Published online Aug 16, 2017. doi: 10.12998/wjcc.v5.i8.333
Peer-review started: October 23, 2016
First decision: December 20, 2016
Revised: April 30, 2017
Accepted: May 18, 2017
Article in press: May 19, 2017
Published online: August 16, 2017
Processing time: 301 Days and 22.2 Hours
Vertebroplasy is considered an alternative and effective treatment of painful oncologic spine disease. Major complications are very rare, but with high morbidity and occur in less than 1% of patients who undergo vertebroplasty. Spinal subdural hematoma (SDH) is an extremely rare complication, usual developing within 12 h to 24 h after the procedure. We report the case of a tardive SDH in an oncologic patient who underwent VP for Myxoid Liposarcoma metastasis. Trying to explain the pathogenesis, we support the hypothesis that both venous congestion of the vertebral venous plexus of the vertebral body and venous congestion due to a traumatic injury can provoke SDH. To our best knowledge, only 4 cases of spinal subdural hematoma following a transpedicular vertebroplasty have been previously described in International literature and only one of them occurred two weeks after that surgical procedures. Percutaneous verteboplasty is a well-known treatment of pain oncologic spine disease, used to provide pain relief and improvement of quality life and is considered a simple surgical procedure, involving a low risk of complications, but related to high morbidity, such as SDH. Therefore it has to be performed by experienced and skilled surgeons, that should also recognize possible risk factors, making SDH more risky.
Core tip: This is an original paper about a rare complication of vertebroplasty: A subdural hematoma. In literature there are only 4 cases described. To our knowledge thid is the first case in which this complication occur after 20 d. In this work we try to explain the pathogenesis and the importance of a correct and rapid diagnosis, and, if needed, an emergency treatment.
- Citation: Tropeano MP, La Pira B, Pescatori L, Piccirilli M. Vertebroplasty and delayed subdural cauda equina hematoma: Review of literature and case report. World J Clin Cases 2017; 5(8): 333-339
- URL: https://www.wjgnet.com/2307-8960/full/v5/i8/333.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v5.i8.333
Myxoid liposarcoma is the most common subtype of liposarcoma, accounting for 10% of all adult tissue sarcomas[1]. The frequency of bone metastasis arising from liposarcoma has been reported to be 14% and 17%[2]. In one of the largest series, which analyze specifically the development of bone metastases, the incidence of spinal metastases was 83%[2]. Treatment options included: Surgical excision, chemotherapy, adjuvant radiotherapy, surgical decompression of spinal metastasis after having their surgery elsewhere.
The first percutaneous vertebroplasty in an oncological patient, was performed at the University Hospital of Amiens, France, to fill a vertebral void after the removal of a benign spinal tumor, then it was quickly adopted also for use in metastatic vertebral lesions and hematologic malignancies such as multiple myeloma and lymphoma. Clinical studies documented the effectiveness of VP as an alternative treatment of painful oncologic spine disease[3].
The first vertebroplasty was performed by Galibert in 1987 for a C2 hemangioma[4]. The first series was reported in 1997 and since[5], it has become a very common surgical technique for the symptomatic treatment of painful osteoporotic vertebral fractures, wedge-compression fractures, vertebral malignancies and painful vertebral angiomas.
The goal is to provide pain relief and bone strengthening, injecting cement or calcium phosphate bone cement into the vertebral body, via a transpedicular or an extrapedicular approach under fluoroscopic guidance. There is strong evidence of pain relief and improvement in the patient’s quality life. Percutaneous vertebroplasty is usually performed in the thoracic and lumbar vertebrae and rarely in the cervical vertebrae and cervico-thoracic junction. Absolute contraindications are: Unstable fractures with posterior element involvement, bleeding disorders, active local infections and sepsis[6]. Relative contraindications are: clinical conditions not allowing to lie prone, neurological signs and symptoms due to vertebral body collapse or tumor extension[7].
