Published online Sep 16, 2015. doi: 10.12998/wjcc.v3.i9.765
Peer-review started: September 24, 2014
First decision: October 28, 2014
Revised: May 24, 2015
Accepted: June 18, 2015
Article in press: June 19, 2015
Published online: September 16, 2015
Processing time: 356 Days and 9.3 Hours
Recently, many surgeons have been using intraoperative neurophysiological monitoring (IOM) in spinal surgery to reduce the incidence of postoperative neurological complications, including level of the spinal cord, cauda equina and nerve root. Several established technologies are available and combined motor and somatosensory evoked potentials are considered mandatory for practical and successful IOM. Spinal cord evoked potentials are elicited compound potentials recorded over the spinal cord. Electrical stimulation is provoked on the dorsal spinal cord from an epidural electrode. Somatosensory evoked potentials assess the functional integrity of sensory pathways from the peripheral nerve through the dorsal column and to the sensory cortex. For identification of the physiological midline, the dorsal column mapping technique can be used. It is helpful for reducing the postoperative morbidity associated with dorsal column dysfunction when distortion of the normal spinal cord anatomy caused by an intramedullary cord lesion results in confusion in localizing the midline for the myelotomy. Motor evoked potentials (MEPs) consist of spinal, neurogenic and muscle MEPs. MEPs allow selective and specific assessment of the functional integrity of descending motor pathways, from the motor cortex to peripheral muscles. Spinal surgeons should understand the concept of the monitoring techniques and interpret monitoring records adequately to use IOM for the decision making during the surgery for safe surgery and a favorable surgical outcome.
Core tip: Recently, many surgeons have used intraoperative neurophysiological monitoring (IOM) in spinal surgery to reduce the incidence of postoperative neurological complications, including level of the spinal cord, cauda equina and nerve root. Several established technologies are available and multimodality combinations are considered necessary for practical and effective IOM. Spinal surgeons should understand the concept of the monitoring techniques and interpret monitoring records adequately to use IOM for the decision making during the surgery for safe surgery and a favorable surgical outcome. In this review, the authors will review the different IOM techniques to provide a fundamental concept for better comprehension.
- Citation: Park JH, Hyun SJ. Intraoperative neurophysiological monitoring in spinal surgery. World J Clin Cases 2015; 3(9): 765-773
- URL: https://www.wjgnet.com/2307-8960/full/v3/i9/765.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v3.i9.765
Recently, many surgeons have used intraoperative monitoring (IOM) in spinal surgery to reduce the incidence of postoperative neurological complications, including level of the spinal cord, cauda equina and nerve root. Its importance has been emphasized to prevent an unsuspected and unpleasant neurological deficit after spinal surgery. Surgeons should understand the rationale and clinical basis for IOM to interpret the monitoring alerts and to utilize them for a better surgical outcome. Several established technologies are available and combined motor and somatosensory evoked potentials are considered mandatory for practical and successful IOM. In this review, the authors will review the different IOM techniques to provide a fundamental concept for better comprehension.
This technique was invented in Japan in the 1970s. Electrical stimulation was provoked on the dorsal spinal cord from an epidural electrode[1]. The evoked compound potentials from the stimulated spinal cord are recorded over the spinal cord. The spinal cord evoked potentials (SCEP) correspond to summation of neural activities originating from the ascending and descending tracts and neurons near the recording electrode. The recorded potentials are very vigorous and most likely represent the combined activity of the tracts of the spinal cord, such as dorsal columns, the corticospinal tracts and others[2]. In a practical setting, SCEP cannot provide sufficient information about motor-related function because sensory-related potentials, which are large in amplitude, mask motor-related potentials. The advantages and disadvantages of each modality, including SCEPs, are summarized in Table 1.
