Published online Nov 16, 2017. doi: 10.12998/wjcc.v5.i11.397
Peer-review started: June 3, 2017
First decision: June 27, 2017
Revised: July 13, 2017
Accepted: September 12, 2017
Article in press: September 13, 2017
Published online: November 16, 2017
Processing time: 170 Days and 5.6 Hours
Here, we report a case of gastric neuroendocrine carcinoma showing an interesting tumorigenic pathway. A 57-year-old Japanese woman presented with epigastric tenderness, and distal gastrectomy was performed. In the surgical specimen, histologically, the tumor tissue was composed of three subtypes of tumor components showing different histological architecture and cellular atypia, diagnosed as neuroendocrine tumor (NET) G2, NET G3, and neuroendocrine carcinoma (NEC) components. Immunohistochemically, the Ki-67-positive rates of NET G2, NET G3, and NEC components were 6.5%, 99.5% and 88.1%, respectively. Although allelic imbalance (AI) on chromosomes 1p, 3p, 8q, TP53, 18q and 22q was commonly found in all components, AI of 4p was found in NET G3 and NEC components (but not in the NET G2 component). In contrast, AIs of 5q and 9p were found in only the NEC component. Thus, we showed the progression from NET G2 to NEC, via NET G3, within the same tumor.
Core tip: Gastric neuroendocrine carcninoma (NEC) is typically generated by dedifferentiation of adenocarcinoma cells to endocrine cells. However, we experienced a case of gastric NEC possibly generated from the neuroendocrine tumor (NET) component. The present case demonstrated an unconventional carcinogenic pathway in neuroendocrine tumorigenesis. In addition, we analyzed allelic imbalance in NET and NEC components and provided important insights into neuroendocrine carcinogenesis.
- Citation: Uesugi N, Sugimoto R, Eizuka M, Fujita Y, Osakabe M, Koeda K, Kosaka T, Yanai S, Ishida K, Sasaki A, Matsumoto T, Sugai T. Case of gastric neuroendocrine carcinoma showing an interesting tumorigenic pathway. World Journal of Clinical Cases 2017; 5(11): 397-402
- URL: https://www.wjgnet.com/2307-8960/full/v5/i11/397.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v5.i11.397
Neuroendocrine neoplasms of the digestive system are classified as neuroendocrine tumors (NETs) or neuroendocrine carcinomas (NECs) based on assessment of the pro-liferative fraction and morphological criteria in the 2010 World Health Organization (WHO) classification. Furthermore, NET can be divided into three tiers (G1, G2, and G3) based on the mitotic count and Ki-67 labeling index[1]. NEC of the stomach is composed of proliferation of poorly differentiated tumorous endocrine cells with marked cellular atypia[1]. In general, gastric NEC may be generated by dedifferentiation of adenocarcinoma cells to endocrine cells, and it is possible that gastric NETs and NECs may have different tumorigenic pathways[1,2]. Here, we report a case of gastric NEC generated from a NET component, with immunohistochemical and molecular studies.
A 57-year-old Japanese woman presented with epigastric tenderness. Gastroendoscopy showed a growing lesion (size, 16 mm × 11 mm) in the greater curvature of the upper gastric body, and the mucosa adjacent to the lesion showed atrophic gastritis. Histopathological examination of a biopsy sample revealed NEC. Helicobactor pylori were not detected by Giemsa staining. In addition, serum levels of chromogranin A and gastrin were not measured. The patient subsequently underwent distal gastrectomy. One year after surgery, liver metastasis was found in abdominal computed tomography examination, and chemotherapy was performed.