Major complications are very rare, but with high morbidity and occur in less than 1% of patients who undergo vertebroplasty. The most common are anaphylaxis and hypotension due to an adverse reaction to the cement, pneumothorax, pulmonary embolism due to cement leakage, spinal cord compression following the cement leakage, epidural or subdural hematoma, vertebral injury, infections and death[8,9]. Most often, complications occur during surgery or immediately following surgery. Late-developing complications are infection, adjacent vertebral body fractures and recurrent fracture; they appear within days to weeks following surgical procedure. Spinal subdural hematoma (SDH) is an extremely rare complication, usual developing within 12 h to 24 h after the procedure. To our knowledge, to date, only 4 cases have been previously reported in International literature[10,11], where only one of them occurred two weeks following transpedicular vertebroplasty[12]. We report the case of a tardive SDH in an oncologic patient who underwent VP for Myxoid Liposarcoma metastasis.
We report the case of a 63-year-old man who presented to our emergency department with bilateral inferior limb numbness and weakness, mainly to the left leg and complaining of bladder retention. Neurological assessment revealed a 1/5 monoparesis of the left inferior limb and 3/5 monoparesis of the right, as well hypoesthesia and dysesthesia in the same region. Perineal reflexes were absent. The patient was on anticoagulants.
Three weeks prior to the onset of neurological symptoms, the patient underwent percutaneous VP of L1 and L3 vertebrae, in an oncology institute, for pathological compression fractures, due to secondary localization of a retroperitoneal myxoid liposarcoma, removed several years before. VP was indicated by an oncologist and performed at the above-mentioned institute of oncology. Pathological anamnesis revealed that the patient underwent surgery several times for the removal of a retroperitoneal liposarcoma. In 1997 the patient underwent the first surgical procedure for the removal of the lesion located in the upper left quadrant of the retroperitoneal space. During the same procedure, the left colon was also removed. In 2004 a second surgical procedure was performed for the removal of a local relapse of the lesion as well as for the removal of the spleen. In February 2005 a follow up abdominal magnetic resonance imaging (MRI) showed the presence of another local relapse of the pathology. In consequence, another surgical excision of the lesion was performed, including excision of the pancreatic tail. The procedure was proceeded by the administration of a chemotherapeutic protocol consisting of Antracicline and Ifosfamide. In November 2011 another surgical excision was performed. It included the left part of the diaphragm as well as a portion of the small intestine and the left half of the transverse colon. Furthermore, on November 2013 the patient underwent cyberknife radiotherapy.
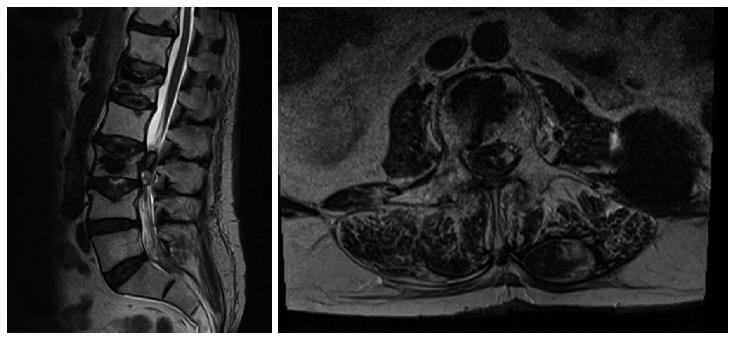
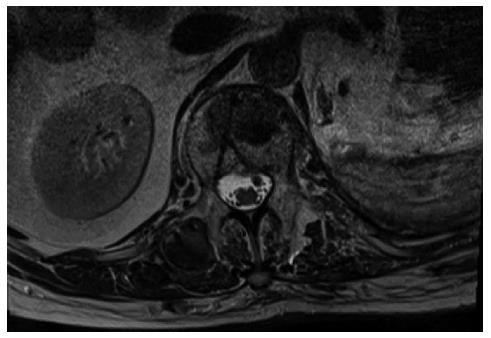
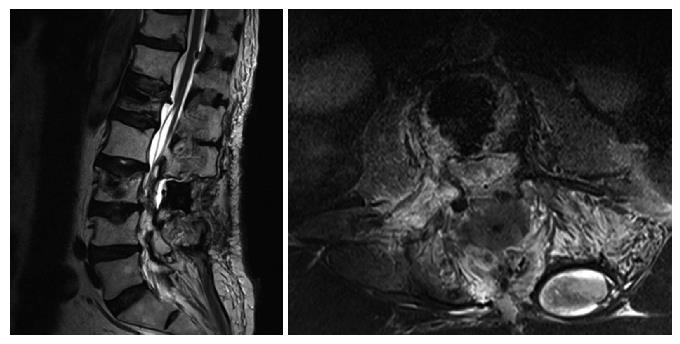
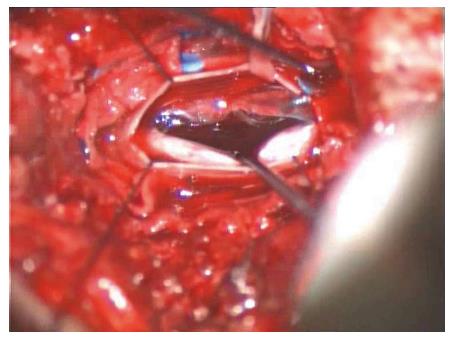
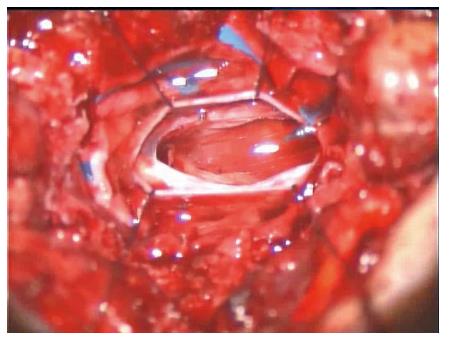