| Advantages | Disadvantages | |
| SCEP | Easy to record using very simple hardware | The electrode used to deliver stimulation to the spinal cord should be located in the epidural space and the recording electrode in the intrathecal space |
| Provides real-time information because its potentials are large enough without averaging | The malposition of the electrode can occur | |
| Previous scarring can sometimes impair electrode placement | ||
| SEP | Broadly available | Does not directly monitor corticospinal tract. Only assess the functional integrity of spinal cord dorsal columns. In the case of anterior spinal artery syndrome, postoperative paraplegia despite intraoperative SEP preservation has been reported |
| Easy to implement | When approaching the intramedullary tumor during the initial dorsal myelotomy, SEPs can completely disappear | |
| Has no contraindications | SEP recording requires signal averaging, which results in a time delay until data interpretation can generate a response to the surgeon. Therefore, an injury can be irreversible before it is even detected | |
| Can be combined with other monitoring techniques | ||
| Allows continuous monitoring throughout case | ||
| Excellent specificity (approaching 100%) | ||
| Neurogenic | Fast and easy to implement | Their specificity remains relative because they correspond to the joined activation of motor and sensory pathways |
| MEP | Resistant to most anesthetics | Require curarization |
| Sensitive in detecting a lesion | The terminal medullary cone is not monitored | |
| In case of alert, the lesional level can be determined by displacing the stimulation electrode along the intervertebral spaces | ||
| D waves | Very rapid acquisition | The recording electrode is in the surgical field and its use by the surgeon can produce artifacts |
| D waves are specific of motor pathways | Laterality cannot be distinguished | |
| They can establish a lesional level by displacing the spinal electrode along the intervertebral spaces | D waves cannot be used in small children, generally under 4 yr of age (incomplete maturation of motor path-ways) | |
| D waves have prognostic value | Cannot be recorded below the level of T12 because there are not enough corticospinal tract fibers | |
| Correlates most accurately with long-term motor function following | Previous scarring can sometimes impair electrode placement | |
| intramedullary spinal cord tumor resection | ||
| Muscle MEP | Do not require an averaging. Thus immediate feedback can be available | Require at least partially functional motor pathways |
| Preserved sensitivity and sensitivity even after posterior myelotomy | Incompatible with prolonged curarization | |
| Exceptional adverse effects have been described: tongue or lip laceration, mandibular fracture, cardiac arrhythmia, epileptic seizures, scalp burn and intraoperative awareness | ||
| Often difficult to carry out on patients under the age of 6 yr because of incomplete maturation of motor pathways | ||
| Pedicle screw testing | Rapid and easy technique | Sensitive to a large number of anesthetics |
| Can be combined with new surgical instruments used during screw placement | Can be distorted by curarization | |
| High sensitivity for medial pedicle breach | Less sensitive for thoracic pedicle screws than for lumbar pedicle screw | |
| Useful in minimally invasive surgery where anatomical landmarks may be challenging to visualize | Optimal alarm criteria not firmly established | |
| Does not directly assess for neurological injury, only provides information regarding pedicle integrity |
Somatosensory evoked potentials (SEPs) assess the functional integrity of sensory pathways from the peripheral nerve, through the dorsal column and to the sensory cortex. SEPs were first used in the 1970s to monitor the spinal cord function during surgery for scoliosis correction. After stimulation of peripheral nerves, SEPs are recorded both from the spinal cord with an epidural electrode and/or from the cortex. SEPs can be applied for monitoring the peripheral sensory pathways consistently[3]. From a technical point of view, the posterior tibial nerves are stimulated (duration of stimulus, 0.2 ms; frequency, -3 Hz; intensity, -25 mA). SEPs are the result of averaging. The acquisition time is on the order of 1 min[4]. Data are measured to determine latency and amplitude. Latency is a measure of time and is related to distance. Amplitude is a measure of power and characteristically more variable than latency. This potential indicates activities from the dorsal column such as the sensory tract. By shifting the stimulus site, one can identify the laterality of dorsal column lesions. Monitoring the laterality can provide important information during posterior myelotomy for removing intramedullary spinal cord tumors. An increase in latencies greater than 10% and a decrease in amplitudes greater than 50% constitute warning signals. SEPs are altered by the surgical procedure due to a mechanical factor or secondarily by ischemia. SEP alterations can also be related to patient’s age, height and length of the limbs, systemic hypotension, hematocrit decrease, hypothermia and anesthesia (volatile agents such as isoflurane, halothane, nitrous oxide attenuate SEPs and should not be used if monitoring is employed). Body temperature is a common factor affecting spinal somatosensory evoked potential (SSEP) latency readings.
Monitoring of dorsal column integrity with SSEP is the most commonly used technique in spinal surgery. Large diameter, myelinated and fast conducting cutaneous and muscle afferents carry the peripheral SSEP. SSEP can monitor the dorsal column-medial lemniscus pathway, which mediates tactile discrimination, vibration sensation, form recognition and joint/muscle sensation (conscious proprioception). In SSEP monitoring, stimulation electrodes excite controlled repetitive action potentials that propagate from peripheral nerves to the contralateral sensory cortex through the dorsal roots and the dorsomedial tracts of the spinal cord.
These signals can be recorded at various anatomically accessible locations, such as the peripheral nerve, spinal cord, brainstem and its endpoint and the somatosensory cortex. Platinum subdermal needle electrodes are used for both stimulation and recording. Generally, a 50% decrease in amplitude with an associated 10% increase in latency in comparison to the patient’s baseline values constitute a warning signal. A previous study reported that false negative SEP monitoring occurred during surgery in only 0.063% of patients[5]. A large multicenter study has reported that postoperative paraplegia was reduced more than 50%-60% with SEP monitoring[6]. Although SSEP signals are good basic indicators of spinal cord function, they cannot provide much information regarding nerve root function.
