Published online Dec 16, 2017. doi: 10.12998/wjcc.v5.i12.432
Peer-review started: June 30, 2017
First decision: August 10, 2017
Revised: August 25, 2017
Accepted: September 13, 2017
Article in press: September 13, 2017
Published online: December 16, 2017
Processing time: 162 Days and 0.1 Hours
Taenia spp. are flatworms of the class Cestoda, whose definitive hosts are humans and primates. Human infestation (taeniasis) results from the ingestion of raw meat contaminated with encysted larval tapeworms and is considered relatively harmless and mostly asymptomatic. Anemia is not recognized as a possible sign of taeniasis and taeniasis-induced hemorrhage is not described in medical books. Its therapy is based on anthelmintics such praziquantel, niclosamide or albendazole. Here we describe a case of acute ileal bleeding in an Italian man affected with both Taenia spp. infestation resistant to albendazole and Helicobacter pylori-associated duodenal ulcers.
Core tip: The novel contribution of our paper is to draw attention to taeniasis as a possible cause of gastrointestinal bleeding, since anemia is so far not recognized as a possible sign of taeniasis nor is taeniasis-induced hemorrhage described in medical text books. With this report we describe a case of ileal bleeding most probably caused by this kind of infestation. Our objective is to make clinicians aware of this rare but possible situation. Taeniasis should be therefore taken into account in the differential diagnosis of melena and/or hematochezia.
- Citation: Settesoldi A, Tozzi A, Tarantino O. Taeniasis: A possible cause of ileal bleeding. World J Clin Cases 2017; 5(12): 432-436
- URL: https://www.wjgnet.com/2307-8960/full/v5/i12/432.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v5.i12.432
Taenia spp. are flatworms of the class Cestoda, whose definitive hosts are humans and primates. Human infestation (taeniasis) results from the ingestion of raw or undercooked meat contaminated with encysted larval tapeworms (cysticercosis), which subsequently exit the cyst and reach lengths of 3.6-7.5 m in the human gut. In Italy taeniasis incidence is 0.02%-0.04%, while cysticercosis prevalence is 0.02%-2.4%[1]. Taeniasis is relatively harmless and clinically asymptomatic. In sporadic cases it can cause loss of appetite, weight loss, abdominal pain, diarrhea or constipation, dizziness, headaches or nausea. Anemia is not recognized as a possible sign of taeniasis nor is taeniasis-induced hemorrhage described in medical text books[2]. Nevertheless there are some reports in medical literature which consider such associations possible[2-6]. Taeniasis therapy is commonly based on the use of anthelmintics like praziquantel, niclosamide or albendazole. Here we describe a case of acute ileal bleeding in an Italian man affected with both Taenia spp. infestation resistant to albendazole and Helicobacter pylori (H. pylori)-associated duodenal ulcers.
In March 2016 a 77-year-old Italian man attended the Emergency Room of San Giuseppe Hospital in Empoli. He was a retired farmer, who had been suffering from hematochezia together with melena for two weeks. His vital parameters were normal. In his medical history there was chronic atrial fibrillation, for which he was taking rivaroxaban 20 mg/d and digoxin 0.125 mg/d, and prior cholecystectomy. He weighed 68 kg, was 170 cm tall (body mass index 23.5). Digital rectal examination revealed the presence of melena. A nasogastric tube was inserted, with no evidence of gastric blood traces. Laboratory tests showed anemia (Hb 5.1 g/dL). The patient underwent blood transfusions and esophagogastroduodenoscopy (EGD), which showed a small hiatal hernia, bile in the stomach and a 10 mm duodenal fibrinous ulcer. We decided to suspend rivaroxaban and start low molecular weight heparin. Continuous intravenous infusion of pantoprazole was administered and the patient was admitted to the Gastroenterology Department.
In the following days he continued to bleed and required further blood transfusions. He also underwent several EGDs, which confirmed the previous findings and allowed us to get biopsies in the antrum for rapid urease test and on the ulcer margins for histology assessment. We also used argon plasma coagulation on the oozing borders of the ulcer, with consequent bleeding cessation. The rapid urease test was positive for H. pylori and the patient started eradication treatment. The biopsies of the ulcer margins demonstrated regenerative hyperplasia on productive inflammation.