Upon admittance at our emergency department for paraparesis, an emergency spinal MRI with gadolinium was obtained. Results showed the presence of a high signal lesion in the intradural extramedullary space, at the conus medullaris (Figure 1). Furthermore, the trajectory of the needle used to perform the vertebroplasty was detected at L1 and L3 levels and it suggested that the needle had passed through the dura into the subarachnoid space and then into the vertebral body (Figure 2). An emergency decompressive bilateral laminectomy of L2 and L3 vertebrae was performed. No epidural bleeding was observed. A longitudinal durotomy revealed a blood clot, tightly adherent to the cauda equina rootlets (Figure 3). The hemorrhagic lesion was completely removed with the assistance of a surgical microscope (Figure 4). After the procedure, neurological symptoms progressively disappeared and 5 d later, the patient completely recovered both motor and sensory deficits, as well as bladder functions. Postoperative MRI documented adequate surgical decompression and removal of the intradural lesion (Figure 5). Histological examination confirmed the haemorrhagic origin of the lesion, constituted by clots and fibrin, with no evidence of tumor.
Liposarcoma is a common malignant soft tissue tumor, accounting for 10% to 16% of all sarcomas[1]. It typically affects patients between the fifth and seventh decade of life and usually develops in the extremities or retroperitoneum[13]. It can be classified into five distinct histological subtypes (WHO 1994): Well-differentiated, dedifferentiated, mixed, round cell and pleomorphic. Myxoid liposarcoma (MLS) is the second most common subtype, accounting for 10% of all adult soft tissue sarcoma, occurring more frequently during the fourth and fifth decades of life[14]. It is considered as a clinicopathologically and genetically distinct type, characterized by its common occurrence in young patient, its location in the thigh and the presence of at translocation[12,15,16]. Specifically, it is common associated with TLS-CHOP fusion transcript. Differently from other soft-tissue sarcomas, that show a tendency for metastasis to the lung, MLS has a propensity to spread to extrapulmonary sites, including bone. The frequency of bone metastasis is reported at 14%[2] and 17%[16]. Furthermore, MLS presents often as a multifocal disease, either synchronous or metachronous. The degree to which MLS spreads to bone has not been specifically studied and it is still unclear if skeletal metastasis represents the usual pattern of spread in MLS, or if it is the mark of specific molecular subset. In a large series, including 40 patients who developed skeletal metastasis, 33 (83%) were diagnosed with spine metastasis[2]. The spine metastases demonstrated the typical MRI findings of MLP. T1 weight images were heterogenous with areas go high signal intensity corresponding to the lipid component and low signal to the mixed component, as T2 images as well. The treatment of metastasis is individualized to each patient. Surgical excision is the treatment of choice; chemotherapy and radiotherapy are also utilized. Percutaneous verteboplasty (VP) is a well-known treatment of pain oncologic spine disease, used to provide pain relief and improvement of quality life. It was introduced for the first time to fill a vertebral void after a the removal of a benign spinal tumor, since then it was introduced as a treatment option also for primary and metastatic spinal tumor[17]. During the last few decades, improvement in surgical strategies and technologies, have increased disease-free survival rates, in patient with a wide variety of malignant tumors that were once considered inoperable. Despite these advances, many patients present with widespread tumor and minimal life expectancy and surgical or any other aggressive treatment