Published online Dec 6, 2018. doi: 10.12998/wjcc.v6.i15.1036
Peer-review started: August 17, 2018
First decision: October 4, 2018
Revised: November 3, 2018
Accepted: November 7, 2018
Article in press: November 7, 2018
Published online: December 6, 2018
Processing time: 113 Days and 21.4 Hours
Solid pseudopapillary tumor of the pancreas (SPTP), also known as solid and papillary epithelial neoplasm of the pancreas, is a rare pancreatic exocrine tumor that is difficult to diagnose before surgery. Pancreatic panniculitis is a rare type that occurs in less than 3% of all patients with pancreatic diseases. We here report a 19-year-old woman who presented with persistent left upper quadrant pain without obvious cause for 1 d. The patient also developed subcutaneous nodules involving lower abdomen bilaterally and lower limbs, and subcutaneous nodules were pathologically diagnosed as pancreatic panniculitis. Plain abdominal computed tomography revealed a soft-tissue mass in the body and tail of the pancreas, which was closely associated with the gastric wall. Contrast-enhanced ultrasound showed inhomogeneous echogenicity in the anterior pancreatic body, which had blurred parenchymal demarcation of the body and tail of the pancreas. Contrast-enhanced abdominal computed tomography revealed a mixed density mass with solid and cystic components in the body and tail of the pancreas, and the solid component was markedly enhanced. The lesion was pathologically diagnosed as SPTP after laparoscopic resection. Clinicians should be aware of the clinical manifestation, diagnosis, and treatment of pancreatic panniculitis and SPTP.
Core tip: Pancreatic panniculitis is a rare complication of pancreas diseases, and solid pseudopapillary tumor of the pancreas is a rare pancreatic exocrine tumor that is difficult to diagnose before surgery. We describe the diagnosis and treatment of solid pseudopapillary tumor of the pancreas accompanied with pancreatic panniculitis in a young woman. Plain abdominal computed tomography, contrast-enhanced ultrasound, contrast-enhanced abdominal computed tomography and pathological examinations were performed.
- Citation: Zhang MY, Tian BL. Pancreatic panniculitis and solid pseudopapillary tumor of the pancreas: A case report. World J Clin Cases 2018; 6(15): 1036-1041
- URL: https://www.wjgnet.com/2307-8960/full/v6/i15/1036.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v6.i15.1036
Pancreatic panniculitis is a rare complication of pancreas disorders occurring in 1%-3% of patients, most often accompanied by the pancreatic carcinoma. It often presents multiple and red-brown subcutaneous nodules. The pathogenesis is not fully understood, but it is thought to result from lipolysis and fat necrosis with secondary tissue inflammation induced by pancreatic enzymes[1]. Solid pseudopapillary tumor of the pancreas (SPTP) often occurs in young women, with an average age of 25 years. While the histogenetic origin of SPTP is still unclear, it may arise from the cells related with the gonadal ridge/ovarian primordium during embryogenesis or the embryonic neural crest cells. SPTP exhibits two histological features: Solid and pseudopapillary. In fact, the papillary structure is composed of pseudopapillae that are formed due to degeneration of tumor cells, decreased adhesion of cells, and cystic cavity formation. Clinically, it is usually manifested as an epigastric mass or epigastric pain, and some patients present with both epigastric mass and pain. Some patients may only have epigastric discomfort or fatigue, while others do not have any symptoms. In those asymptomatic patients, pancreatic masses are accidentally found only during routine examinations, and the results of routine biochemical tests are normal[2]. SPTP is unresponsive to radiotherapy or chemotherapy, and surgical resection is the primary treatment of choice. In this report, we describe the diagnosis and treatment of SPTP accompanied with pancreatic panniculitis in a young woman.