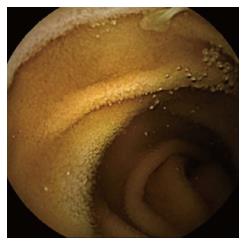
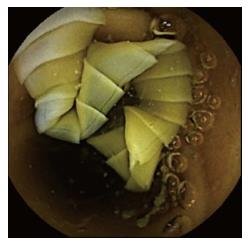
Despite endoscopic therapy, the patient still complained of melena and hematochezia. He underwent ileocolonoscopy, which showed bright red blood in ileum and colon, without identification of the bleeding source. We performed a contrast-enhanced computed tomography (CT) scan of the abdomen and of the abdominal aorta, which showed no bleeding cause and only minor findings like parietal calcifications of blood vessels, a small hepatic cyst, diffuse moderate intra- and extrahepatic biliary ducts dilation and benign prostatic hyperplasia. We then decided to use wireless capsule endoscopy (WCE), which described two duodenal fibrinous ulcers (5 and 10 mm) without signs of recent bleeding, the presence of a tapeworm, starting from 1 h 45 min until 4 h 28 min after WCE ingestion (Figures 1 and 2) and plentiful dark red blood in the colon, lighter in the proximal regions.
The patient reported only then, that he had occasionally eaten raw beef and that he had taken mebendazole 5 mo before, because of suspected oxyuriasis. The patient received albendazole 400 mg/d for five days, since this drug was suitable for taeniasis and already available in the department.
While on albendazole, he still complained about melena and iron-deficiency anemia (IDA) (Hb 9 g/dL, Hct 27%, RDW-CV 17%, iron 27 μg/dL, ferritin 53 ng/mL), with constant need of blood transfusions. He also stated he had not discharged the head of the tapeworm yet. We performed the last EGD, which demonstrated healing of the ulcers, with no bleeding signs. The second ileocolonoscopy was comparable to the first one, hence we decided to give him a second WCE after discontinuation of albendazole, which demonstrated persistence of the tapeworm starting from jejunum to the whole ileum (last visualization at 4 h 25 min) and plentiful dark red blood in the colon, lighter in the proximal regions (Figures 1 and 2).
We consulted an infectious disease specialist for a change of therapy after albendazole failure. He advised to give the patient niclosamide 2 g in a single administration and then to initiate bowel preparation with macrogol. After niclosamide and bowel preparation we performed the last ileocolonoscopy, which displayed no blood in ileum or colon and 2 non polypoid lesions in the caecum that we removed via loop electrosurgical excision procedure. Histology revealed those lesions to be intestinal tubular adenomas with low grade dysplasia.
The day after niclosamide administration, the patient felt a lot better. Melena and rectal bleeding had finally stopped and after two more days he noticed the complete excretion of the tapeworm head. Since his condition was improving, we decided to dismiss him.
He was seen at follow-up visit in mid-June 2016, where he stated he was feeling good and had no more rectal bleeding. He performed fecal obscure blood test and H. pylori stool antigen test, both showing negative results. His hemoglobin had reached 11.3 g/dL by then, with a positive trend. He underwent ova and parasite fecal exams at 2, 4 and 6 mo after dismission, none of which showed relapse of the infestation.
Among the currently accepted causes of iron-deficiency anemia, infestations with Ascaris lumbricoides, Trichuris trichiura, Necator americanus and Ancylostoma duodenale are reported, while taeniasis is not mentioned[7].
Likewise, no parasitic infection is officially listed as a cause of upper or lower gastrointestinal (GI) bleeding[8,9].
Even if not conventionally accepted, the association between anemia and/or hemorrhage and taeniasis has already been reported in a few other case reports.
In 1989 Ali et al[3] found anemia in 50 urban and rural Egyptians, suffering from various parasitic infestations, among which T. saginata.
De Simone et al[4] described T. solium as the only cause of IDA secondary to acute intestinal bleeding in a woman, who had underwent surgical enterotomy for suspected angiodysplasia.
A case of T. saginata infestation causing macrocytic anemia was reported by Vuylsteke et al[5] in 2004. The tapeworm and adjacent erosions were seen in the terminal ileum at colonoscopy and the patient recovered after therapy with niclosamide[5].
In 2007 Barnett et al[2] published a case of a 7-year old boy suffering from IDA, whose colonoscopy showed taeniasis after repeated normal stool examination. The tapeworm was not regarded as the cause of his anemia and 7 years later anemia recurred. Upper and lower endoscopy were negative and the boy underwent WCE, that found Taenia spp. in the mid jejunum, together with ulcers and areas of denuded mucosa.
A case of melena similar to ours was reported by Howell et al[6] in 2008. Their patient showed a pylorus ulcer and a vascular duodenal lesion at EGD, which were treated with adrenaline, electrocautery and H. pylori eradication, without recovery of the symptoms. A later WCE revealed taeniasis in mid-jejunum, with small erosions, without active bleeding.