cannot be medically or ethically considered[18]. Palliative strategies are recommended in this cases, such methods include medical management, pain management, vertebroplasty, radiotherapy. Vertebroplasty and Kyphoplasty are usually indicated for the treatment of metastatic spinal tumors without epidural compression, to improve the anterior column stably of the spine in conjunction with medical and radiation therapies and to obtain pain relief[19]. In particular, these conservative procedures are recommended in elderly patients, at high anesthetic risk, because less operating time under anesthesia and minimal blood loss. Randomized, multi centered and controlled trials, demonstrated that the use of VP, specifically for spinal metastasis, had an improvement in pain (among 73% to 100% of patients), mobility and vertebral height restoration[20,21]. To our knowledge, Yang et al[22] conduct- ed the largest vertebroplasty study in patients with metastatic spinal disease. A total of 196 patients were treated during the study and a 98.5% improvement in pain was seen, as well as statistically significant improvements in vertebral body height[22].
Percutaneous vertebroplasty is a therapeutic strategy, that gained increasing popularity among the neurosurgical community for the treatment of refractory axial mechanical pain due to osteoporotic fractures, malignancy fractures and painful hemangiomas. With time, the indications for vertebroplasty were extended to include acute traumatic vertebral compression fractures[23]. The therapeutic mechanism of action consists of injecting polymethyl methacrylate into the fractured vertebral body. There is evidence of the ability of vertebroplasty to provide pain relief and improvement of patient’s quality of life. Although it is a safe procedure, the rate of major complications is from 0.5% to 1%, when it is conducted by experienced spinal surgeons. Complications reported in literature are often related to the cement extravasation into the epidural space[24] (some series reported up to 20% extravasation rates, occasionally requiring surgical decompression) causing spinal cord compression, or related to the cement migration through the epidural veins to the venous system leading to pulmonary embolism[25]. To our knowledge only 5 cases of SDH, including our own, have been reported in literature (Table 1). Cosar et al[10] reported two cases: An 18-year-old man with an acute compression fracture of the L2 and L4 vertebrae (AO Type A1.1), in whom both levels were treated with vertebroplasty. The patient complained of severe back pain immediately after the surgical procedure and paraparesis developed in both his legs 12 h later. Postoperative MRI showed spinal SDH extending from T1 to L2, evacuated via cross-hemilaminectomy from T-1 to L2. The second case reports a 75-year-old woman with an osteoporotic compression fracture at L1. The patient suffered psychosomatic symptoms with paraparesis 24 h after the procedure. The Postoperative MRI revealed spinal SDH extending from T-10 to L-3, evacuated via T-12 laminectomy. Both patients improved after the second surgical procedure, but reported back pain after a few months, with an MRI showing spinal arachnoiditis, controlled with steroids and anti-inflammatory drug therapy. They hypothesized that the spinal SDH developed after puncture of the spinal dura mater and that venous blood began to enter the subdural space slowly after this trauma. This is reasonable, according to the time of onset of symptom presentation.
