Published online Apr 16, 2016. doi: 10.12998/wjcc.v4.i4.118
Peer-review started: November 6, 2015
First decision: December 23, 2015
Revised: December 30, 2015
Accepted: February 14, 2016
Article in press: February 16, 2016
Published online: April 16, 2016
Processing time: 159 Days and 22.2 Hours
Gastrointestinal (GI) stromal tumor is the most common mesenchymal neoplasm of the GI tract but also occurs with a lower frequency in extragastrointestinal regions and is called extragastrointestinal stromal tumor (EGIST). We report an unusual case of EGIST presenting as a vaginal mass. A 41-year-old woman presented with a gradually enlarging vaginal mass for the last 2 years. Physical examination revealed an elliptical, non-tender mass about 7.5 cm × 7 cm in size in the posterior vaginal wall and was resected completely. Under histological examination, the tumor showed a spindle cell type with coagulation necrosis, hemorrhage and high mitotic count. Immunohistochemical analysis revealed tumor cells were positive for DOG1, CD117, CD34 and p53 protein. Ki-67 labeling was 8%. Genetic analysis showed a deletion of exon 11 of the c-kit gene at codons 557-558. EGISTs should be kept in mind in the differential diagnosis in patients presenting with solid mass of the vaginal wall.
Core tip: Gastrointestinal (GI) stromal tumor is the most common mesenchymal neoplasm of the GI tract but also occurs with a lower frequency in extragastrointestinal regions and is called extragastrointestinal stromal tumor (EGIST). We report an unusual case of EGIST presenting as a vaginal mass and describe its clinicopathological, immunohistochemical and genetic features. Our data shows that this case was a primary malignant EGIST in the vaginal wall but few cases of primary vaginal EGIST have been reported to date. EGISTs should be kept in mind in the differential diagnosis of patients presenting with a solid mass of the vaginal wall.
- Citation: Liu QY, Kan YZ, Zhang MY, Sun TY, Kong LF. Primary extragastrointestinal stromal tumor arising in the vaginal wall: Significant clinicopathological characteristics of a rare aggressive soft tissue neoplasm. World J Clin Cases 2016; 4(4): 118-123
- URL: https://www.wjgnet.com/2307-8960/full/v4/i4/118.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v4.i4.118
Gastrointestinal (GI) stromal tumors (GISTs) are the most common mesenchymal neoplasms of the GI tract. They affect all segments of the digestive tract, usually originate in the wall of the stomach or small intestine and develop from the interstitial cells of Cajal[1]. The pathogenesis of GISTs is related to c-kit gene mutation, which results in activation of a c-kit receptor tyrosine kinase (KIT, also called CD117 or stem cell factor receptor), then cell proliferation induction and apoptosis inhibition. Most GISTs harbor gene mutations in either the c-kit or platelet-derived growth factor receptor alpha (PDGFRA) gene. Rarely, GISTs arise primarily in the omentum, mesentery, retroperitoneum or undefined abdominal sites, which are referred to as “extragastrointestinal stromal tumors” (EGISTs)[2]. To the best of our knowledge, few cases of primary EGIST have been reported arising in the vaginal wall. In this study, we present a rare case of vaginal EGIST and a brief literature review.
A 41-year-old woman complained of a painless mass in the perineal region which had gradually increased in size, initially from about 3 cm × 3 cm to 7.5 cm × 7 cm over a period of 2 years. The patient had no significant past medical or surgical history.
The gynecological examination revealed that the vagina was unobstructed and found a mass measuring about 7.5 cm × 7 cm in size with poor mobility and tenderness in the posterior vaginal wall. Its interior pole was closed to the vaginal opening with no discomfort symptoms such as bearing down. The ultrasonograph diagnosed cervix leiomyoma. The magnetic resonance imaging (MRI) revealed an elliptical mass in the cervix and posterior vaginal wall with a clear margin measuring about 9 cm × 7 cm × 6 cm in size, with equal T1 and T2 signals and a few punctate long T2 signal. The MRI also diagnosed leiomyoma. Other laboratory examinations were normal. The mass was completely removed along with part of the vaginal wall using vaginal resection and during the operation gynecologists found the margin of the mass was from the vaginal opening to the fornix vaginae, which was close to the rectum but the mass did not invade the bowel wall.