In Western countries intestinal parasite infestations are rarely taken into account in the diagnostic work-up of anaemia or GI hemorrhage[4]. In our case anemia could have been attributed at first to the presence of H. pylori-associated duodenal ulcers, but melena and hematochezia persisted despite the healing of the ulcers, observed at repeated EGDs and at WCE. While, on the contrary, these two signs stopped after niclosamide therapy and subsequent expulsion of the tapeworm.
The mechanisms that cause digestive bleeding in taeniasis are still poorly understood and could be related to erosions of the bowel mucosa caused by the parasite[4]. Mucosal injury might be determined either directly, by the movement and feeding of the parasite or indirectly, by the host’s immune response[5]. Healing of the ulcers has been described after eradication of the tapeworm[2].
Our patient had already taken mebendazole for suspected oxyuriasis 5 mo before. We can speculate that he had taeniasis rather than oxyuriasis and that he saw proglottids instead of pinworms in his feces. Mebendazole is, in fact, not indicated in taeniasis. The majority of people with taeniasis have a single tapeworm in their GI tract and Taenia spp. can survive up to 30 years[6].
Stool examination is not a very sensitive test for the diagnosis of taeniasis, because of the need of full maturation of the tapeworm, that can take a lot of months. Differentiation between T. saginata and T. solium can be obtained from a careful examination of fecal proglottids[6]. Unfortunately we could not perform such examination, since the tapeworm and its proglottids were never collected.
The finding of a tapeworm during an EGD is quite rare. Taenia spp. usually attach to the upper jejunum, because their scolex becomes evaginated under digestive enzyme stimuli in that site[10]. Most often, the diagnosis is made during a colonoscopy or a WCE. According to international guidelines for the management of obscure GI bleeding or unexplained IDA, WCE is indicated after negative upper and lower GI endoscopy[11]. De Simone, Barnett and Howell initially used upper and/or lower endoscopy to diagnose the cause of the blood loss. These procedures didn’t reveal the tapeworm. The diagnosis could only be obtained using WCE[2,4,6]. WCE allows physicians to have a look at the entire bowel, especially at those intestinal tracts that are not reachable by EGD or colonoscopy. Our case, as well, highlights the ability of WCE to diagnose taeniasis and to follow abnormalities after treatment[2].
Our patient was a retired farmer who occasionally ate raw beef. We can speculate his rural family background and poor education have led him to take this habit. In any case, we have no evidence for any specific demographics, that could include an increased number of raw meat eaters.
Treatment of taeniasis includes praziquantel (5-10 mg/kg, single-administration) or niclosamide (2 g, single-administration after a light breakfast followed after 2 h by a laxative). Treatment of human cysticercosis includes praziquantel and/or albendazole, corticosteroids and/or anti-epileptic drugs[12]. Asymptomatic cysticercosis requires no treatment. In 1991 De Kaminsky RG treated 56 individuals suffering from taeniasis with albendazole 400 mg for 3 d. All of them discharged the tapeworm and remained stool-negative after 60 and 90 d. Of the 21 Taenia spp. recovered, 4 were T. saginata, 15 were T. solium and 2 could not be identified. Albendazole seemed to be well-tolerated and very effective[13]. Nevertheless albendazole resistance cases have been described. Màrquez-Navarro et al[14] reported a case of albendazole failure in a child with 5-year long-lasting infection. Niclosamide is not absorbed by the GI tract. Therefore it has no activity against cysts and is very safe. Its efficacy is high, with cure rates of approximately 90% against T. saginata and T. solium. Unfortunately, niclosamide is not easy to find[15]. We also had to wait some days to get it, since our hospital pharmacy didn’t have it.
Our case underlines the possible association between taeniasis and digestive bleeding. We recommend investigating raw meat consumption in every patients suffering from obscure anemia. In case of positive history, it is advisable to perform ova and parasite fecal tests, whose negative result should not erase the suspicion of a GI infestation. The use of WCE as diagnostic tool in obscure anemia should be supported, as its ability to reveal the presence of tapeworms, after negative EGD and colonoscopy, has been described in this and other case reports. We suggest to be aware of possible drug resistance, which could be demonstrated by the persistence of the tapeworm, and could be overcome through switching therapy. Taking into account the possible association between taeniasis and small bowel bleeding could spare hospitalization days and allow a better and faster recovery of the patients.
The man had been suffering of hematochezia and melena for two weeks, together with fatigue, pallor, exertional dyspnea and retrosternal pain receding with rest.
The patient weighed 68 kg and was 170 cm tall (body mass index 23.5), his vital parameters were good; he showed melena at the digital rectal examination but no gastric blood traces.
Peptic ulcer, Mallory-Weiss lesion, Dieulafoy lesion, neoplasms, esophagitis, gastritis, duodenitis, polyps, inflammatory bowel disease, diverticula, infectious colitis, angiodysplasia, ischemic colitis.