| Case | Age, gender | Fracture level | Fracture cause | SDH symptoms onset | Symptoms | SDH level | Treatment | Recovery |
| Lee et al[12] | 40 yr, female | T11-T12 | Traumatic | 2 wk | Back pain, radiating both legs | SDH T10-L5 | No surgery, corticosteroid therapy | Good |
| Cosar et al[10] | 75 yr, female | L1 | Osteoporotic | 12 h | Paraparesis, incontinence | SDH T12-L3 | Laminectomy T12 | Good with arachnoiditis |
| Cosar et al[10] | 18 yr, male | L2-L4 | Traumatic | 12 h | Paraparesis | SDH T1-L2 | Hemilaminectomy T1-L2 | Good with arachnoiditis |
| Mattei et al[11] | 49 yr, female | T8 | Traumatic | Immediate | Motor deficit left leg | SDH T9-C7 | Laminectomy T7-T9 | Good |
| Our case | 63 yr, male | L1-L3 | Oncological fracture | 2 wk | Paraparesis | SDH conus | Laminectomy L2-L3 | Good |
Lee et al[12] reported a 40-year-old female with an acute compression fracture of the T11 and T12 vertebrae, treated with successful transpedicular VP, under continuous visualization with fluoroscopic guidance. After two weeks, during which the patient’s conditions were improving, she complained of acute back pain. MRI imaging showed a high signal intensity mass lesion in the intradural extra medullary space, located at the lower thoracic, lumbar and sacral area. No coagulation disturbances were detected. Open surgery was recommended but she refused. Following 10 d of intravenous therapy with dexamethasone, she improved. The authors did not give a precise explanation and concluded that pathogenesis is still unclear. Among possible theories explaining the pathogenesis of SDH after vertebroplasty, the authors hypothesize the increase in thoracic and/or abdominal pressure, due to leakage of bone cement, increasing the pressure within the intraspinal vessels, particularly the valveless radiculomedullary veins, that cross subdural and subarachnoid space (but leakage was not enough), the development after spinal puncture of dura mater, as Cosar et al[10] proposed and the possibility that SDH may originate directly from the subarachnoid space, dissecting through the arachnoid membrane and eventually break into the spinal subdural space.
Mattei et al[11] reported the case of a 49-year-old woman with a T8 compression fracture, previously treated conservatively and with a VP after 3 mo follow-up, when she complained of severe deep axial pain. After cannulation of the left T8 pedicle and the initial injection of PPMA, a small posterior extravasation of cement to the epidural veins was observed. Surgical procedure was stopped, and, after awaking, she presented diffuse numbness on the left side (both in the superior and inferior limbs) and diffuse weakness in the left leg. An emergency CT scan showed a very small posterior leakage of PMMA towards the epidural space and into the adjacent costotransverse joint and a hyperdense collection anterior to the spinal cord from T7 to the upper cervical spine. decompressive laminectomy was performed, at T8, T7, T9. Postoperative MRI confirmed the presence of SDH. The authors commented on the anatomy of spinal venous drainage and focused on the possible etiologic role of venous congestion caused by the venous obstruction.
SDHs can be divided into traumatic and spontaneous. Traumatic SDHs usually occur after minor spinal trauma, spinal anesthesia lumbar puncture and spinal surgery, especially in the presence of intraoperative dural tears[26,27]. Spontaneous (non traumatic) SDHs are much more rare, with a recent review having identified 106 cases reported in the English literature[28]. Most of them are located anteriorly to the spinal cord, differently from epidural haematomas located posteriorly, at the lower thoracic region and lumbar region. Predisposing factors are considered coagulation abnormalities, anticoagulation therapy, platelet disfunction, polycythemia vera, pregnancy, arterial wall abnormalities and spinal arteriovenous malformations[29-33], but the pathophysiology still remains unclear. The management of SDH is still controversial as well. Some authors propose emergency spinal decompression and evacuation of the hematoma, while other wait for the recovery of incomplete neurological deficits, especially in the absence of spinal cord compression. Several theories have been proposed to explain the pathogenesis, most of them stressing the anatomy of spinal venous drainage, involving venous congestion. Although some authors have suggested that thin and delicate extra-arachnoid vessels on the inner surface of dura can give rise to SDH, it is confined to specific cases occurring in association with a subarachnoid hemorrhage of traumatic origin[34]. Alternatively, other authors have reported cases of sudden episodes of increased intra-abdominal or intra-thoracic pressure (coughing or straining) associated with SDH, suggesting the presence of a locus minoris resistentiae, that, when submitted to high pressure for venous congestion, would possibly rupture, causing extravasation of blood into the subdural space[35,36]. According to this theory, both venous congestion of the vertebral venous plexus of the vertebral body and venous congestion due to a traumatic injury can provoke SDH.