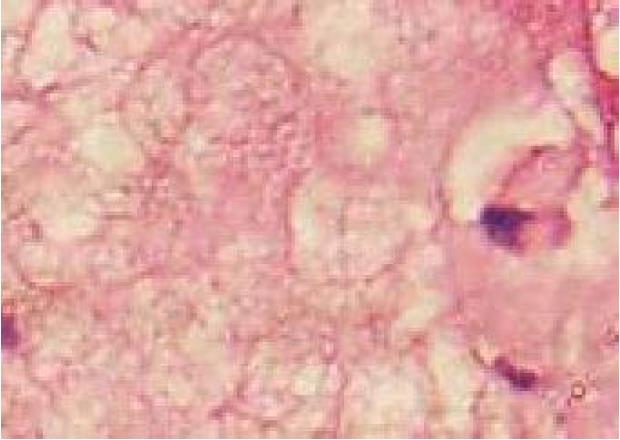
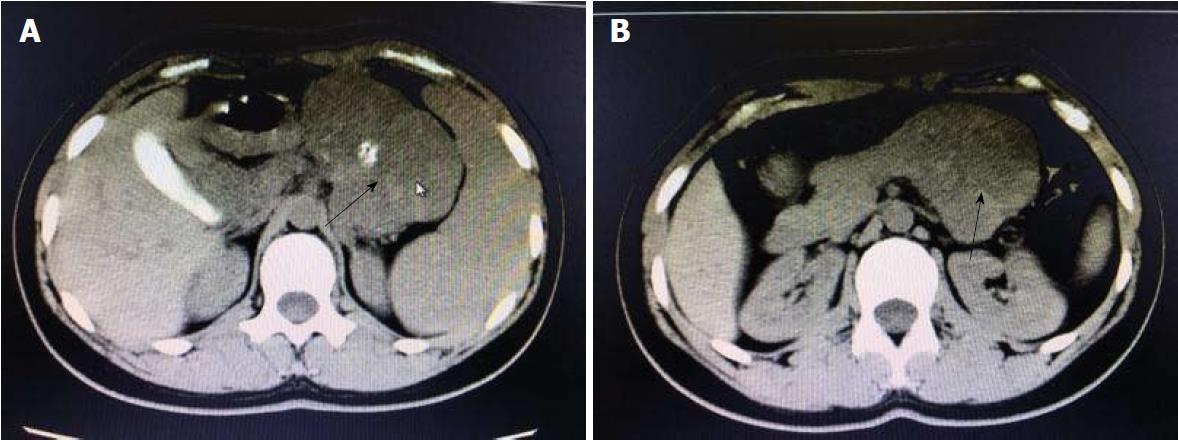
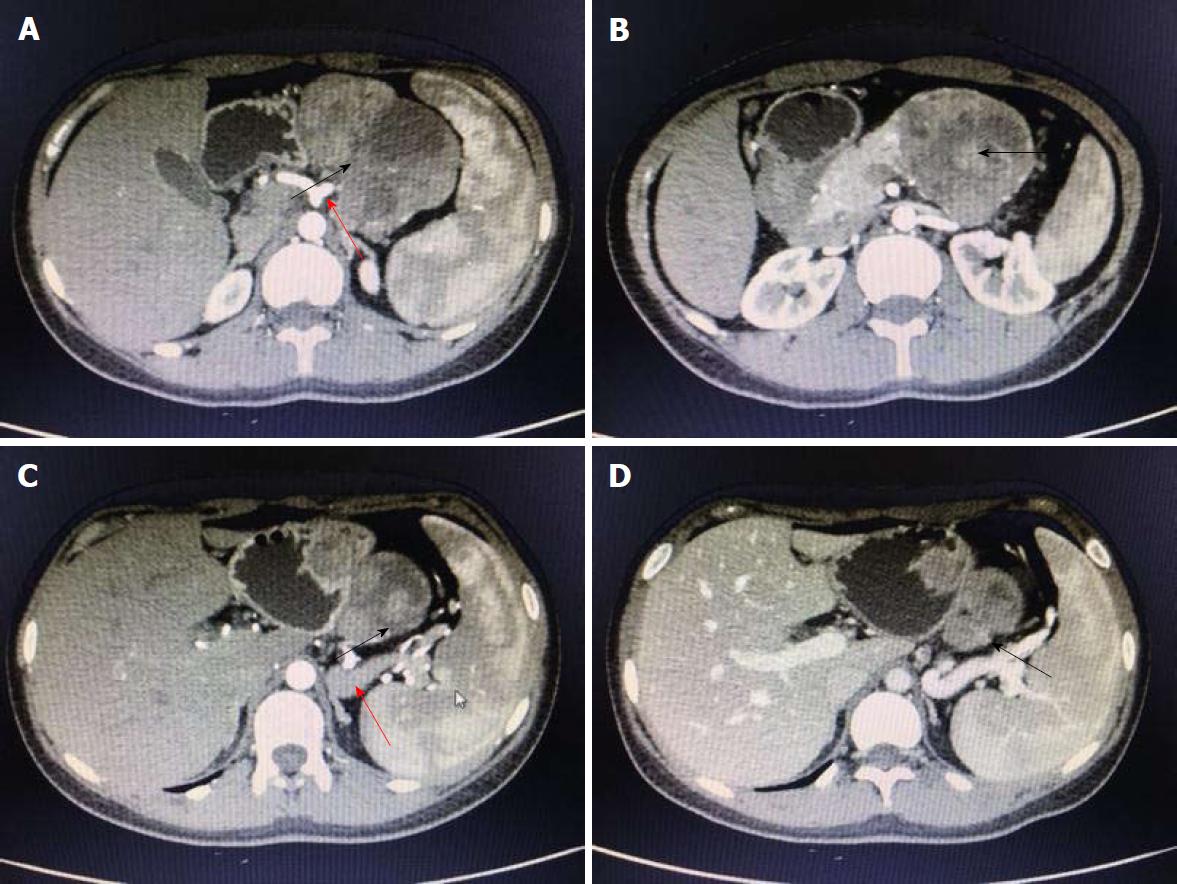
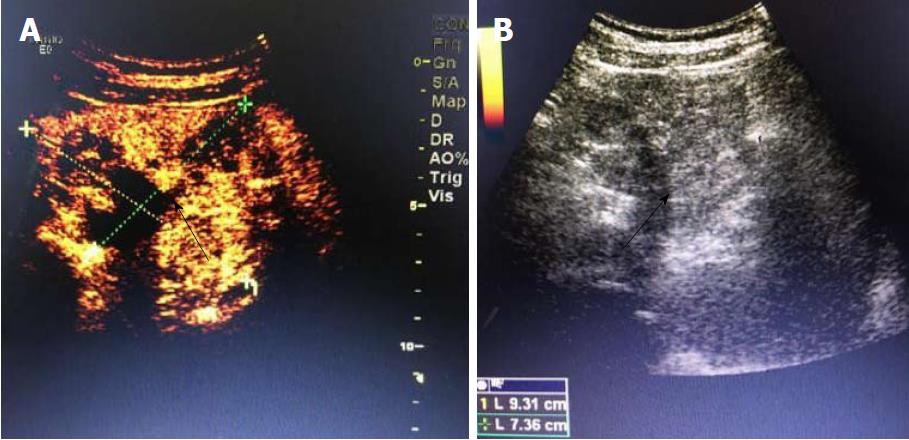
A 19-year-old woman was presented with persistent left upper abdominal pain without obvious cause for 1 d. She also developed subcutaneous nodules involving lower abdomen bilaterally and lower limbs. She had no abdominal distention, vomiting, or radiative and referred pain. Physical examination revealed a palpable mass with slight tenderness in the left upper quadrant of the abdomen. Subcutaneous nodules were pathologically diagnosed as pancreatic panniculitis (Figure 1). The results of routine blood test at admission were as follows: white blood cell 7.84 × 109/L (normal range: 4-10 × 109/L), neutrophil-to-lymphocyte ratio 85.5% (normal range: 40%-75%), and hemoglobin 88 g/L (normal range: 115-150 g/L). Liver function test showed albumin 30 g/L (normal range: 40-55 g/L) and alanine aminotransferase 62 U/L (normal range: 8-40 U/L). The result of serum amylase was 869 U/L (normal range: 40-100 U/L), serum lipase was 759 U/L (normal range: 0-110 U/L). Plain abdominal computed tomography (CT) revealed a mass in the body and tail of the pancreas, which required further examinations (Figure 2). After admission, antimicrobial treatment, somatostatin, and hepatoprotective therapies were administered. Contrast-enhanced abdominal CT was highly suggestive of a neoplastic lesion in the body and tail of the pancreas. A SPTP was suspected. The lesion was fed by the common hepatic artery and branches of splenic artery, along with regional portal hypertension (Figure 3). Abdominal contrast-enhanced ultrasound showed a solid mass in the body and tail of the pancreas, and a diagnosis of SPTP was considered (Figure 4).
