Published online Sep 16, 2015. doi: 10.12998/wjcc.v3.i9.757
Peer-review started: May 4, 2015
First decision: June 3, 2015
Revised: June 20, 2015
Accepted: July 24, 2015
Article in press: July 27, 2015
Published online: September 16, 2015
Processing time: 143 Days and 8.6 Hours
Vitamin K (VK), which was originally identified as a cofactor involved in the production of functional coagulation factors in the liver, has been shown to be involved in various aspects of physiological and pathological events, including bone metabolism, cardiovascular diseases and tumor biology. The mechanisms and roles of VK are gradually becoming clear. Several novel enzymes involved in the VK cycle were identified and have been shown to be linked to tumorigenesis. The VKs have been shown to suppress liver cancer cell growth through multiple signaling pathways via the transcription factors and protein kinases. A VK2 analog was applied to the chemoprevention of hepatocellular carcinoma (HCC) recurrence after curative therapy and was shown to have beneficial effects, both in the suppression of HCC recurrence and in patient survival. Although a large scale randomized control study failed to demonstrate the suppression of HCC recurrence, a meta-analysis suggested a beneficial effect on the long-term survival of HCC patients. However, the beneficial effects of VK administration alone were not sufficient to prevent or treat HCC in clinical settings. Thus its combination with other anti-cancer reagents and the development of more potent novel VK derivatives are the focus of ongoing research which seeks to achieve satisfactory therapeutic effects against HCC.
Core tip: Vitamin K (VK) is essential nutrient initially identified as a cofactor to produce functional coagulation factors. In addition to the roles in hemostasis, pleiotropic effects of VK in bone health, atherosclerotic diseases and cancer have been attracting. VK has been shown to play tumor-suppressive roles in several cancers including hepatocellular carcinoma (HCC) and reported to have beneficial effects in the treatment of HCC although its anti-tumor effects remain to be improved. Currently novel VK derivatives are under developing and will be applied to cancer treatment in the future.
- Citation: Jinghe X, Mizuta T, Ozaki I. Vitamin K and hepatocellular carcinoma: The basic and clinic. World J Clin Cases 2015; 3(9): 757-764
- URL: https://www.wjgnet.com/2307-8960/full/v3/i9/757.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v3.i9.757
Vitamin K (VK) is a well-known lipid-soluble vitamin that includes two natural types: VK1 and VK2, and synthetic types, known as VK3, VK4, and VK5. VK1, also known as phylloquinone, was first accidently identified by Dam[1] and Dam et al[2] for its anti-hemorrhagic activities in 1929. VK1 is ubiquitous and abundant in green leafy vegetables because it plays a direct role in photosynthesis. It performs the classic functions of VK that help the production of blood-clotting proteins[3-5]. VK2 is also known as menaquinone (MK), the subtypes of which are mainly synthesized by limited bacteria. They are mainly stored in animal products. The VK2 subtypes, which are characterized by the isoprenoid side chain length. MK-4 differs from the other MKs, which are synthesized by bacteria, such as MK-7, MK-8, and MK-9, in that it is the most common VK2 subtype in animals. It is a unique subtype because it is normally synthesized from VK1 in vivo[6-8]. In addition to VK1 and VK2, VK3 is an efficient coagulant[9]. Unlike the safe natural forms and other synthetic forms of VK, VK3 (menadione) is considered to be toxic because large doses have been shown to cause various adverse effects, such as allergic reactions, hemolytic anemia, and cytotoxicity in liver cells[10]. Aside from its clinical use as a hemostasis medicine, VK4 has recently been reported to have inhibitory effects on prostate cancer[11]. VK5 is used in many areas including the pet food industry to inhibit fungal growth[12] and has been shown to mimic the effect of insulin[13].