It is important to decide where to perform the myelotomy during intramedullary spinal cord surgery. Anatomic landmarks are often utilized as an indicator for midline intraoperatively. The typical anatomical landmarks for midline of the spinal cord include the dorsal median sulcus between the dorsal columns and the median dorsal sulcal vein which enters the midline raphe. Dorsal column mapping technique can be applied to identify the “physiological midline”. It is helpful for reducing the postoperative morbidity associated with dorsal column dysfunction when an intramedullary cord lesion distorts the normal spinal cord anatomy that results in confusion in distinguishing the midline for the myelotomy[7-9]. The multielectrode grid is placed transversely over the dorsal surface of the cord. It has multiple wires and each wire has been stripped of its insulating coating. These recording wires pass parallel to the long axis of the spinal cord and a reference needle electrode is inserted in a nearby muscle. This multielectrode grid can record the traveling SEP waves from the dorsal surface of the exposed spinal cord very selectively with the amplitude gradient corresponding to the topographic arrangement of the dorsal column. Because of the somatotopic distribution of ascending fibers in the dorsal column, the highest amplitude close to the midline will be recorded after SEP stimulation on the right posterior tibial nerve. By the same reaction from the contralateral side, we can identify ‘‘physiological midline’’ between these two amplitude peaks.
D (direct) waves are compound corticospinal action potentials initiated by direct axonal activation with conduction velocity approximately 50 m/s[10], making it possible to monitor the motor pathways from the cortex to the level of the spinal electrode. D waves are obtained by a single transcranial electrical stimulation (intensity, 80-100 mA; duration of stimulus, 0.5-1 ms; frequency, 0.5-2 Hz) recorded from the epidural or subdural space of the spinal cord[11]. It is generated directly by the electrical pulse. Therefore, they are called “single stimulus technique” or “spinal motor evoked potentials (MEPs)”. D waves usually do not need averaging, although a few averages improve its quality of recording. This provides clinically real-time feedback. If the D wave amplitude decreases more than 50% of the baseline value or cannot be detectable, there is high probability of severe neurological deficit such as permanent paraplegia.
Neurogenic MEP is an elicited potential that is electrically stimulated at the spinal cord with epidural electrodes and then recorded from the peripheral nerves. Neurogenic MEPs are recorded by stimulating the spinal cord through electrodes inserted by the surgical team: A flexible spinal electrode inserted into the epidural space proximal to the operating field. The stimulation parameters are the following: intensity, 20-50 mA; duration of stimulation, 1 ms; frequency, 4.1 Hz. Recordings are performed at the internal popliteal sciatic nerves or the posterior tibial nerves. This technique allows monitoring of the overall spinal cord. In 1988, recording neurogenic evoked potentials from peripheral nerves in lower extremities after spinal cord stimulation was suggested[12] to monitor spinal motor pathways. It is now widely revealed that these potentials contain at least a significant antidromical sensory component[13-15]. Neurophysiological collision studies have shown that the biphasic component corresponds to antidromical activation of the sensory pathways, whereas the polyphasic component corresponds to activation of the motor pathways. Neurogenic MEPs provide combined sensory and motor spinal pathway monitoring[16,17].
Motor potentials are evoked with transcranial electrodes which are placed on the scalp over the motor cortex area of the skull. The stimulus points are C3, C4, C1, C2, Cz and a point 6 cm in front of Cz (international 10-20 system)[11]. Muscle MEPs are derived from transcranial electrical stimulation (five to seven pulses, 2-4 ms apart; intensity, 250-750 V; duration of each pulse, 0.5 ms) over the same electrodes as for the D-wave. The technique is called the “multipulse technique’’ or ‘‘train stimulus technique’’. Compound muscle action potentials (CMAP) are recorded with needle electrodes from target belly muscles in all four limbs. Muscles are selected based on the surgical procedure and spinal levels involved. Typical muscles for arm MEP recordings include the abductor pollicis brevis (thenar muscle); the 1st dorsal interosseous and hypothenar muscles are alternatives. Typical muscles for leg MEP recordings include the tibialis anterior (TA) and abductor hallucis (AH). Other muscles, such as the quadriceps, deltoids, biceps, even the diaphragm, and the external anal sphincter can be selected. Muscle MEPs allow selective and specific analysis of the functional integrity of descending motor tracts, from the motor cortex to muscles. Recordings are lateralized for each limb. Muscle MEPs do not require averaging and can be repeated at a rate of 0.5-2 Hz. A reduction of more than 50% of the baseline amplitude can be an alarm point for postoperative motor deficit. However, even though muscle MEPs are lost, there may be a transient deficit but no permanent postoperative deficit if the D wave is preserved[18]. A temporary postoperative motor deficit can occur when muscle MEP amplitude is lost but D wave amplitude preserved. In this situation, surgeons can continue the resection or stop and wait for recordings to recover, which they often do. Muscle MEP loss without D wave changes or the D wave decrease without muscle MEP changes in muscle MEP occurs. A D wave maintained over 50% of the baseline indicates that the neurons of the corticospinal tract which controls delicate voluntary movements remain. In any event, if the D wave was preserved over 50% of the baseline value, it is considered that the voluntary motor control in the lower extremities are preserved[19]. A decrease of the amplitude of muscle MEP is not always associated with postoperative neurological deficit; however, it is valuable to evaluate the early ischemic or mechanical damage to the spinal cord. Despite several reports that refer the accuracy of D-wave monitoring during surgeries for intramedullary spinal cord tumors, muscle MEP monitoring is maintained as a valuable tool. This is because muscle MEP does not need an epidural electrode, has a higher generation rate of MEP and it is more accurate in monitoring for scoliosis surgery[20].