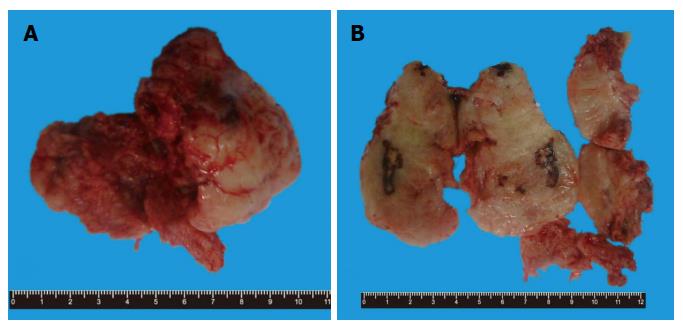
Grossly, the mass was elliptical, gray-white and grey-red, about 8 cm × 7.5 cm × 5 cm in size. The specimen was a well circumscribed mass and was surrounded by a fibrous capsule with pushing borders. Its section was also gray-white and grey-red in color with medium texture, containing multiple hemorrhages and necrosis (Figure 1).
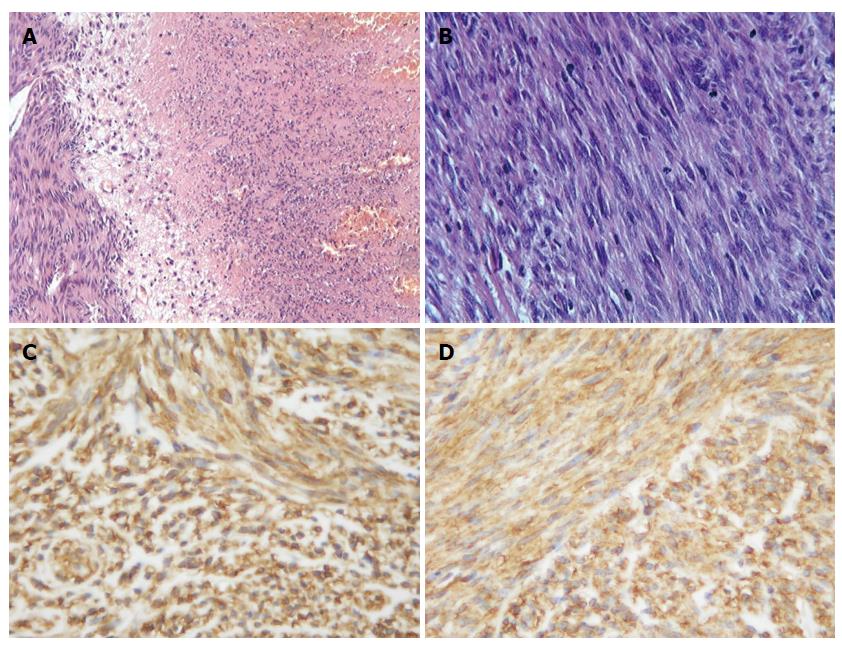
The fresh specimen was fixed in 10% formalin buffer solution and embedded in paraffin. Sections (3-4 μm) were cut from the block and stained with hematoxylin and eosin. Microscopic examination showed that the mass was mainly composed of spindle cells which were arranged in fasciculation or paliform, like leiomyoma or neurilemmoma. The cytoplasm of the tumor cells was eosinophilic and some had clear cytoplasm or paranuclear vacuoles. The nucleus of tumor cells was oval or rod shape with some vesicular chromatin and small prominent nucleoli. There were multiple large or small areas of coagulation necrosis and hemorrhage, accompanied by variable amounts of myxoid stroma (Figure 2A and B). Average mitotic figures were about 25 mitoses per 50 HPFs.
Immunohistochemical study was performed and the following antigens were used. CD117 (c-kit oncogene production, No.A4502, polyclonal, 1:70), CD34 (QBEND10, No.M7165, monoclonal, 1:150), S-100 protein (No.Z0311, polyclonal, 1:300), smooth muscle antibody (SMA, No.M0851, monoclonal, 1:200), desmin (No.M0760, monoclonal, 1:150), p53 protein (DO-7, No.M7001, monoclonal, 1:300) and Ki-67 (No.M7248, monoclonal, 1:100) were purchased from Dako Corp. Discovered on GIST 1 (DOG1) (DOG1, clone SP31, Cat.#RM-9132, monoclonal, ready to use) was applied by LabVision Corp. The slides were inserted in 10 mmol/L citrate buffer solution, pH 6.0 and heated in a microwave oven at a high setting for 5 min for epitope retrieval. The slides were incubated with 3,3-diaminobenzidine tetrahydrochloride, following the use of the EnVision staining technique, and counterstained with hematoxylin. The tumor cells showed diffusely strong positivity for DOG1, CD117 (Figure 2C and D) and CD34 at both the cytoplasmic and membranous components and p53 protein at the nucleus, while tumor cells were completely negative for SMA, desmin and S-100 protein. Ki-67 labeling was about 8%.