Dietary VK is absorbed from the small intestine along with dietary fat[14]. The latest findings have demonstrated that a cholesterol transporter, Niemann-Pick C1-like 1, is a key regulator of intestinal VKs absorption[15]. Despite VK’s rapid metabolism in tissue, which results in comparatively low body storage[16], primary VK deficiency is rare in healthy adults. In addition to the average diet, which provides plenty of VK, other mechanisms maintain its balance within the human body. The VK cycle plays a critical role in maintaining VK function. The cycle proceeds through the coupled carboxylation and epoxidation carried out by gamma-glutamyl carboxylase (GGCX) and VK epoxide reductase (VKOR)[17,18]. The product, VK epoxide, plays an important role as a cofactor in blood coagulation factor production and is then reconverted to VK by VKOR[19]. It is well-known that warfarin and other 4-hydroxycoumarins block the activity of VKOR to inhibit coagulation[20,21], however, the identification of the VKOR gene was a recent finding[22,23]. More recently, it was demonstrated that VKOR deficiency caused early postnatal lethality in a knockout mice model due to severe intracerebral hemorrhage[24]. For decades it was believed that VK1 had the potential to transform into MK-4 endogenously in animals[8,25]. This was proven by mouse experiments[26,27]. Recently, the same group found that UbiA prenyltransferase containing 1 (UBIAD1), a human homologue of prenyltransferase menA, is a human MK-4 biosynthetic enzyme. Furthermore, they demonstrated that UBIAD1 is located in the ER and that it is not suppressed by warfarin[7].
In addition to the initially identified role of VK as a cofactor in the production of functional clotting factors through Gla residue formation in the liver, Gla protein was identified in the bone matrix proteins such as osteocalcin in 1975[28], and the involvement of VK in the bone physiology has been studied[29]. Furthermore, since the 1980s researchers have shown the anti-proliferative effects of VK in several cancer cell lines, including (HCC)[30-33]. Although the novel attractive functions of VK were reported, the mechanisms of VK function beyond their role in activating hepatic coagulation factors remained unknown. In 2003, Tabb et al[34] identified the steroid and xenobiotic receptor (SXR), also known as PXR, as a ligand of VK2 and showed that SXR mediated gene expression in an osteosarcoma cell line. Interestingly, this research group further demonstrated that SXR is abundantly expressed in the liver and that it reciprocally regulates nuclear factor-kappa B (NF-κB)-regulated gene expression[35].
The anti-tumor effects of VK were attractive to investigators studying cancer biology. Otsuka et al[36] reported that VK2 inhibited the growth of HCC cells as well as their invasiveness via the activation of protein kinase A (PKA) and the subsequent inhibition of Rho activation. They also demonstrated the activation of the transcription factors AP-2-, USF-1- and CREB in HCC cells by showing the nuclear accumulation of Ser-phosphorylated CREB, although the roles of these factors in the VK2-induced suppression of cell growth and invasion are not known.
We have revealed that VK2 inhibits the growth of human HCC cells by suppressing cyclin D1 expression through the inhibition of NF-κB activation by suppressing IKK activity[37]. The suppression of NF-κB activation by VK2 was also observed in lipopolysaccharide-mediated macrophage activation[38] and in the VK-mediated suppression of the osteoclastogenesis of bone cells through the RANK/RANKL pathway[39,40]. It has been demonstrated that VK2 inhibits the expression of matrix metalloproteinases that contain NF-κB binding motifs in their promoter region[41], and augments the 5-fluorouracil-induced growth inhibition of HCC cells by inhibiting NF-κB activation[42]. Furthermore, we elucidated that VK2 inhibited the NF-κB activation through the inhibition of protein kinase C (PKC)-alpha and -epsilon kinase activities, as well as through the subsequent inhibition of PKD1 activation[43]. We have recently found that VK2 suppressed hypoxia inducible factor (HIF)-1 alpha activity through the inhibition of PKC by inhibiting the translocation of HIF to the nucleus[44].