Both SSEP and MEPs are affected by various pharmacological and physiological factors. Any drug or physical parameter that influences electrical conduction along an axon may alter the evoked potential waveform. In general, the longer with more synapses tracts are, the more sensitive they are. Furthermore, a greater number of pulses will be needed for lower extremity recordings compared with upper extremity sites because it is easier to obtain signals from the upper extremity than from the lower extremity. This is because the hand area occupies a larger representation on the motor cortex[21]. Inhaled anesthetics reduce the amplitude and increase latency, while intravenous anesthetics have the same effect but to a lesser degree. Halogenated or nitrous oxide-based agents influence SSEP amplitude and latency. MEPs are generally more sensitive to anesthetics than SSEPs. Total intravenous anesthesia without neuromuscular blockade is material to muscle MEPs to allow CMAP monitoring. Typically, induction with short-acting muscle relaxants, a continuous infusion of propofol and fentanyl and low level nitrous oxide use (not exceeding 50% by volume) are mandatory for MEP monitoring. In our institute, when we need IOM for the surgery, anesthesia is maintained with a continuous infusion of propofol (10 mg/kg per hour) and remifentanil (0.25 mg/kg per minute). At induction, a single bolus of non-depolarizing short acting muscle relaxant (rocuronium) is given to facilitate tracheal intubation and ventilation. The level of neuromuscular block is monitored by recording the CMAP to a train of 4 stimuli.
Spontaneous or free-running electromyography (EMG) is widely applied to monitor selective nerve root function during spinal cord surgery. Unlike SEP and SSEP data, EMG is “real-time” recording from peripheral musculature. Spontaneous EMG can help to prevent postoperative radiculopathy during spinal instrumentation surgery, including pedicle screw placement. This technique does not require stimulation and can be recorded continuously from preselected muscle groups based on the nerve roots at risk[22]. One muscle group per nerve is generally considered appropriate. Common EMG recording sites by spinal levels are as follows: C4 - supraspinatus; C5 - deltoid and biceps; C6 - biceps and wrist extensors; C7 - triceps, wrist flexors and finger extensors; C8 - hand intrinsics and finger flexors; T1 - hand intrinsics; T6-12 - rectus abdominis; L1 - iliopsoas; L2 - adductor longus; L3 - adductor longus and vastus medialis; L4 - vastus medialis and vastus lateralis; L5 - TA and extensor hallucis longus; S1 - medial gastrocnemius and peroneus longus; S2-5 - perianal musculature and urethral sphincter. However, in case of cervical spinal surgery, many surgeons favor monitoring 2 muscle groups, deltoid (predominantly C5, also C6) and biceps brachii (predominantly C6, also C5), due to the risk of C5 palsy[23]. At baseline, no muscle activity is recorded from an intact nerve root. Surgical manipulations such as pulling, stretching or compression of nerves provokes spikes or bursts of activity termed neurotonic discharges, resulting in activity in the corresponding innervated muscle(s). Spontaneous EMG is quite sensitive to irritation of the nerve root, such as retraction of spinal cord or nerve root, saline irrigation and manipulation during surgery. However, false spontaneous EMG activation commonly occurs during irrigation with cold water, cauterization and use of a high-speed drill because it is sensitive to temperature changes.
Spontaneous EMG trains are continuous, repetitive EMG firing caused by continuous mechanical stress on the nerve root. Trains of higher frequency and/or amplitude are likely to indicate a high probability of nerve injury unless a prompt corrective management is performed. On the other hand, spontaneous EMG spikes and bursts indicate the proximity of the nerve root.
It is also important to note that EMG signals are interfered with in patients with various neurological disorders, such as myasthenia gravis, botulinum toxin treatments for dystonia and muscular dystrophy.
The introduction of triggered EMG for evaluating the integrity of lumbar pedicles during screw placement and the accuracy of pedicle screw placement was first described by Calancie et al[24] in 1992 using a porcine model. The concept of triggered EMG is that intact cortical bone should be electrical insulation for a well-placed pedicle screw from the adjacent nerve root. In contrast, the pedicle screw would be relatively poorly insulated when medial pedicle breach occurred[22]. Intraoperative triggered EMG detects root irritation or the post injury condition of the root after medial pedicle breach. An irritated or damaged nerve root causes a decrease in electric threshold followed by sudden appearance of CMAPs of the specific muscles of the myotome after stimulation via the screw[25]. Each pedicle screw is electrically stimulated with an increasing intensity from 5 to 30 mA (duration, 0.2 ms; frequency, 0.8 Hz). Recordings are made at the level of the lower limb muscles with or without the rectus abdominis muscles (depending on the root levels to test). The recording of muscle activity at an intensity under 10 mA (the classically accepted threshold) argues in favor of a medial breach (close proximity of the screw to the nerve root).