In conclusion, there are still questions that remain unclear. How can the differences in time of onset be explained? Why do certain SDH cases present immediately following intervention with neurological deficits (within 24 h), while others presented later (2 wk after)? Is it possible that there is no difference, but that the SDH already present in both cases and becomes symptomatic within 24 h or 2 wk. Can we postulate that other conditions are superimposed? Concerning our case, both theories have been proposed. The late onset of SDH at the same level of a vertebral boy previously treated by VP, without extension to the upper and lower levels, is extremely rare. It is most likely related to the wrong insertion of the needle, but also to the anticoagulants, with a delay in the onset probably due to the mechanism of venous congestion. We definitely consider VP a simple surgical procedure, involving a low risk of complications, but related to high morbidity. Therefore it has to be performed by experienced and skilled surgeons. Furthermore, surgical iatrogenic complications must be known, correctly and rapidly diagnosed and, if needed, receive emergency treatment. Experienced surgeons should also consider and evaluate possible risk factors, making SDH more risky.
This is the case of a 63-year-old man who presented to our emergency department with bilateral inferior limb numbness and weakness, mainly to the left leg and complaining of bladder retention. Three weeks prior to the onset of neurological symptoms, the patient underwent percutaneous vertebroplasty (VP) of L1 and L3 vertebrae, in an oncology institute, for pathological compression fractures, due to secondary localization of a retroperitoneal myxoid liposarcoma, removed several years before.
Neurological assessment revealed a 1/5 monoparesis of the left inferior limb and 3/5 monoparesis of the right, as well hypoesthesia and dysesthesia in the same region. Perineal reflexes were absent.
Haemorrhage, concussion injury, spinal contusion, Guillain- Barrè Sindrome.
All labs were within normal limits.
A magnetic resonance imaging scan showed the presence of a high signal lesion in the intradural extramedullary space, at the conus medullaris.
An emergency decompressive bilateral laminectomy of L2 and L3 vertebrae was performed. A longitudinal durotomy revealed a blood clot, tightly adherent to the cauda equina rootlets. The hemorrhagic lesion was completely removed with the assistance of a surgical microscope.
Spinal subdural hematoma is an extremely rare complication, usual developing within 12 to 24 h after the procedure. To our knowledge, to date, only 4 cases have been previously reported in International literature.
Vertebroplasty is usually indicated for the treatment of metastatic spinal tumors without epidural compression, to improve the anterior column stably of the spine in conjunction with medical and radiation therapies and to obtain pain relief.
VP a simple surgical procedure, involving a low risk of complications, but related to high morbidity. Therefore it has to be performed by experienced and skilled surgeons. Furthermore, surgical iatrogenic complications must be known, correctly and rapidly diagnosed, and, if needed, receive emergency treatment.
The manuscript reports a rare case and is clear, comprehensive and convincing. It is an interesting review about the complications following the percutaneous vertebroplasty, mainly about the occurrence of spinal subdural hematoma.