Another interesting function of VK in the suppression of tumor development is its ability to induce apoptosis in certain cancer cells. Matsumoto et al[45] showed that VK2 induced apoptosis in Hep3B cells through the activation of AP-1. VK2-induced apoptosis is shown to be associated with p53 status in the human HCC cell line[46]. Recently Karasawa et al[47] demonstrated that VK2 covalently binds to Bcl-2 antagonist killer 1, a mitochondrial-mediated proapoptotic factor. The enhancement of apoptosis when VK2 was used in combination with acyclic retinoid (ACR) has been reported[48,49]. Kanamori et al[49] treated Huh7 cells with the combination of VK2 and ACR and found that VK2 plus ACR synergistically inhibited the growth of Huh7 cells by increasing apoptosis. When combined with ACR, VK2 inhibited Ras activation, followed by the inhibition of ERK phosphorylation. Interestingly, Suzuki et al[50] reported that des-gamma-carboxy prothrombin (DCP), also called protein induced by VK absence or antagonist II, which is widely used as a tumor marker of HCC, has a binding affinity to c-Met, a hepatocyte growth factor receptor, and that it transmits aberrant STAT3 signaling[50]. They also reported the involvement of variant GGCX mRNA expression in the production of DCP in liver cancer[51]. Furthermore, Ma et al[52] showed the DCP-dependent growth advantage of HCC cells.
An interesting topic that has recently been reported in tumor biology is the role of UBIAD1, also called TERE1. UBIAD1 was recently identified as the menaquinone-4 biosynthetic enzyme[7]. UBIAD1 mRNA has been reported to be downregulated in prostate carcinoma cells and the overexpression of UBIAD1 inhibits the proliferation of tumor cell lines. UBIAD1 has therefore been considered to be a tumor suppressor in prostate cancer tumors[53]. Fredericks et al[54,55] reported that UBIAD1 controlled SXR-dependent gene expression in prostate cancer cells through several mechanisms. Since SXR transcription factor is a ligand of VK2 and because it has been shown to be involved in HCC cell growth[56,57], UBIAD1 expression might be linked to the effects of VK in HCC cells. More recently, UBIAD1 has been reported to be essential for embryonic development in mice[58] and VK2 has been shown to drive the metabolic maturation of pluripotent stem cells and fetal hepatocytes[59]. These findings suggest a novel role of VK metabolism in stem cells and that VK might be involved in cancer stem cell biology.
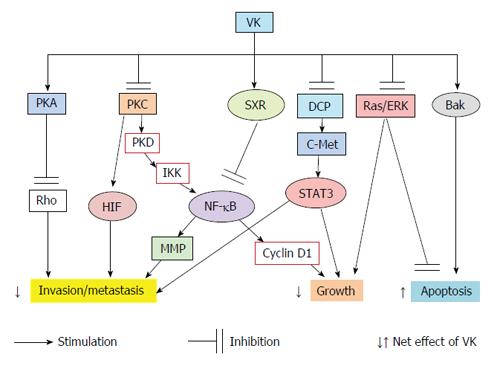
Collectively, the possible signal mechanisms of VK are summarized in Figure 1. VKs have been shown to have diverse effects on the phosphorylation states of various proteins[60]. Although the involvement of PKA, PKC, NF-κB, STAT, SXR and MAPK pathways are reported, the mechanisms by which VK2 modulates the protein kinases and/or phosphatases still remain to be elucidated. VK2 may reduce the growth and invasion of cancer cells through the modulation of protein kinases/phosphatases cascades.
