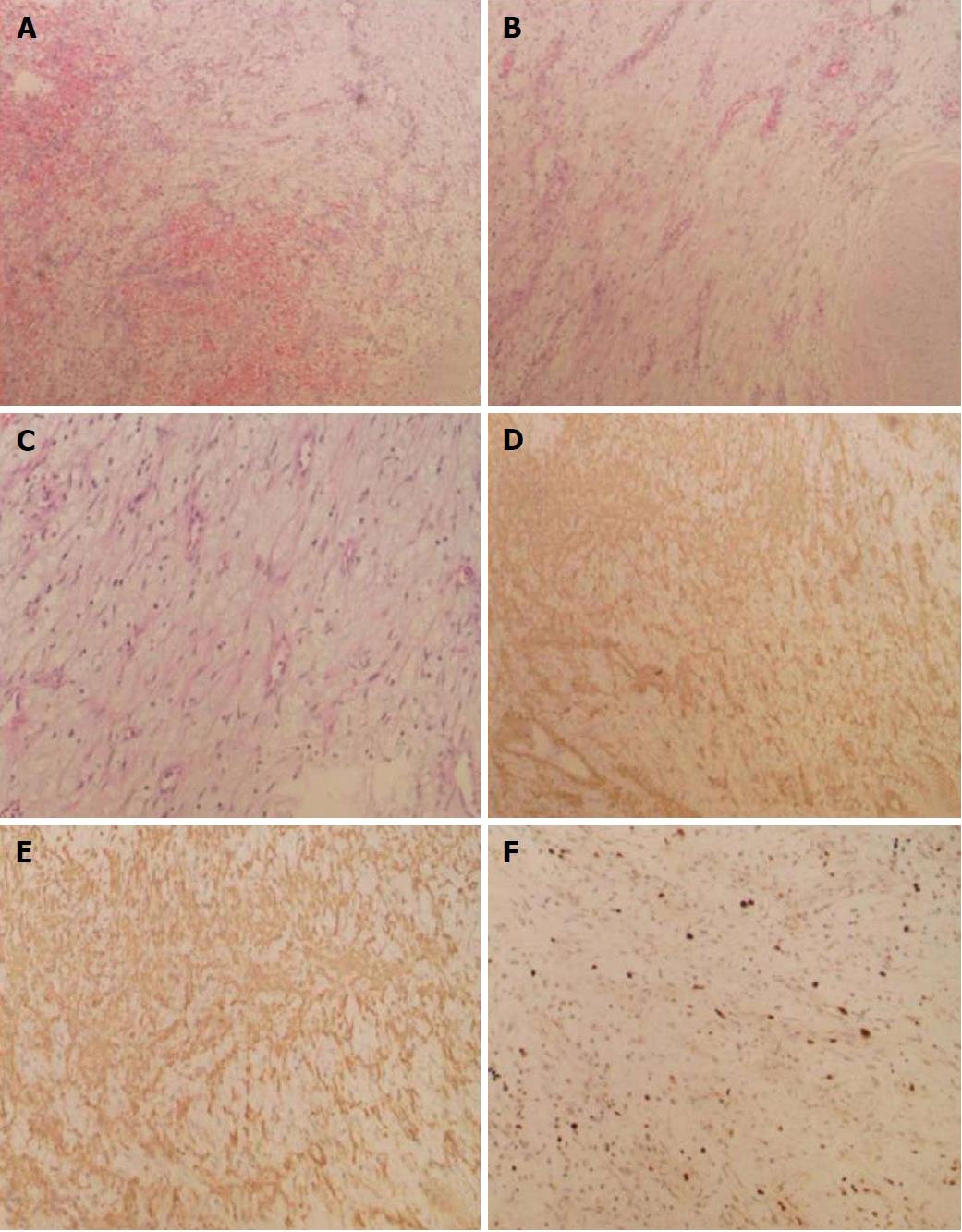
The patient underwent surgical exploratory laparotomy and resection of the upper jejunal tumor, including local intestinal resection. The size of the resected tumor was about 1.2 × 1.0 cm. Postoperative pathological examination confirmed the presence of proliferative spindle cells in the mucosal and submucosal layers of the small intestine. Immunohistochemical staining indicated spindle cells that were positive for smooth muscle actin (SMA) and CD10 (few cells) and negative for cytokeratin, CD117, DOG-1 (discovered on GIST-1), desmin, S-100, epithelial membrane antigen, and CD34. The Ki-67 labeling index was < 5%, and no vascular invasion was observed. The results supported the diagnosis of small intestinal plexiform fibromyxoma (Figure 3).