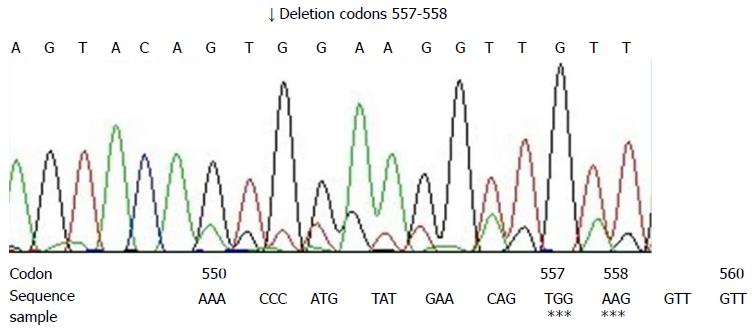
Direct sequencing of polymerase chain reaction (PCR) productions was applied to detect the gene mutations of the c-kit gene (exons 9, 11, 13, 17) and the PDGFRA gene (exons 12, 18). Sections (10 μm) were cut and DNA was extracted from paraffin embedded tissue, according to the protocol of the Dneasy Tissue Kit (Cat.69504, Qiagen, Hilden, Germany). The product was stored at -20 °C. Based on the c-kit gene and PDGFRA gene, 6 pairs of primers were designed and are shown in Table 1[3]. The PCR reaction conditions, recommended by Perkin Elmer, were the standard ones. The annealing temperature was set at 55 °C. PCR productions were fractionated on 50 g/L polyacrylamide gelatins and then stained with ethidium bromide. The ABI Prism 310 DNA sequencer (Applied Biosystems) was used to perform the direct sequencing by use of the same primers mentioned above. The Big Dye Terminator cycle sequencing ready reaction kit (Applied Biosystems Inc., Foster City, Calif.) was used for the sequencing reactions, according to the manufacturer’s instructions. PCR amplification and DNA sequencing revealed exon 11 mutation of c-kit gene. Sequence analysis showed the deletion of 6 bp at codons 557-558 (Figure 3). Other mutations were not found.
| Forward | Reverse |
| c-kit exon 9 | |
| 5’-TCCTAGAGTAAGCCAGGGCTT-3’ | 5’-TGGTAGACAGAGCCTAAACATCC-3’ |
| c-kit exon 11 | |
| 5’-CCAGAGTGCTCTAATGACTG-3’ | 5’-TGACATGGAAAGCCCCTGTT-3’ |
| c-kit exon 13 | |
| 5’-GCTTGACATCAGTTTGCCAG-3’ | 5’-AAAGGCAGCTTGGACACGGCTTTA-3’ |
| c-kit exon 17 | |
| 5’-CTCCTCCAACCTAATAGTGT-3’ | 5’-GTCAAGCAGAGAATGGGTAC-3’ |
| PDGFRA exon 12 | |
| 5’-TTGGATATTCACCAGTTACCTGTC-3’ | 5’-CAAGGGAAAAGCTCTTGG-3’ |
| PDGFRA exon 18 | |
| 5’-ACCATGGATCAGCCAGTCTT-3’ | 5’-TGAAGGAGGATGAGCCTGACC-3’ |
The maximal diameter of this mass was 8 cm and average mitotic figures were about 25 mitoses per 50 HPFs. There was multiple coagulation necrosis and hemorrhage. According to the results of immunohistochemistry and genetic mutation in exon 11 of c-kit gene, the histopathological diagnosis of this case was primary malignant EGIST in the vaginal wall. The patient was well after surgery and discharged with imatinib (STI-571) treatment. After 5 mo of follow-up, no recurrence or metastasis was found by clinical or radiological examination.
GIST is the most common mesenchymal tumor of the GI tract and can occur anywhere in the GI-tract, most commonly in the stomach (60%) and small intestine (30%)[4]. The occurrence of most EGIST is related to the metastasis of primary GIST. Primary EGIST is very rare and occurs in the omentum, mesentery, retroperitoneum, pleura, liver, spleen, pancreas, pelvis, rectovaginal septum and vagina with clinicopathological and molecular features similar to GISTs[5]. However, little is known about their actual origin and pathogenesis. There are three reported cases of primary vaginal EGIST[6-8] and Weppler et al[7] supposed they might be derived from the rectovaginal septum. Our case was a primary vaginal EGIST because it was an isolated tumor with a clear boundary and no adherence to the intestine. Furthermore, there was no abnormal affect in other organs, especially the GI tract.