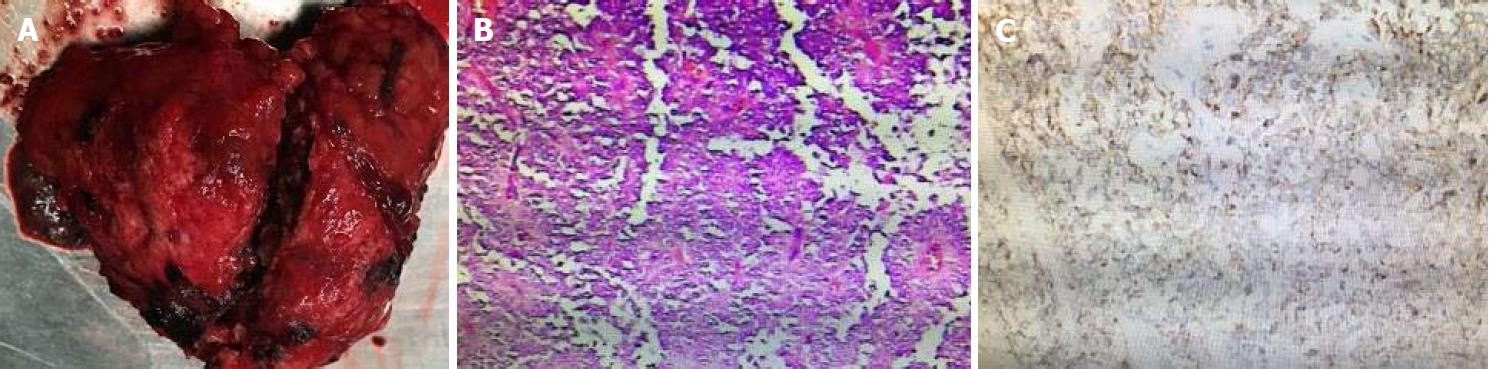
After antimicrobial drug, somatostatin, and liver-protective drug treatments, routine blood test showed white blood cell 5.62 × 109/L, neutrophil-to-lymphocyte ratio 72.8%, and hemoglobin 108 g/L, and liver function test showed albumin 39 g/L and alanine aminotransferase 30 U/L. Serum amylase and lipase, however, declined slowly, showing 479 U/L and 325 U/L. Therefore, traditional Chinese medicine was added to the treatment. Three days later, serum amylase and lipase declined obviously, showing 92 U/L and 98 U/L. After adequate preoperative preparation, the patient underwent laparoscopic resection of the lesion in the pancreatic body and tail with preservation of the spleen. During the operation, a mass sized about 8.5 cm × 8 cm × 7.0 cm was found at the tail of the pancreas. It had an irregular shape and a poorly-defined border with pancreatic body and tail (Figure 5). It was tightly adhered to the splenic vein, and several of its nourished vessels joined the splenic vein. Postoperative pathology suggested that the mass was an SPTP (Figure 5).
Pancreatic panniculitis is a rare cause of subcutaneous fat necrosis secondary to elevated serum levels of pancreatic enzymes. It is most often associated with pancreatic cell carcinoma, but has also been seen in patients with pancreatitis. Systematic evidence on pancreatic panniculitis is limited[3]. SPTP is a rare tumor, accounting for about 3% of all pancreatic tumors. It is a low-grade malignancy, and usually occurs in young women.
The clinical manifestations of SPTP are atypical. In most cases, a mass is often found within the abdomen accidentally or during physical examination, with or without gastrointestinal symptoms, such as the upper abdominal discomfort and dyspepsia[4-6]. These non-specific gastrointestinal symptoms are mostly associated with compression of nearby organs by tumors. In some special cases, SPTP can cause acute pancreatitis or spontaneous rupture of the pancreatic pseudocyst, which is clinically manifested as acute abdominal pain. Routine blood test, liver function test, renal function test, and tests for the endocrine/exocrine functions of pancreas often show normal results, and the levels of various tumor markers are usually within the normal ranges.