Seven days after surgery, the patient’s condition improved, and she was discharged from hospital. No gastrointestinal hemorrhage was found during 3 years consecutive follow-up. No signs of tumor recurrence and metastasis were found by imaging examination (enhanced CT and CTA of the abdomen) at 6 mo and 1, 2, and 3 years after surgery.
Plexiform fibromyxoma is a rare mesenchymal tumor. It was previously reported[2,7] to occur in the gastric antrum and was thought to be derived from cells in this location. However, as the number of reported cases has increased, this is no longer thought to be the case. So far, > 60 cases of PAMT or plexiform fibromyxoma have been reported worldwide[5]. The published literature suggests that plexiform fibromyxoma is mainly located in the gastric antrum and prepyloric area, but some reports indicate that the tumor originates from the gastric fundus[8], gastric body[9], the duodenum, and even the cecum[10] and posterior mediastinum may be involved[11]. Takahashi et al[7] reported six cases of plexiform fibromyxoma that originated from the pyloric area but extended into the duodenal bulb, and one of those cases came from the duodenal stump[12]. In the present case, plexiform fibromyxoma originated from the upper segment of the jejunum. There are no previous reports of plexiform fibromyxoma originating from the jejunum or ileum, and the present case may be the first jejunal plexiform fibromyxoma.
Plexiform fibromyxoma has a wide range of onset age, from 7 to 75 years[7]. It also has a balanced gender distribution. The clinical symptoms of patients with plexiform fibromyxoma are atypical, so patients often present to a hospital for treatment due to upper gastrointestinal symptoms, including hematemesis, melena, anemia, vomiting, abdominal pain, abdominal distension, abdominal mass, and other abdominal discomfort. There are also individual reports of gastrointestinal perforation[13]. The majority of patients attend hospital for treatment for gastrointestinal hemorrhage, which is induced by ulceration that forms on the surface of the tumor.
In the present case, the patient presented with hematochezia as the initial symptom. At the local hospital and our hospital, gastroscopy, colonoscopy, ultrasound examination of the upper abdomen, and enhanced abdominal CT were suspicious for gastrointestinal hemorrhage induced by small intestinal disease. Further CT and CTA of the small intestine still did not detect any lesions that could reasonably explain the gastrointestinal hemorrhage. A gastrointestinal hemorrhage due to small intestinal disease was suspected and capsule endoscopy was performed. One protuberant lesion was found in the small intestine, which was coupled with ulceration on its surface. Initially, we considered that it may have been a small intestinal stromal tumor, so we performed peroral single balloon enteroscopy again to assist with tumor orientation and resected the lesion completely. Surprisingly, the results of final pathological examination indicated plexiform fibromyxoma.
Plexiform fibromyxoma has been described as subserosal nodules[4] and polypoid projections, and it has smooth mucosal surface or ulceration[14]. The tumor size is 1.5 cm-15.0 cm[15]. Plexiform fibromyxoma and other subserosal nodules are indistinguishable macroscopically. The diagnosis of plexiform fibromyxoma mainly depends on pathological examination. The gross findings are characterized by a lobulated or nodular solid mass, accompanied by ulcer, erosion, and even cystic changes[16]. Histologically, plexiform fibromyxoma shows multinodular or plexiform growth. The cells in the tumor node are spindle-shaped but few in number and mitotic figures. Rich small vessels and mucous matrix can be found among the tumor cells. In most areas, the tumor cells are arranged loosely. Immunohistochemical staining shows that plexiform fibromyxoma cells are positive for SMA[17], focally positive for CD10, and negative for cytokeratin, CD117, DOG-1, desmin, S-100, epithelial membrane antigen, and CD34[1,2,6,7]. Mitoses are rare (up to 7/50 HPF). The positive expression rate of Ki-67 is low[2,3].