The diagnosis of GIST is established based on the morphology characteristics, distinctive immunophenotype and molecular genetic features. Histologically, GIST usually presents as a nodular mass and is solid on cross section, frequently with hemorrhage. There are three types of GISTs: Spindle cell (70%), epithelioid (20%) and mixed[9]. The spindle type often presents with paranuclear vacuoles and is similar to leiomyoma or neurilemmoma. The National Institute of Health developed a new classification system for GIST for the risk of malignant behavior in 2001, which ranged from very low to high risk and was based on tumor size, mitotic counts and anatomical position, the three factors that are the most important reference index for judging malignant degree and prognosis[4,10,11]. In general, tumors larger than 5 cm in size with more than 5 mitoses per 50 HPFs are considered to be high risk. The tumor in our case was 8 cm in diameter with active mitotic counts about 25 mitoses per 50 HPFs and Ki-67 labeling was about 8%, so it was considered a high risk tumor and presented with malignant biological behavior. Hou et al[12] reported that most EGISTs from the abdominal cavity or retroperitoneum were borderline or malignant. Two of the three reported cases of primary vaginal EGISTs were malignant. Our present case was also malignant.
Approximately 95% of GISTs show a distinctive immunohistochemical feature of positive staining for CD117 on membrane, cytoplasm and the paranuclear region (Golgi pattern)[1]. CD117 is the product of proto-oncogene c-kit, a tyrosine kinase transmembrane receptor located on chromosome 4 (4q11-q12)[9]. CD117 positive tumors are most responsive to the treatment with c-kit selective tyrosine kinase inhibitor, imatinib (STI-571). DOG1, known also as TMEM16A and ANO1, is currently considered the most specific and sensitive marker of GIST[13]. Also, its expression does not depend on the gene mutation state of c-kit or PDGFRA gene and it is a very valuable marker, especially in CD117-negative cases[14]. In molecular genetics, 86% of GISTs present with mutations in the c-kit gene and 15% in PDGFRA gene[1]. In our case, immunohistochemical analysis showed diffusely strong positivity for DOG1, CD117, CD34 and p53 protein, while it was completely negative for SMA, desmin and S-100 protein. Mutational analysis revealed exon 11 mutation of c-kit gene that was deletion mutation at codons 557-558. He et al[15] reported that c-kit mutations were identified in 76.1% of CD117 positive GISTs and most were mutations of exon 11 (67.1%). Hou et al[16] discovered that many mutational sites of exon 11 were not fixed and had a central tendency. Point mutations and frame deletions were most frequently concentrated at codons 550-560 but duplications were most observed at codons 570-585. Terada[3] reported that primary EGIST of the omentum showed a deletion mutation of exon 11 of the c-kit gene at codons 552-558. Zheng et al[17] analyzed gene mutations from 25 cases of EGIST and found that the pattern of c-kit and PDGFRA mutation in EGISTs was essentially similar to that in GISTs, that is, c-kit mutations were detected in 44% of EGISTs and all were exon 11 mutations. The c-kit gene mutation state and type was not only correlated to malignant degree and prognosis of GIST, but also a predictive factor of imatinib (STI-571) therapeutic reaction, which has important significance in the clinical diagnosis of GIST. Exon 11 gene mutation occurred especially in malignant GISTs and might be a clinical useful adjunct marker in the evaluation of GISTs[16]. In our case, exon 11 mutation also occurred and presented with deletion mutations at codons 557-558, identical to those reported in the literature.
The diagnosis of primary EGIST should be based on morphology, immunophenotype and molecular genetic features, and at the same time differentiated from other mesenchymal tumors of GI tract. The present data on EGIST is insufficient to make a final conclusion regarding pathogenesis, biological behavior, treatment, prognosis and recurrence. Thus, follow-up for a long period of time is required.
A rare extragastrointestinal stromal tumor (EGIST) arising in the vaginal wall.
Leiomyoma.
Leiomyoma, malignant peripheral nerve sheath tumor and stromal sarcoma.
EGIST.
Excision and administration of imatinib.
In early reports, extragastrointestinal stromal tumor presented as a recurrent vulvar mass.
EGIST is a subtype of GIST and occurs in extragastrointestinal regions.
EGIST mostly has the same biological behavior as GIST.