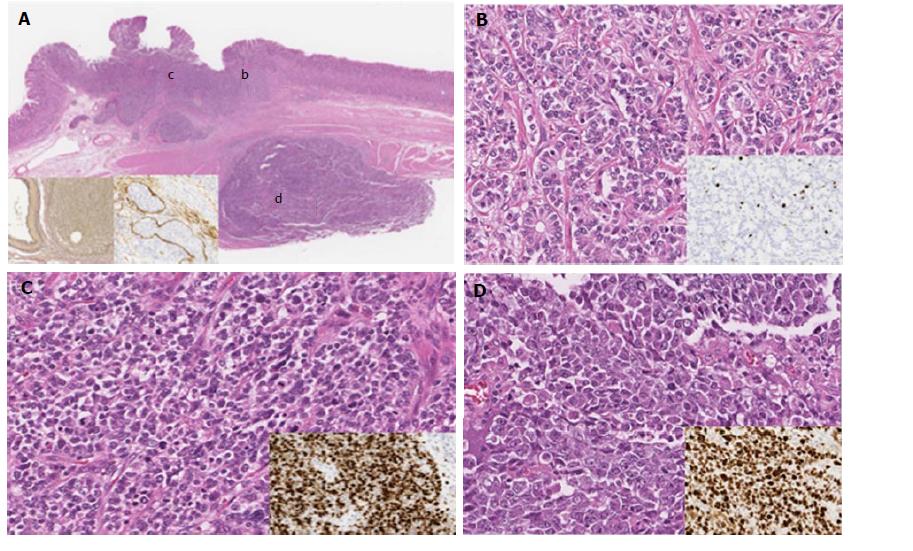
In low-magnification images, tumor cells were distributed in two lesions. One lesion was constructed by tumor cell proliferation within the submucosal layer, and another lesion was found in the muscle layer; these two lesions were discontinuous (Figure 1A).
Microscopic examination revealed that the tumor tissue was composed of three subtypes of tumor components as follows. The first component was composed of cuboidal epithelial cells with uniform, oval nuclei showing fine nuclear chromatin and arranged in a trabecular growth pattern with focal rosettes. Tumor cells had very few mitotic figures [< 2 per 10 high-powered fields (HPF)] and no necrosis. This component corresponded to the NET G2 component (Figure 1B). The second component consisted of diffuse proliferation of tumor cells with small nuclei showing irregular, dense nuclear chromatin and high mitotic counts, corresponding to the NET G3 component (Figure 1C). The third component was composed of diffuse proliferation of tumor cells with large, irregular, coarse nuclei, high mitotic counts, and geographic necrosis, similar to the large cell NEC component (Figure 1D). Although the transition between the NET G2 component and the NET G3 component was confirmed, the submucosal lesion and intramuscular lesion were separated by the muscle layer (Figure 1A). Marked lymph vessel and venous invasion were observed in the submucosal layer, as determined by D2-40 immunohistochemical staining and EVG staining (Figure 1A, inset).
In addition, the mucosa adjacent to the tumor showed atrophic gastritis with intestinal metaplasia, but not type A gastritis. No endocrine cell micronests were found in the surrounding mucosa.
Immunohistochemical examination was performed using an auto-immunostaining system (Dako EnVision System, Denmark) for neuroendocrine differentiation, cell proliferation activity, p53 overexpression, and mucin phenotype (Muc2, Muc5AC, Muc6, CD10). The positive rate of Ki-67 was calculated using an APERIO virtual slide system (AT2; Leica Biosystems, United States).
All tumor components were immunopositive for chromogranin A, synaptophysin, and CD56, and decreased immunoreactivity was found in the NEC component. Differences in Ki-67-positive rates were found among NET G2, NET G3, and NEC components (NET G2, 6.5%; NET G3, 99.5%; NEC, 88.1%). Overexpression of p53 protein was not found in any component. With regard to the mucin phenotype, no mucin markers were expressed in any tumor component (Table 1).
| Antibody | Clone | Dilution | Source | NET G2 component | NET G3 component | NEC component |
| Chromogranin A | DAK-A3 | 1:100 | DAKO, CA, Unites States | Positive | Positive | Weakly positive |
| Synaptophysin | SY38 | 1:20 | DAKO, CA, Unites States | Positive | Positive | Weakly positive |
| NCAM | 1B6 | 1:100 | DAKO, CA, Unites States | Positive | Positive | Weakly positive |
| Ki-67 | MIB-1 | 1:50 | DAKO, CA, Unites States | 6.50% | 99.50% | 88.10% |
| p53 | DO-7 | 1:100 | Novocastra, United Kingdom | Negative | Negative | Negative |
| Muc2 | Ccp58 | 1:200 | Novocastra, United Kingdom | Negative | Negative | Negative |
| Muc5AC | CLH2 | 1:100 | Novocastra, United Kingdom | Negative | Negative | Negative |
| Muc6 | CLH5 | 1:100 | Novocastra, United Kingdom | Negative | Negative | Negative |
| CD10 | 56C6 | 1:50 | Novocastra, United Kingdom | Negative | Negative | Negative |
DNA from NET G2, NET G3, and NEC components was extracted separately. PCR-allelic imbalance (AI) analyses were performed using a thermal cycler (GeneAmp PCR System 9600; Perkin-Elmer, CA, United States) according to previously reported procedures[3]. AIs on chromosomes 1p, 3p, 4p, 5q, 8p, 9p, 13q, 17p, 18p, and 22q were examined using 22 highly pleomorphic microsatellite markers (D1S228, D1S548, D3S2402, D3S1234, D4S2639, D4S1601, D5S107, D5S346, D5S299, D5S82, D8S201, D8S513, D8S532, D9S171, D9S1118, D13S162, TP53, D18S487, D18S34, D22S274, D22S1140, and D22S1168).