MEP changes occur most frequently towards the end of the tumor resection when the interface between the tumor and normal tissue comes close. Some of the authors of this study have previously reported that the type of muscle recorded affects the changing patterns and prognostic values of muscle MEP[26]. Muscle MEPs recorded from the AH muscle were relatively resistant to perioperative neural damage, otherwise the muscle MEPs from the TA were relatively vulnerable to perioperative neural damage at an earlier time and/or more sensitive compared with the single-muscle recordings in the AH. Thus, the loss of muscle MEP in the TA, even though muscle MEPs in the AH were preserved, should be considered a potential early warning sign for possible postoperative neurological deficit. This result is a meaningful point of view in that using a combination of muscle MEPs from different muscles with individual sensitivities and clinical significances will improve intra-operative muscle MEP monitoring interpretations and surgical strategies.
There are some procedures useful for promoting the recovery of deteriorated or lost MEPs during spinal surgery[8,10,11,27,28]. First, transient stopping the surgical manipulations immediately after MEP and observation may recover MEP spontaneously. Second, irrigation of the surgical field with warm saline solution generally removes irritating blood products and metabolites. It is believed that accumulated extracellular potassium which is derived from local tissue damage by surgery acts as a strong axonal blocking agent. Third, local application of papaverine and increasing the mean arterial pressure (MAP) more than 90 mmHg can facilitate local perfusion to resist local ischemia. Sustained hypotension may affect MEPs and may result in an unfavorable postoperative outcome. Temporary moderate elevation of MAP may restore blood flow and may lead to improving both D-waves and muscle MEPs successfully if ischemia due to insufficient MAP is the primary cause. Fourth, when dissection in a particular location results in MEP changes, the resection at a different site can proceed without further aggravation. Fifth, increasing body temperature also may help recover MEPs because deep hypothermia obliterates muscle MEPs. Sixth, when all the previous methods have failed to regain MEP, applied correction is reduced or anchoring hardware removed in case of the correction of the spinal deformity. If there is still no significant signal recovery, a steroid bolus (methylprednisolone or dexamethasone) could be considered. Finally, if there is no noticeable neurophysiological improvement in response to all methods described above, consider removing the spinal implant or discontinuing tumor resection.
For many years, only SEPs were monitored during spinal cord surgeries before MEP techniques were developed. Since the multipulse technique was introduced in the mid-1990s, combined muscle MEP and SEP monitoring have been routinely used intraoperatively in various spinal operations, including from deformity correction to intramedullary cord tumors. Many authors have reported that combined SEP/MEP monitoring provided higher sensitivity and higher positive/negative predictive values than single-modality monitoring techniques and that optimal monitoring requires both SEPs and MEPs[27-30].
The reliability of intraoperative MEP to identify impending motor deficits has been improved, mainly as a result of accumulated knowledge interpreting the MEP changes. Monitoring muscle MEPs became the gold standard due to their increased sensitivity and the importance of motor function. It has been believed most widely that MEP recordings are stable if changes were less than 50% in amplitude and less than 10% in latency. However, there is no definite alarm point. Various different alarm points have been reported. From a surgical viewpoint, the 50% criterion could have the effect that the surgical correction of the deformity is performed incompletely or tumor resection is forsaken too early and the 80% criterion that the operation proceeded too aggressively[30]. The benefit of more sensitive MEP criteria and the risk of stopping resection too early should be always considered in parallel. This is important especially for tumors of which the most important prognostic factor is complete resection, such as spinal cord ependymomas.
Some authors have attempted to establish a more sensitive and specific warning criteria recently[31]. They recommend an alarm point of a 70% decrease in amplitude for routine spinal cord monitoring, particularly during surgery for spinal deformity, ossification of posterior longitudinal ligament and extramedullary spinal cord tumor, but not in cases of intramedullary spinal cord tumor. Different MEP methods and warning criteria still exist (muscle MEP, D-wave, absence/presence criteria, amplitude criteria and morphology criteria, etc.) and neither definite monitoring method nor definite warning criteria have been established. Future study should establish a more confident and accurate IOM method or criteria which prosecutes an aggressive tumor removal while preserving function of the spinal cord.
Reliable IOM enables surgeons to perform more difficult and challenging surgical procedures to the spine and spinal cord safely. Its role is to accurately identify the topography of neural structures and to avoid surgical insults by giving real-time alarms to the surgical team so that there is a response immediately. The role of IOM is increasing not only in deformity correction and intramedullary tumors but also in various spinal surgeries. It is well known that a rapid decompression of cervical medulla or cervical cord could result in neurological deficits such as C5 palsy, paresis or even plegia. It is possible to have a stretching of the lumbar roots during the reduction of the high dysplastic lumbar spondylolisthesis. IOM can help the surgical team accomplish a safe and successful outcome in spinal surgery which affects the spinal cord or spinal roots directly or indirectly. Multimodal IOM is a useful method to prevent spinal cord injury during neck positioning in cervical spine surgical procedures. It should not only be considered for detecting intra-operative warnings, but also during positioning[32].