This case (GIST in the vaginal wall) is an extremely rare case and well organized. The presentation of the case is clear and the discussion conducted well with updated references.
| 1. | Sekkate S, Kairouani M, Abahssain H, Serji B, Boutayeb S, Mrabti H, Errihani H. [Gastrointestinal stromal tumors]. Presse Med. 2012;41:917-926. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 2. | Yang HJ, Kim TH, Park MK, Lim CH, Lee KH, Kim CW, Han SW, Kim JA. [A case of primary extragastrointestinal stromal tumor presenting as peritoneal dissemination]. Korean J Gastroenterol. 2010;56:319-323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 3. | Terada T. Primary multiple extragastrointestinal stromal tumors of the omentum with different mutations of c-kit gene. World J Gastroenterol. 2008;14:7256-7259. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 32] [Cited by in RCA: 59] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 4. | Miettinen M, Lasota J. Histopathology of gastrointestinal stromal tumor. J Surg Oncol. 2011;104:865-873. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 138] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 5. | Barros A, Linhares E, Valadão M, Gonçalves R, Vilhena B, Gil C, Ramos C. Extragastrointestinal stromal tumors (EGIST): a series of case reports. Hepatogastroenterology. 2011;58:865-868. [PubMed] |
| 6. | Kim YJ, Jeong YY, Kim SM. Extragastrointestinal stromal tumor arising from the vagina: MR findings. Eur Radiol. 2006;16:1860-1861. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 7. | Weppler EH, Gaertner EM. Malignant extragastrointestinal stromal tumor presenting as a vaginal mass: report of an unusual case with literature review. Int J Gynecol Cancer. 2005;15:1169-1172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 29] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 8. | Ceballos KM, Francis JA, Mazurka JL. Gastrointestinal stromal tumor presenting as a recurrent vaginal mass. Arch Pathol Lab Med. 2004;128:1442-1444. [PubMed] |
| 9. | Alkhatib L, Albtoush O, Bataineh N, Gharaibeh K, Matalka I, Tokuda Y. Extragastrointestinal Stromal Tumor (EGIST) in the abdominal wall: Case report and literature review. Int J Surg Case Rep. 2011;2:253-255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 27] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 10. | Raut CP, Morgan JA, Ashley SW. Current issues in gastrointestinal stromal tumors: incidence, molecular biology, and contemporary treatment of localized and advanced disease. Curr Opin Gastroenterol. 2007;23:149-158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 35] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 11. | Kostka R, Gürlich R, Koldová L. Gastrointestinal stromal tumors (GIST): a single center experience. Acta Chir Belg. 2012;112:33-39. [PubMed] |
| 12. | Hou YY, Sun MH, Wei YK, Tan YS, Lu XY, Wang J, Zhu XZ, Zheng AH. [Clinicopathological, immunohistochemical and molecular genetic study of intra-abdomen extra-gastrointestinal stromal tumors]. Zhonghua Binglixue Zazhi. 2003;32:422-426. [PubMed] |
| 13. | Lee CH, Liang CW, Espinosa I. The utility of discovered on gastrointestinal stromal tumor 1 (DOG1) antibody in surgical pathology-the GIST of it. Adv Anat Pathol. 2010;17:222-232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 56] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 14. | González-Cámpora R, Delgado MD, Amate AH, Gallardo SP, León MS, Beltrán AL. Old and new immunohistochemical markers for the diagnosis of gastrointestinal stromal tumors. Anal Quant Cytol Histol. 2011;33:1-11. [PubMed] |
| 15. | He HY, Fang WG, Zhong HH, Li Y, Zheng J, Du J, Heng WJ, Wu BQ. [Status and clinical implication of c-kit and PDGFRA mutations in 165 cases of gastrointestinal stromal tumor (GIST)]. Zhonghua Binglixue Zazhi. 2006;35:262-266. [PubMed] |
| 16. | Hou YY, Tan YS, Sun MH, Wei YK, Xu JF, Lu SH, A-Ke-Su SJ, Zhou YN, Gao F, Zheng AH. C-kit gene mutation in human gastrointestinal stromal tumors. World J Gastroenterol. 2004;10:1310-1314. [PubMed] |
| 17. | Zheng S, Huang KE, Tao DY, Pan YL. Gene mutations and prognostic factors analysis in extragastrointestinal stromal tumor of a Chinese three-center study. J Gastrointest Surg. 2011;15:675-681. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 19] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
Open-Access: This article is an open access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Lee HW, Peparini N S- Editor: Gong ZM L- Editor: Roemmele A E- Editor: Jiao XK