In addition, PCR-MSI analysis was performed as described previously[4]. Five different loci were assessed for MSI, including all those recommended by the Bethesda panel for colon cancer (BAT25, BAT26, D5S346, D2S123, and D17S250)[4].
DNA methylation at the six specific promoters originally described by Yagi and colleagues was quantified[5]. Methylation of three markers (RUNX3, MINT31, and LOX) was analyzed, and samples with at least two methylated markers were defined as highly methylated epigenotype (HME) tumors. The remaining tumors were also screened for methylation at three other markers (NEUROG1, ELMO1, and THBD) and were defined as intermediate methylation epigenotype (IME) tumors if they had at least two methylated markers out of the three markers proposed as a second panel. Tumors not classified as HME or IME were designated as low methylation epigenotype (LME).
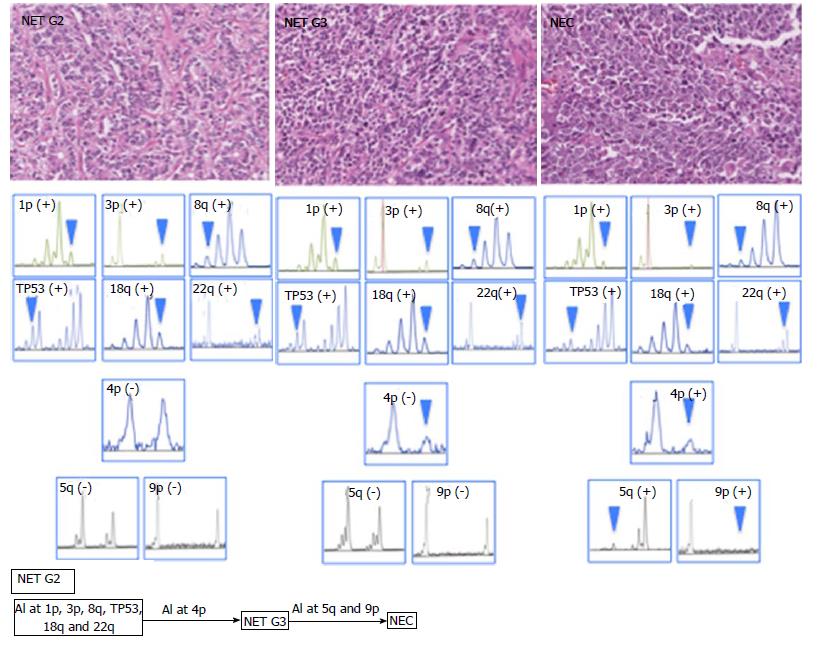
In the present case, DNA methylation status was LME, and MSI was not found in all tumor components. AIs at 1p, 3p, 8q, TP53, 18q and 22q were commonly found in all tumor components. Although AI of 4p was found in NET G3 and NEC components, it was not observed in the NET G2 component. In addition, AIs at 5q and 9p were found in the NEC component only (negative for NET G2 and G3 components; Figure 2).
Most cases of gastric NEC may be developed from endocrine precursor cell clones occurring in preceding adenocarcinoma components[1,2]. In the present case, histological examination revealed that the tumor tissue was composed of three subtype components. In addition, the transition between NET G2 and G3 components was confirmed. No adenocarcinoma components were found in serial sections of all specimens. Although a clear transition between the NET and NEC components was not found, we assumed that the tumor mass in the muscle layer may be an intramural metastasis because the tumor tissue showed marked vessel invasion (Figure 1A). Therefore, we speculated that the morphological change from NET to NEC could have occurred through the process of tumor progression. Additionally, NEC may arise from the NET component, suggesting that the tumor may have developed through an unconventional pathway in neuroendocrine tumorigenesis in our case[2].
With regard to the tumorigenesis of the NET G2 component, although serum chromogranin A and gastrin had been measured, no endocrine cell micronests or findings of type A gastritis were observed in the mucosa adjacent to the tumor. Therefore, we assumed that the tumor in this case may be sporadic NET, namely non-endochromaffin-like cell tumor, defined as type 3 tumor by Rindi et al[1].