| 1. | Conesa X, Seijas R, Ares O, Huguet P, Perez-Dominguez M. Multicentric liposarcoma. Acta Orthop Belg. 2011;77:9-14. [PubMed] |
| 2. | Schwab JH, Boland P, Guo T, Brennan MF, Singer S, Healey JH, Antonescu CR. Skeletal metastases in myxoid liposarcoma: an unusual pattern of distant spread. Ann Surg Oncol. 2007;14:1507-1514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 89] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 3. | John MM, Herve D, Stephen MB, eds . [First edition published 2002]. Percutaneous Vertebroplasty and Kyphoplasty (2nd ed.). Springer Science Business Media, 266: 3-5. |
| 4. | Galibert P, Deramond H, Rosat P, Le Gars D. [Preliminary note on the treatment of vertebral angioma by percutaneous acrylic vertebroplasty]. Neurochirurgie. 1987;33:166-168. [PubMed] |
| 5. | Jensen ME, Evans AJ, Mathis JM, Kallmes DF, Cloft HJ, Dion JE. Percutaneous polymethylmethacrylate vertebroplasty in the treatment of osteoporotic vertebral body compression fractures: technical aspects. AJNR Am J Neuroradiol. 1997;18:1897-1904. [PubMed] |
| 6. | Peh WC, Gilula LA. Percutaneous vertebroplasty: indications, contraindications, and technique. Br J Radiol. 2003;76:69-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 64] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 7. | Burton AW, Mendel E. Vertebroplasty and kyphoplasty. Pain Physician. 2003;6:335-341. [PubMed] |
| 8. | Moreland DB, Landi MK, Grand W. Vertebroplasty: techniques to avoid complications. Spine J. 2001;1:66-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 70] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 9. | Vats HS, McKiernan FE. Infected vertebroplasty: case report and review of literature. Spine (Phila Pa 1976). 2006;31:E859-E862. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 44] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 10. | Cosar M, Sasani M, Oktenoglu T, Kaner T, Ercelen O, Kose KC, Ozer AF. The major complications of transpedicular vertebroplasty. J Neurosurg Spine. 2009;11:607-613. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 41] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 11. | Mattei TA, Rehman AA, Dinh DH. Acute Spinal Subdural Hematoma after Vertebroplasty: A Case Report Emphasizing the Possible Etiologic Role of Venous Congestion. Global Spine J. 2015;5:e52-e58. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 12. | Lee KD, Sim HB, Lyo IU, Kwon SC, Park JB. Delayed onset of spinal subdural hematoma after vertebroplasty for compression fracture: a case report. Korean J Spine. 2012;9:285-288. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 13. | Gharehdaghi M, Hassani M, Khooei AR, Ghodsi E, Taghizadeh A. Multicentric myxoid liposarcoma; a case report and literature review. Arch Bone Jt Surg. 2014;2:79-81. [PubMed] |
| 14. | Cho SH, Rhim SC, Hyun SJ, Bae CW, Khang SK. Intradural involvement of multicentric myxoid liposarcoma. J Korean Neurosurg Soc. 2010;48:276-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 15. | Antonescu CR, Tschernyavsky SJ, Decuseara R, Leung DH, Woodruff JM, Brennan MF, Bridge JA, Neff JR, Goldblum JR, Ladanyi M. Prognostic impact of P53 status, TLS-CHOP fusion transcript structure, and histological grade in myxoid liposarcoma: a molecular and clinicopathologic study of 82 cases. Clin Cancer Res. 2001;7:3977-3987. [PubMed] |
| 16. | Schwab JH, Boland PJ, Antonescu C, Bilsky MH, Healey JH. Spinal metastases from myxoid liposarcoma warrant screening with magnetic resonance imaging. Cancer. 2007;110:1815-1822. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 76] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 17. | Fourney DR, Schomer DF, Nader R, Chlan-Fourney J, Suki D, Ahrar K, Rhines LD, Gokaslan ZL. Percutaneous vertebroplasty and kyphoplasty for painful vertebral body fractures in cancer patients. J Neurosurg. 2003;98:21-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 211] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 18. | Kaloostian PE, Yurter A, Etame AB, Vrionis FD, Sciubba DM, Gokaslan ZL. Palliative strategies for the management of primary and metastatic spinal tumors. Cancer Control. 2014;21:140-143. [PubMed] |
| 19. | Ryska P, Rehák S, Odráka K, Maisnar V, Raupach J, Málek V, Renc O, Kaltofen K. [Role of percutaneous vertebroplasty and kyphoplasty in the treatment of oncology disorders of the spine]. Cas Lek Cesk. 2006;145:804-809; discussion 809-810. [PubMed] |