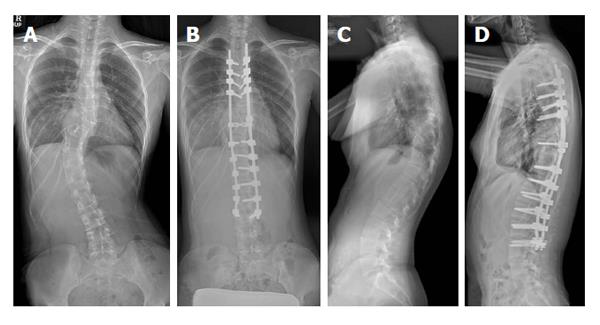
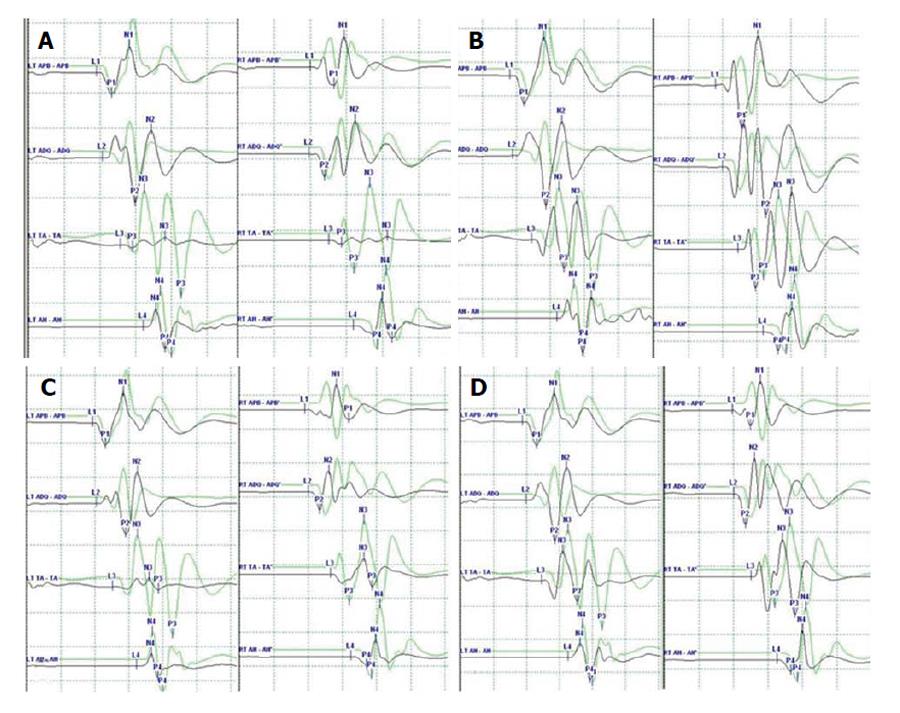
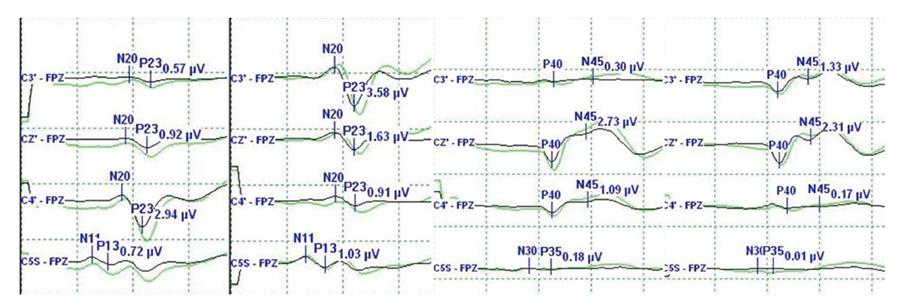
A 17-year-old girl visited our institute with back pain. She was diagnosed as a scoliosis associated with neurofibromatosis (Figure 1). We planned to perform a deformity correction by posterior column osteotomies and posterior segmental spinal instrumented fusion with intraoperative combined MEP and SSEP monitoring. When we applied the rod to the screw heads using a derotation maneuver and cantilever maneuver, the amplitude of MEP at both lower extremities decreased more than 50% compared with the baseline amplitude (Figure 2A). However, SSEP showed no change compared with the baseline amplitude (Figure 3). The amplitude of MEP recovered after correction release by removal of the rods and set screws (Figure 2B). The amplitude of MEP re-deteriorated after reassembly of the implants (Figure 2C). The amplitude of MEP recovered finally after raising MAP and administration of dexamethasone (Figure 2D). After the corrective surgery (Figure 1), she woke up without any postoperative neurological deficit and was discharged from the hospital on foot.