Laboratory tests showed anemia (Hb 5,1 g/dL), that was later defined as iron-deficiency anemia (Hb 9 g/dL, Hct 27%, RDW-CV 17%, iron 27 μg/dL, ferritin 53 ng/mL).
Esophagogastroduodenoscopy (EGD) showed a duodenal fibrinous ulcer, colonoscopy showed blood in ileum and colon, computed tomography scan showed only minor findings, while wireless capsule endoscopy (WCE) showed the presence of a tapeworm in the jejunum- ileum.
The rapid urease test was positive for Helicobacter pylori, the biopsies of the ulcer margins demonstrated regenerative hyperplasia on productive inflammation, the polyps of the colon turned out to be intestinal tubular adenomas with low grade dysplasia.
The patient received albendazole 400 mg/d for five days, and then niclosamide 2 g in a single administration.
The association between anemia and/or hemorrhage and taeniasis has already been reported in a few other case reports.
Taeniasis is the human infestation of Taenia spp., and results from the ingestion of raw or undercooked meat contaminated with encysted larval tapeworms (cysticercosis).
This case underlines the possible association between taeniasis and acute post-hemorrhagic anemia. The authros recommend investigating raw meat consumption in every patient suffering from obscure anemia, and using WCE as a diagnostic tool in case of negative EGD and colonoscopy.
The authors have described a case of gastrointestinal bleeding in a man infestated with Taenia spp., that resolved after taeniasis treatment. The novel contribution of this paper is to draw attention to taeniasis as a possible cause of gastrointestinal bleeding that should be therefore taken into account in the differential diagnosis of melena and/or hematochezia.
| 1. | Cabaret J, Bouilhol M, Mage C. Managing helminths of ruminants in organic farming. Vet Res. 2002;33:625-640. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 32] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 2. | Barnett K, Emder P, Day AS, Selby WS. Tapeworm infestation: a cause of iron deficiency anemia shown by capsule endoscopy. Gastrointest Endosc. 2007;66:625-627. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 3. | Ali AA, Mahmoud LH, el-Zoheiry AA. A study on intestinal helminths causing human anaemia in Cairo. J Egypt Soc Parasitol. 1989;19:251-256. [PubMed] |
| 4. | De Simone P, Féron P, Loi P, Van Nuffelen M, Nagy N, Van Gossum A, Gelin M. [Acute intestinal bleeding due to Taenia solium infection]. Chir Ital. 2004;56:151-156. [PubMed] |
| 5. | Vuylsteke P, Bertrand C, Verhoef GE, Vandenberghe P. Case of megaloblastic anemia caused by intestinal taeniasis. Ann Hematol. 2004;83:487-488. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 6. | Howell J, Brown G. Education and imaging. Gastrointestinal: beef tapeworm (Taenia saginata). J Gastroenterol Hepatol. 2008;23:1769. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 7. | WHO. Soil-transmitted helminth infections: World Health Organization 2017. [updated January 2017]. Available. Available from: http://www.who.int/mediacentre/factsheets/fs366/en/. |
| 8. | Bogitsh B, Carter C. Human Parasitology- 4th Edition 2012. Available from: https://www.k-state.edu/michellab/lab%20manual_biol546-2012.pdf. |
| 9. | Shorbagi A, Efe C, Ozseker B, Kav T, Bayraktar Y. Education and Imaging. Gastrointestinal: An unexpected cause of refractory iron deficiency anemia; Taenia SPP. on capsule endoscopy. J Gastroenterol Hepatol. 2012;27:843. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 10. | WHO. Taeniasis Signs, symptoms and treatment of taeniasis/cysticercosis. Available from: http://www.who.int/taeniasis/symptoms/en/. |
| 11. | de Kaminsky RG. Albendazole treatment in human taeniasis. Trans R Soc Trop Med Hyg. 1991;85:648-650. [PubMed] |
| 12. | Márquez-Navarro A, Cornejo-Coria Mdel C, Cebada-López F, Sánchez-Manzano RM, Díaz-Chiguer DL, Nogueda-Torres B. Taenia saginata: failure treatment in a child with 5-year long-lasting infection. Gastroenterol Nurs. 2012;35:125-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 13. | Vermund SH, MacLeod S, Goldstein RG. Taeniasis unresponsive to a single dose of niclosamide: case report of persistent infection with Taenia saginata and a review of therapy. Rev Infect Dis. 1986;8:423-426. [PubMed] |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country of origin: Italy
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Burke DA, Kim JM, Okello M S- Editor: Ma YJ L- Editor: A E- Editor: Lu YJ