In previous reports, most cases of gastric NEC with an adenocarcinomatous component were found to exhibit immunopositivity of mucin markers, and the mucin phenotype of the NEC component was found to correspond with that of the adenocarcinoma component[2,6]. In our case, both the NET and NEC components showed negative immunoreactivity for all mucin markers examined. Thus, it was unlikely that the NEC component was generated from adenocarcinoma in the present case.
Fur-thermore, Nishikura et al[7] reported that most cases of NEC with an ad-enocarcinoma component showed p53 mutations, consistent with the adenocarcinoma component. This finding strongly supported the hypothesis that most cases of gastric NEC are developed from precursor cell clones that occur in a preceding adenocarcinoma component.
The molecular features of NET and NEC have not been clarified[8]. In the present case, AIs at 1p, 3p, 8q, TP53, 18q, and 22q were commonly found in three components (NET G2, NET G3, and NEC). These findings supported that the tumor in our case had been generated by progression from the NET G2 to NEC component. In contrast, AI at 4p was acquired during the progression from NET G2 to G3. In addition, AIs at 5q and 8q play an important role in the development from NET G3 to NEC. These findings suggested that acquisition of multiple AIs contributed to the progression from NET to NEC (Figure 2).
In conclusion, we experienced a case of gastric NEC possibly generated from the NET component. The present case demonstrated an unconventional carcinogenic pathway in neuroendocrine tumorigenesis, providing important insights into neuroendocrine carcinogenesis.
Gastric neuroendocrine carcinoma showing unconventional tumorigenic pathway.
Gastric carcinoma.
Gastric carcinoma.
Gastric carcinoma.
Gastric neuroendocrine carcinoma.
Distal gastrectomy.
This is an interesting case of gastric neuroendocrine carcinoma showing a tumorigenic pathway. This report presents a case of gastric NEC possibly generated from NET component by analyzing allelic imbalance and shows unconventional carcinogenic pathway in neuroendocrine tumorigenesis.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country of origin: Japan
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Fukuchi M, Tsolakis AV, Yang F S-Editor: Gong ZM L-Editor: A E-Editor:Zhao LM
| 1. | Bosman FT, Carneiro F, Hruban RH. WHO Classification of Tumours of the Digestive System. Lyon: IARC Press 2010; . |
| 2. | Domori K, Nishikura K, Ajioka Y, Aoyagi Y. Mucin phenotype expression of gastric neuroendocrine neoplasms: analysis of histopathology and carcinogenesis. Gastric Cancer. 2014;17:263-272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 25] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 3. | Sugai T, Habano W, Jiao YF, Toyota M, Suzuki H, Tsukahara M, Koizuka H, Akasaka R, Koeda K, Wakabayashi G. Molecular analysis of single isolated glands in gastric cancers and their surrounding gastric intestinal metaplastic mucosa. Oncol Rep. 2010;23:25-33. [PubMed] |
| 4. | Sugai T, Habano W, Uesugi N, Jao YF, Nakamura S, Abe K, Takagane A, Terashima M. Three independent genetic profiles based on mucin expression in early differentiated-type gastric cancers--a new concept of genetic carcinogenesis of early differentiated-type adenocarcinomas. Mod Pathol. 2004;17:1223-1234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 41] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 5. | Kaneda A, Yagi K. Two groups of DNA methylation markers to classify colorectal cancer into three epigenotypes. Cancer Sci. 2011;102:18-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 64] [Article Influence: 4.3] [Reference Citation Analysis (1)] |
| 6. | Takenaka Y, Tsukamoto T, Mizoshita T, Ogasawara N, Hirano N, Otsuka T, Ban H, Nakamura T, Yamamura Y, Kaminishi M. Gastric and intestinal phenotypic correlation between exocrine and endocrine components in human stomach tumors. Histol Histopathol. 2007;22:273-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 7. | Nishikura K, Watanabe H, Iwafuchi M, Fujiwara T, Kojima K, Ajioka Y. Carcinogenesis of gastric endocrine cell carcinoma: analysis of histopathology and p53 gene alteration. Gastric Cancer. 2003;6:203-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 73] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 8. | Leotlela PD, Jauch A, Holtgreve-Grez H, Thakker RV. Genetics of neuroendocrine and carcinoid tumours. Endocr Relat Cancer. 2003;10:437-450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 103] [Article Influence: 4.5] [Reference Citation Analysis (0)] |