Although we cannot monitor every function of the spinal cord during spinal surgery, the technology of IOM has markedly developed. It is certain that the significance and utilization of IOM during spinal surgery will increase because of medicolegal issues as well as its usefulness. Spinal surgeons should understand the concept of the monitoring techniques and interpret monitoring records adequately to use IOM for decision making during the surgery for safe surgery, favorable surgical outcome and the surgeon’s medicolegal safety.
| 1. | Tamaki T, Kubota S. History of the development of intraoperative spinal cord monitoring. Eur Spine J. 2007;16 Suppl 2:S140-S146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 63] [Article Influence: 3.3] [Reference Citation Analysis (1)] |
| 2. | Deletis V, Sala F. Intraoperative neurophysiological monitoring of the spinal cord during spinal cord and spine surgery: a review focus on the corticospinal tracts. Clin Neurophysiol. 2008;119:248-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 279] [Cited by in RCA: 269] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 3. | Sala F, Squintani G, Tramontano V, Arcaro C, Faccioli F, Mazza C. Intraoperative neurophysiology in tethered cord surgery: techniques and results. Childs Nerv Syst. 2013;29:1611-1624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 43] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 4. | Gavaret M, Jouve JL, Péréon Y, Accadbled F, André-Obadia N, Azabou E, Blondel B, Bollini G, Delécrin J, Farcy JP, Fournet-Fayard J, Garin C, Henry P, Manel V, Mutschler V, Perrin G, Sales de Gauzy J. Intraoperative neurophysiologic monitoring in spine surgery. Developments and state of the art in France in 2011. Orthop Traumatol Surg Res. 2013;99:S319-S327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 33] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 5. | Nuwer MR, Dawson EG, Carlson LG, Kanim LE, Sherman JE. Somatosensory evoked potential spinal cord monitoring reduces neurologic deficits after scoliosis surgery: results of a large multicenter survey. Electroencephalogr Clin Neurophysiol. 1995;96:6-11. [PubMed] |
| 6. | Nuwer MR. Spinal cord monitoring. Muscle Nerve. 1999;22:1620-1630. [PubMed] |
| 7. | Mehta AI, Mohrhaus CA, Husain AM, Karikari IO, Hughes B, Hodges T, Gottfried O, Bagley CA. Dorsal column mapping for intramedullary spinal cord tumor resection decreases dorsal column dysfunction. J Spinal Disord Tech. 2012;25:205-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 34] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 8. | Sala F, Bricolo A, Faccioli F, Lanteri P, Gerosa M. Surgery for intramedullary spinal cord tumors: the role of intraoperative (neurophysiological) monitoring. Eur Spine J. 2007;16 Suppl 2:S130-S139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 146] [Article Influence: 7.7] [Reference Citation Analysis (1)] |
| 9. | Yanni DS, Ulkatan S, Deletis V, Barrenechea IJ, Sen C, Perin NI. Utility of neurophysiological monitoring using dorsal column mapping in intramedullary spinal cord surgery. J Neurosurg Spine. 2010;12:623-628. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 42] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 10. | Macdonald DB, Skinner S, Shils J, Yingling C. Intraoperative motor evoked potential monitoring - a position statement by the American Society of Neurophysiological Monitoring. Clin Neurophysiol. 2013;124:2291-2316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 311] [Cited by in RCA: 329] [Article Influence: 25.3] [Reference Citation Analysis (0)] |
| 11. | Kothbauer KF. Intraoperative neurophysiologic monitoring for intramedullary spinal-cord tumor surgery. Neurophysiol Clin. 2007;37:407-414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 71] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 12. | Owen JH, Laschinger J, Bridwell K, Shimon S, Nielsen C, Dunlap J, Kain C. Sensitivity and specificity of somatosensory and neurogenic-motor evoked potentials in animals and humans. Spine (Phila Pa 1976). 1988;13:1111-1118. [PubMed] |
| 13. | Deletis V. Intraoperative monitoring of the functional integrity of the motor pathways. Adv Neurol. 1993;63:201-214. [PubMed] |
| 14. | Haghighi SS, York DH, Gaines RW, Oro JJ. Monitoring of motor tracts with spinal cord stimulation. Spine (Phila Pa 1976). 1994;19:1518-1524. [PubMed] |
| 15. | Su CF, Haghighi SS, Oro JJ, Gaines RW. “Backfiring” in spinal cord monitoring. High thoracic spinal cord stimulation evokes sciatic response by antidromic sensory pathway conduction, not motor tract conduction. Spine (Phila Pa 1976). 1992;17:504-508. [PubMed] |
| 16. | Péréon Y, Nguyen The Tich S, Delécrin J, Pham Dang C, Bodin J, Drouet JC, Passuti N. Combined spinal cord monitoring using neurogenic mixed evoked potentials and collision techniques. Spine (Phila Pa 1976). 2002;27:1571-1576. [PubMed] |