Small intestinal plexiform fibromyxoma has a unique histological appearance, which is easy to distinguish from other mesenchymal tumors in the small intestine: (1) Gastrointestinal stromal tumor (GIST): Plexiform fibromyxoma shows plexiform or nodular growth, with few cells. The capillary vessels proliferate obviously. Histologically, few small intestinal stromal tumors show plexiform or nodular growth, while they are positive for DOG-1 and CD117. Plexiform or nodular growth can be seen in succinate-dehydrogenase-deficient GIST. Genetic detection can reveal mutation of kit or PDGFR-α genes, which can contribute to the identification of plexiform fibromyxoma and GIST; (2) Small intestinal leiomyoma: A rare tumor that mainly occurs in the esophagus, and leiomyomas derived from the stomach and colorectum are even rarer. Leiomyomas comprise irregular fascicular smooth muscle cells. Except for SMA(+), immunohistochemical staining shows desmin(+) and h-caldesmom(+) as well as CD117(+) and CD34(+), which can distinguish leiomyoma from plexiform fibromyxoma; (3) Small intestinal schwannoma: A rare disease that is similar to gastric schwannoma. It has benign biological behavior. The tumor comprises diversely arranged tumor cells that often form a microtrabecular structure against a background of collagen. Immunohistochemical staining is S-100(+) and vimentin(+), which can distinguish schwannoma from plexiform fibromyxoma; and (4) Inflammatory myofibroblastic tumor: A rare tumor that is composed of spindle myofibroblasts, lymphocytes, and plasma cells. Immunohistochemical staining indicates anaplastic lymphoma kinase[18], which can contribute to the identification of inflammatory myofibroblastic tumor and plexiform fibromyxoma.
Plexiform fibromyxoma has benign biological behavior[1,6,7-18], and tumor resection is considered to be effective. To date, no cases with local recurrence or distal metastasis after resection have been reported, except for abdominal dilatation and vascular invasion. In the present case, imaging showed no obvious signs of metastasis or recurrence during 3 years consecutive follow-up. Currently, there are few reports on plexiform fibromyxoma, so more cases and close follow-up observation are needed. Obscure gastrointestinal bleeding occurs in approximately 5% of all patients with gastrointestinal bleeding. In 41%–75% of patients with obscure gastrointestinal bleeding, further evaluation can confirm the lesions that cause bleeding in the small intestine[19]. Capsule endoscopy is preferred for patients with high suspicion of small intestinal disease. In particular, capsule endoscopy should be performed for initial screening in patients with gastrointestinal hemorrhage caused by suspicious small intestinal disease. After finding the lesion by capsule endoscopy, enteroscopy can be considered to assist with orientation if the location of the lesion is inaccurate. Small intestinal disease or postoperative follow-up needs imaging examination as well as capsule endoscopic or enteroscopic re-examination, which could be used for direct observation of small intestinal disease or postoperative anastomosis.
Plexiform fibromyxoma is a rare, special type of mesenchymal tumor. The diagnosis mainly depends on pathological examination, and it should be distinguished from other gastrointestinal mesenchymal tumors. At present, plexiform fibromyxoma is reported to occur in the stomach, duodenum, cecum, and small intestine. According to the existing literature, plexiform fibromyxoma has benign biological behavior. Currently, there are few reports on plexiform fibromyxoma, so more cases and close follow-up observation are needed.