| 20. | Weill A, Chiras J, Simon JM, Rose M, Sola-Martinez T, Enkaoua E. Spinal metastases: indications for and results of percutaneous injection of acrylic surgical cement. Radiology. 1996;199:241-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 577] [Cited by in RCA: 454] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 21. | Mikami Y, Numaguchi Y, Kobayashi N, Fuwa S, Hoshikawa Y, Saida Y. Therapeutic effects of percutaneous vertebroplasty for vertebral metastases. Jpn J Radiol. 2011;29:202-206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 22. | Yang Z, Xu J, Sang C. [Clinical studies on treatment of patients with malignant spinal tumors by percutaneous vertebroplasty under guidance of digital subtraction angiography]. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2006;20:999-1003. [PubMed] |
| 23. | Lieberman I, Reinhardt MK. Vertebroplasty and kyphoplasty for osteolytic vertebral collapse. Clin Orthop Relat Res. 2003;S176-S186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 140] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 24. | Zheng ZM. [The disaster complication of percutaneous vertebroplasty and kyphoplasty: cement leakage and its prevention]. Zhonghua Yi Xue Za Zhi. 2006;86:3027-3030. [PubMed] |
| 25. | Baumann A, Tauss J, Baumann G, Tomka M, Hessinger M, Tiesenhausen K. Cement embolization into the vena cava and pulmonal arteries after vertebroplasty: interdisciplinary management. Eur J Vasc Endovasc Surg. 2006;31:558-561. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 62] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 26. | Moussallem CD, El-Yahchouchi CA, Charbel AC, Nohra G. Late spinal subdural haematoma after spinal anaesthesia for total hip replacement. J Bone Joint Surg Br. 2009;91:1531-1532. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 27. | Gakhar H, Bommireddy R, Klezl Z, Calthorpe D. Spinal subdural hematoma as a complication of spinal surgery: can it happen without dural tear? Eur Spine J. 2013;22 Suppl 3:S346-S349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 28. | Domenicucci M, Ramieri A, Ciappetta P, Delfini R. Nontraumatic acute spinal subdural hematoma: report of five cases and review of the literature. J Neurosurg. 1999;91:65-73. [PubMed] |
| 29. | Konitsiotis S, Glantzouni A, Argyropoulou MI, Tsapoga T, Elisaf M, Efremidis SC. Acute spontaneous spinal subdural haematomas in a patient with essential thrombocythaemia. J Neurol. 2003;250:1109-1111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 11] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 30. | Kalina P, Drehobl KE, Black K, Woldenberg R, Sapan M. Spinal cord compression by spontaneous spinal subdural haematoma in polycythemia vera. Postgrad Med J. 1995;71:378-379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 31. | Haraga I, Sugi Y, Higa K, Shono S, Katori K, Nitahara K. [Spontaneous spinal subdural and epidural haematoma in a pregnant patient]. Masui. 2010;59:773-775. [PubMed] |
| 32. | Kim SD, Park JO, Kim SH, Lee YH, Lim DJ, Park JY. Spontaneous thoracic spinal subdural hematoma associated with fibromuscular dysplasia. J Neurosurg Spine. 2008;8:478-481. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 33. | Abut Y, Erkalp K, Bay B. Spinal subdural hematoma: a pre-eclamptic patient with a spinal arteriovenous malformation. Anesth Analg. 2006;103:1610. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 34. | Morandi X, Riffaud L, Chabert E, Brassier G. Acute nontraumatic spinal subdural hematomas in three patients. Spine (Phila Pa 1976). 2001;26:E547-E551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 65] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 35. | Kim JS, Lee SH. Spontaneous spinal subarachnoid hemorrhage with spontaneous resolution. J Korean Neurosurg Soc. 2009;45:253-255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 18] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 36. | Morandi X, Carsin-Nicol B, Brassier G, Scarabin JM. MR demonstration of spontaneous acute spinal subdural hematoma. J Neuroradiol. 1998;25:46-48. [PubMed] |
Manuscript Source: Unsolicited Manuscript
Specialty type: Medicine, research and experimental
Country of origin: Italy
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Araujo AMF, Palacios-Eito A S- Editor: Kong JX L- Editor: A E- Editor: Wang S