Imaging examination is crucial for the preoperative diagnosis of SPTP. It can determine the size and location of the tumor and its relationship with surrounding tissues, thus providing detailed information to make a surgical protocol[2,7,8]. The typical ultrasonographic finding of SPTP is a cystic/solid mass with mixed density in the pancreas. When the tumor is small, homogeneous hypoechoic echoes can be detected. Color doppler flow imaging often reveals a few blood flow signals on the capsule or parenchyma of the tumor. CT is the most commonly used imaging method for SPTP. On CT scans, SPTP is often round and oval, with lobulation. It has a well-defined border with the pancreas, and its margin is smooth. The solid part is often located in the periphery of the tumor, and the cystic part inside the tumor. The tumor can be located at any part of the pancreas. After enhancement, the parenchyma part is slightly enhanced during arterial phase and markedly enhanced during portal venous phase. The cystic part is hypodense before and after enhancement[9-11].
In our case, routine blood test, serum amylase and lipase test, and liver function test showed slight or obvious abnormalities before the operation, which were significantly improved after treatment. Subcutaneous nodules involving lower abdomen bilaterally and lower limbs reduced after treatment. The diagnosis of SPTP and the differential diagnosis of this lesion from other pancreatic diseases were mainly based on abdominal contrast-enhanced CT and abdominal contrast-enhanced ultrasound. A cystic/solid mass with mixed density was found in the body and tail of the pancreas, with a well-defined border. Nodular calcifications were seen inside the lesion, and the lesion was fed by the common hepatic artery and a branch of splenic artery. The spleen was slightly enlarged, along with regional portal hypertension. Surgery was performed after adequate preparation, during which a mass with areas of necrosis was found in the body and tail of the pancreas. The tumor had a poorly-defined border with pancreatic body and tail. It was tightly adhered to the splenic vein, and several of its nourished vessels joined the splenic vein.
Postoperative pathology confirmed the result of preoperative imaging diagnosis. Immunophenotyping showed synaptophysin (+), CD56(+), CD10(+), β-catenin(+), vimentin (+), and AE1/AE3(+), which met the diagnostic criteria for SPTP, and was consistent with the histopathological diversity of SPTP[12-14]. Vimentin, β-catenin, synaptophysin, neuron-specific enolase, α1-antitrypsin (α1-AT), S-100, neural cell adhesion molecule CD56, and cluster of differentiation 10 (CD10) can all be expressed in SPTP[15-17]. At present, surgical resection is the only radical treatment for SPTP. However, laparoscopic surgery is a challenging technique, and the selection of specific surgical approaches depends on the location and size of tumor and the findings of intraoperative pathological examination[18]. The most common postoperative complication is pancreatic fistula, followed by pancreatitis, gastrointestinal bleeding, and pseudocyst. The prognosis of SPTP is good, and the postoperative survival rate is high. If the tumor is located in the body and tail of the pancreas, resection of the body and tail of the pancreas is feasible. If the tumor invades or is closely related to the splenic vessels, pancreatectomy combined with resection of the body and tail of the pancreas can be performed[19-22]. No evidence is yet available that local resection has a higher risk of recurrence or metastasis than radical resection. Therefore, clinicians should be aware of the clinical manifestation and treatment of pancreatic panniculitis[23,24]. Although SPTP has good prognosis and the postoperative 5-year survival rate is high, efforts should be made to increase further diagnostic accuracy and optimize therapeutic methods, so as to improve the quality of life of SPTP patients.
A 19-year-old woman presented with persistent left upper abdominal pain without obvious cause for 1 d. The patient also developed subcutaneous nodules involving lower abdomen bilaterally and lower limbs. An irregular mass was found in the pancreatic body and tail on plain abdominal computed tomography (CT), contrast-enhanced abdominal CT, and contrast-enhanced ultrasound.
Solid pseudopapillary tumor of the pancreas (SPTP), pancreatic panniculitis.