| 1. | Takahashi Y, Shimizu S, Ishida T, Aita K, Toida S, Fukusato T, Mori S. Plexiform angiomyxoid myofibroblastic tumor of the stomach. Am J Surg Pathol. 2007;31:724-728. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 81] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 2. | Miettinen M, Makhlouf HR, Sobin LH, Lasota J. Plexiform fibromyxoma: a distinctive benign gastric antral neoplasm not to be confused with a myxoid GIST. Am J Surg Pathol. 2009;33:1624-1632. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 97] [Article Influence: 5.7] [Reference Citation Analysis (1)] |
| 3. | Fléjou JF. WHO Classification of digestive tumors: the fourth edition. Ann Pathol. 2011;31:S27-S31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 197] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 4. | Jonaitis L, Kiudelis M, Slepavicius P, Poskienė L, Kupcinskas L. Plexiform angiomyxoid myofibroblastic tumor of stomach: A rare case. World J Gastrointest Endosc. 2016;8:674-678. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 9] [Cited by in RCA: 8] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 5. | Kim SM, An JY, Choi MG, Lee JH, Sohn TS, Kim KM, Kim S, Bae JM. Plexiform Angiomyxoid Myofibroblastic Tumor of the Stomach: a Rare Case. J Gastric Cancer. 2017;17:277-281. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 6. | Banerjee N, Gupta S, Dash S, Ghosh S. Plexiform angiomyxoid myofibroblastic tumour of the duodenum: a rare entity. BMJ Case Rep. 2015;2015:pii: bcr2015210004. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 7. | Takahashi Y, Suzuki M, Fukusato T. Plexiform angiomyxoid myofibroblastic tumor of the stomach. World J Gastroenterol. 2010;16:2835-2840. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 48] [Cited by in RCA: 53] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 8. | Wang WY, Li JN, Li GD. Plexiform angiomyxoid myofibroblastic tumour of the gastric fundus: successful diagnosis and treatment by endoscopy. J Clin Pathol. 2010;63:569-570. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 9. | Kang Y, Jung W, Do IG, Lee EJ, Lee MH, Kim KM, Choi J. Plexiform angiomyxoid myofibroblastic tumor of the stomach: report of two cases and review of the literature. Korean J Pathol. 2012;46:292-296. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 32] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 10. | Daum O, Jirasek T, Grossmann P, Mukensnabl P, Michal M. Plexiform fibroma of the colon. Appl Immunohistochem Mol Morphol. 2010;18:483-484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 11. | Duckworth LV, Gonzalez RS, Martelli M, Liu C, Coffin CM, Reith JD. Plexiform fibromyxoma: report of two pediatric cases and review of the literature. Pediatr Dev Pathol. 2014;17:21-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 35] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 12. | Moris D, Spanou E, Sougioultzis S, Dimitrokallis N, Kalisperati P, Delladetsima I, Felekouras E. Duodenal plexiform fibromyxoma as a cause of obscure upper gastrointestinal bleeding: A case report. Medicine (Baltimore). 2017;96:e5883. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 13. | Li B, Zhang QF, Han YN, Ouyang L. Plexiform myxoid gastrointestinal stromal tumor: a potential diagnostic pitfall in pathological findings. Int J Clin Exp Pathol. 2015;8:13613-13618. [PubMed] |
| 14. | Yoshida A, Klimstra DS, Antonescu CR. Plexiform angiomyxoid tumor of the stomach. Am J Surg Pathol. 2008;32:1910-1912; author reply 1912-1913. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 31] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 15. | Kane JR, Lewis N, Lin R, Villa C, Larson A, Wayne JD, Yeldandi AV, Laskin WB. Plexiform fibromyxoma with cotyledon-like serosal growth: A case report of a rare gastric tumor and review of the literature. Oncol Lett. 2016;11:2189-2194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 16. | Lee PW, Yau DT, Lau PP, Chan JK. Plexiform fibromyxoma (plexiform angiomyxoid myofibroblastic tumor) of stomach: an unusual presentation as a fistulating abscess. Int J Surg Pathol. 2014;22:286-290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 17. | Ikemura M, Maeda E, Hatao F, Aikou S, Seto Y, Fukayama M. Plexiform angiomyxoid myofibroblastic tumor (PAMT) of the stomach. A case report focusing on its characteristic growth pattern. Int J Clin Exp Pathol. 2014;7:685-689. [PubMed] |
| 18. | Cook JR, Dehner LP, Collins MH, Ma Z, Morris SW, Coffin CM, Hill DA. Anaplastic lymphoma kinase (ALK) expression in the inflammatory myofibroblastic tumor: a comparative immunohistochemical study. Am J Surg Pathol. 2001;25:1364-1371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 403] [Cited by in RCA: 363] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 19. | Raju GS, Gerson L, Das A, Lewis B; American Gastroenterological Association. American Gastroenterological Association (AGA) Institute technical review on obscure gastrointestinal bleeding. Gastroenterology. 2007;133:1697-1717. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 379] [Cited by in RCA: 350] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country of origin: China
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Kawara F, Li F, Tanaka S, Zhang H S- Editor: Ji FF L- Editor: Filipodia E- Editor: Song H