| 17. | Wilson-Holden TJ, VanSickle D, Lenke LG. The benefit of neurogenic mixed evoked potentials for intraoperative spinal cord monitoring during correction of severe scoliosis: a case study. Spine (Phila Pa 1976). 2002;27:E258-E265. [PubMed] |
| 18. | Morota N, Deletis V, Constantini S, Kofler M, Cohen H, Epstein FJ. The role of motor evoked potentials during surgery for intramedullary spinal cord tumors. Neurosurgery. 1997;41:1327-1336. [PubMed] |
| 19. | Kothbauer KF, Deletis V, Epstein FJ. Motor-evoked potential monitoring for intramedullary spinal cord tumor surgery: correlation of clinical and neurophysiological data in a series of 100 consecutive procedures. Neurosurg Focus. 1998;4:e1. [PubMed] |
| 20. | Ulkatan S, Neuwirth M, Bitan F, Minardi C, Kokoszka A, Deletis V. Monitoring of scoliosis surgery with epidurally recorded motor evoked potentials (D wave) revealed false results. Clin Neurophysiol. 2006;117:2093-2101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 54] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 21. | Deiner S. Highlights of anesthetic considerations for intraoperative neuromonitoring. Semin Cardiothorac Vasc Anesth. 2010;14:51-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 46] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 22. | Lall RR, Lall RR, Hauptman JS, Munoz C, Cybulski GR, Koski T, Ganju A, Fessler RG, Smith ZA. Intraoperative neurophysiological monitoring in spine surgery: indications, efficacy, and role of the preoperative checklist. Neurosurg Focus. 2012;33:E10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 128] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 23. | Gonzalez AA, Jeyanandarajan D, Hansen C, Zada G, Hsieh PC. Intraoperative neurophysiological monitoring during spine surgery: a review. Neurosurg Focus. 2009;27:E6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 163] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 24. | Calancie B, Lebwohl N, Madsen P, Klose KJ. Intraoperative evoked EMG monitoring in an animal model. A new technique for evaluating pedicle screw placement. Spine (Phila Pa 1976). 1992;17:1229-1235. [PubMed] |
| 25. | Bosnjak R, Dolenc VV. Electrical thresholds for biomechanical response in the ankle to direct stimulation of spinal roots L4, L5, and S1. Implications for intraoperative pedicle screw testing. Spine (Phila Pa 1976). 2000;25:703-708. [PubMed] |
| 26. | Kim SM, Yang H, Park SB, Han SG, Park KW, Yoon SH, Hyun SJ, Kim HJ, Park KS, Lee KW. Pattern-specific changes and discordant prognostic values of individual leg-muscle motor evoked potentials during spinal surgery. Clin Neurophysiol. 2012;123:1465-1470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 20] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 27. | Hyun SJ, Rhim SC. Combined motor and somatosensory evoked potential monitoring for intramedullary spinal cord tumor surgery: correlation of clinical and neurophysiological data in 17 consecutive procedures. Br J Neurosurg. 2009;23:393-400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 46] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 28. | Hyun SJ, Rhim SC, Kang JK, Hong SH, Park BR. Combined motor- and somatosensory-evoked potential monitoring for spine and spinal cord surgery: correlation of clinical and neurophysiological data in 85 consecutive procedures. Spinal Cord. 2009;47:616-622. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 34] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 29. | Pelosi L, Lamb J, Grevitt M, Mehdian SM, Webb JK, Blumhardt LD. Combined monitoring of motor and somatosensory evoked potentials in orthopaedic spinal surgery. Clin Neurophysiol. 2002;113:1082-1091. [PubMed] |
| 30. | Weinzierl MR, Reinacher P, Gilsbach JM, Rohde V. Combined motor and somatosensory evoked potentials for intraoperative monitoring: intra- and postoperative data in a series of 69 operations. Neurosurg Rev. 2007;30:109-116; discussion 116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 58] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 31. | Kobayashi S, Matsuyama Y, Shinomiya K, Kawabata S, Ando M, Kanchiku T, Saito T, Takahashi M, Ito Z, Muramoto A. A new alarm point of transcranial electrical stimulation motor evoked potentials for intraoperative spinal cord monitoring: a prospective multicenter study from the Spinal Cord Monitoring Working Group of the Japanese Society for Spine Surgery and Related Research. J Neurosurg Spine. 2014;20:102-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 113] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 32. | Plata Bello J, Pérez-Lorensu PJ, Roldán-Delgado H, Brage L, Rocha V, Hernández-Hernández V, Dóniz A, García-Marín V. Role of multimodal intraoperative neurophysiological monitoring during positioning of patient prior to cervical spine surgery. Clin Neurophysiol. 2015;126:1264-1270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 37] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
P- Reviewer: Landi A, Restuccia D, Tokuhashi Y S- Editor: Ji FF L- Editor: Roemmele A E- Editor: Liu SQ
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/