Pancreatic cancer.
Abnormal laboratory findings included the results of routine blood: Neutrophil-to-lymphocyte ratio 85.5% (normal range: 40%-75%), hemoglobin 88 g/L (normal range: 115-150 g/L). Liver function test showed albumin 30 g/L (normal range: 40-55 g/L) and alanine aminotransferase 62 U/L (normal range: 8-40 U/L). The result of serum amylase was 869 U/L (normal range: 40-100 U/L), and serum lipase was 759 U/L (normal range: 0-110 U/L).
Contrast-enhanced abdominal CT revealed a cystic/solid mass with mixed density in the body and tail of the pancreas.
SPTP, pancreatic panniculitis.
Laparoscopic resection of the mass in the pancreatic body and tail with preservation of the spleen.
Some articles have described the imaging diagnosis and treatment of SPTP and pancreatic panniculitis, as shown in the References.
Clinicians should be aware of the clinical manifestation and treatment of pancreatic panniculitis. Although SPTP has good prognosis and the postoperative 5-year survival rate is high, efforts should be made to increase further diagnostic accuracy and optimize therapeutic methods, so as to improve the quality of life of SPTP patients.
| 1. | Guanziroli E, Colombo A, Coggi A, Gianotti R, Marzano AV. Pancreatic panniculitis: the “bright” side of the moon in solid cancer patients. BMC Gastroenterol. 2018;18:1. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 25] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 2. | Gouta EL, Khalfallah M, Jerraya H, Dougaz W, Nouira R, Dziri C. Pseudo papillary and solid tumor of the pancreas: a rare tumor and a difficult diagnosis. Tunis Med. 2017;95:229-232. [PubMed] |
| 3. | Kim EJ, Chu MS, Sohn KC, Cho DH, Na GH, Kim HC, Cho EY. Pancreatic Panniculitis in Patients with Chronic Pancreatitis: Case Report and Review of Literature. Korean J Gastroenterol. 2017;69:83-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 4. | Patnayak R, Jena A, Reddy VR, Lakshmi AY, Reddy MK. Solid pseudo-papillary tumor of pancreas: Indian perspective. J Cancer Res Ther. 2015;11:1046. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 5. | Michalova K, Michal M, Sedivcova M, Kazakov DV, Bacchi C, Antic T, Miesbauerova M, Hes O, Michal M. Solid pseudopapillary neoplasm (SPN) of the testis: Comprehensive mutational analysis of 6 testicular and 8 pancreatic SPNs. Ann Diagn Pathol. 2018;35:42-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 6. | Nair Anila KA, Nayak N, Muralee M, Venugopal BP, Mony RP. Solid-pseudopapillary neoplasm of the pancreas: A classical presentation with unique paranuclear dot like immunostaining with CD 99. Indian J Pathol Microbiol. 2015;58:365-367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 7. | Wang X, Tan CL, Song HY, Yao Q, Liu XB. Duodenum and ventral pancreas preserving subtotal pancreatectomy for low-grade malignant neoplasms of the pancreas: An alternative procedure to total pancreatectomy for low-grade pancreatic neoplasms. World J Gastroenterol. 2017;23:6457-6466. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 10] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 8. | Akimoto Y, Kato H, Matsumoto K, Harada R, Oda S, Fushimi S, Mizukawa S, Yabe S, Uchida D, Seki H. Pancreatic Hepatoid Carcinoma Mimicking a Solid Pseudopapillary Neoplasm: A Challenging Case on Endoscopic Ultrasound-guided Fine-needle Aspiration. Intern Med. 2016;55:2405-2411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 9. | Li DL, Li HS, Xu YK, Wang QS, Chen RY, Zhou F. Solid pseudopapillary tumor of the pancreas: clinical features and imaging findings. Clin Imaging. 2018;48:113-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 28] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 10. | Chhadi TS, Deshpande AH, Chhadi SA, Kumbhalkar DT, Raut WK. A Solid Pseudopapillary Tumour of the Head of Pancreas: A Rare Case Report Diagnosed by Fine Needle Aspiration Cytology. J Clin Diagn Res. 2016;10:ED06-ED08. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 11. | Tipmanee V, Pattaranggoon NC, Kanjanapradit K, Saetang J, Sangkhathat S. Molecular dynamic simulation of mutated β-catenin in solid pseudopapillary neoplasia of the pancreas. Oncol Lett. 2018;15:9167-9173. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 12. | Turpin S, Perron M, Vairy S, Bénali S, Damphousse A. Solid Pseudo-Papillary Tumor Mimicking as Complicated Pseudocyst: Multimodality Imaging and Pathological Correlation. Clin Nucl Med. 2018;43:e368-e371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 13. | Wang X, Chen YH, Tan CL, Zhang H, Xiong JJ, Chen HY, Ke NW, Liu XB. Enucleation of pancreatic solid pseudopapillary neoplasm: Short-term and long-term outcomes from a 7-year large single-center experience. Eur J Surg Oncol. 2018;44:644-650. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 40] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 14. | Song S, Wang B, Gu S, Li X, Sun S. Expression of Beclin 1 and Bcl-2 in pancreatic neoplasms and its effect on pancreatic ductal adenocarcinoma prognosis. Oncol Lett. 2017;14:7849-7861. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 15. | Jung MJ, Kim HK, Choi SY, Kim SG, Jin SY. Solid pseudopapillary neoplasm of the pancreas with liver metastasis initially misinterpreted as benign haemorrhagic cyst. Malays J Pathol. 2017;39:327-330. [PubMed] |
| 16. | Leraas HJ, Kim J, Sun Z, Ezekian B, Gulack BC, Reed CR, Tracy ET. Solid Pseudopapillary Neoplasm of the Pancreas in Children and Adults: A National Study of 369 Patients. J Pediatr Hematol Oncol. 2018;40:e233-e236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 22] [Article Influence: 2.8] [Reference Citation Analysis (1)] |
| 17. | Carlotto JR, Torrez FR, Gonzalez AM, Linhares MM, Triviño T, Herani-Filho B, Goldenberg A, Lopes-Filho Gde J, Lobo EJ. Solid pseudopapillary neoplasm of the pancreas. Arq Bras Cir Dig. 2016;29:93-96. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 18. | Cawich SO, Ledesma Z, Sampath L, Sandy S. Clinicopathologic features of solid pseudopapillary pancreatic neoplasms in an Eastern Caribbean population. Trop Doct. 2018;48:224-227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 19. | Zhang J, Liu Y, Bao P, Wang Y, Zhang Y. Rare coexistence of metastatic neuroblastoma of liver and solid pseudo papillary tumor of pancreas: case report and literature review. J Cancer Res Ther. 2013;9:308-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 20. | Marchegiani G, Andrianello S, Massignani M, Malleo G, Maggino L, Paiella S, Ferrone CR, Luchini C, Scarpa A, Capelli P. Solid pseudopapillary tumors of the pancreas: Specific pathological features predict the likelihood of postoperative recurrence. J Surg Oncol. 2016;114:597-601. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 66] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 21. | Pattanshetti VM, Vinchurkar K, Pattanshetti SV. Solid pseudo papillary tumor of pancreas: Presenting as acute abdomen in a female child. Indian J Med Paediatr Oncol. 2014;35:184-186. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 22. | Gahlot GP, Mridha AR, Sable M, Sharma MC, Pramanik R, Kumar L. Solid pseudopapillary neoplasm of the ovary with metastases to the omentum and regional lymph nodes. Indian J Pathol Microbiol. 2016;59:348-350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 13] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 23. | Gu Y, Qian Z. Chronic pancreatitis with multiple pseudocysts and pancreatic panniculitis: A case report. Medicine (Baltimore). 2018;97:e10911. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 24. | Marcos P, Kieselova K, Cunha M. Pancreatic Panniculitis. Am J Gastroenterol. 2017;112:1218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
CARE Checklist (2013) statement: We have read the CARE checklist (2013) and prepared and revised the manuscript according to the CARE checklist (2013).
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country of origin: China
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P- Reviewer: Lei YC, Nagaya M, Teramoto-Matsubara OT S- Editor: Ji FF L- Editor: Filipodia E- Editor: Song H