Before the identification of VK as an essential cofactor for the production of functional coagulation factors, Townsend[61] (1894) reported 50 cases of a generalized bleeding tendency in neonates in a condition that was named the hemorrhagic disease of the newborn (HDN). He described that HDN differed from hemophilia in its earlier presentation, the lack of a family history and in its self-limiting course. Townsend[61] suggested a link between the mother’s capacity to breast-feed and the hemostatic capacity of the newborn infant. After the identification of the role of VK in blood coagulation, the disease was shown to be related to VK nutritional deficiency and was renamed as VK deficiency bleeding (VKDB) by the ISTH Pediatric/Perinatal Subcommittee in 1999[62,63]. Although VK deficiency can occur in adults, it is common in newborns because of their limited VK storage, immature gastrointestinal absorption and due to the low placenta transfer of VK. The diagnosis of VKDB can be made in infants younger than 6 mo of age who present spontaneous bleeding, bruising, or intracranial hemorrhage with a prolonged clotting time but with a normal or elevated platelet count. Since the VKDB patients who present with intracranial bleeding are exclusively breastfed, Greer et al[64] investigated phylloquinone intakes in exclusively breast-fed infants in a North America and found that the average daily intake was one-tenth of that in healthy adults while formulated milk contained 50-fold higher concentration of phylloquinone than human milk. Although VKDB is rare in most developed countries, the consequences for the small number of patients who develop intracranial hemorrhage are often catastrophic. Nearly all cases of HDN/VKDB reported in the literature occur in infants who did not receive prophylactic VK supplementation in the newborn period. Consequently, many countries have introduced the routine prophylactic administration of VK at the time of birth to prevent hemorrhagic events[65,66].
Beyond the originally identified function of VK in blood coagulation system, it has been widely reported that VK has possible benefits on bone health and cardiovascular diseases[6,67]. Menatetrenone, a VK2 analog, has been used safely for the treatment of osteoporosis. Several clinical trials have shown it to be effective for treating osteoporosis in postmenopausal women, although the effects of VK2 alone might not be sufficient[68-70]. The potential benefit of VK in reducing cardiovascular disease risk is also reported and it might due to its function as a cofactor in the post-translational modification of the calcification-inhibiting matrix Gla protein[71,72]. An investigation revealed that UBIAD1-generated VK2 played an essential role in maintaining endothelial cell survival and overall vascular homeostasis[73].
A European large cohort study showed that dietary VK intake was associated with the reduced risk of cancer incidence in the prostate and the lung and that the effects were more pronounced in men than in women[74]. Furthermore, a more recent study demonstrated the association between the dietary intake of VK and the reduced risk of cardiovascular disease, cancer, and all-cause mortality in a Mediterranean population[75].
In 1984, abnormal des-carboxy prothrombin was specifically detected in the plasma of patients with HCC[76]. It has since been used as specific diagnostic marker of HCC independent of α-fetoprotein[77,78]. Since the administration of VKs to patients with increased DCP levels showed a transient reduction of DCP levels, HCC was considered to exist under a condition of VK deficiency[79,80]. Earlier studies showed that the administration of VKs on cancer cells including HCC in vitro, resulted in anti-proliferative effects[30-33]. Thus, the anti-tumor effects of VK on HCC have been expected to be found in vivo.
In 2004 Habu et al[81] demonstrated that menatetrenone, a VK2 analog, suppressed the development of HCC in women with viral hepatitis-related cirrhosis. Since then, several randomized controlled studies reported the suppressive effects of menatetrenone on the recurrence of HCC after curative ablation therapy and surgical resection of the liver[82-86]. Although several initial reports with small study populations showed the favorable effects of VK2 in inhibiting the recurrence of HCC after treatment and improving tumor recurrence-free survival, a large randomized control trial (RCT) in which VK2 was administered after curative treatment, failed to show the advantage of VK2 administration[87].
Zhong et al[88] reviewed six RCTs and one cohort study, with a total of 930 patients and performed a meta-analysis. Although treatment with VK2 did not reduce the 1-year recurrence rate, there was a significant association between VK2 and reduced 2- and 3-year tumor recurrence. VK2 treatment was also associated with a significant improvement of 1-, 2-, and 3-year overall survival. However, the results might be considered to still be preliminary because the large scale RCT was evaluated at only 1 year. Therefore, a longer follow-up will be required to confirm the effects of VK2 on HCC.
Although various studies have reported the anti-HCC effects of VK2, the analogs in current use do not appear to exhibit dramatic anti-tumor effects when administered alone. One way to overcome this situation is with the co-administration of VK and other reagents with anti-cancer properties. Yoshiji et al[85] reported the beneficial effects of VK2 combined with ACE inhibitor. Recently acyclic retinoid peretinoin showed beneficial effects on the recurrence and survival of hepatitis C virus-infected HCC patients after curative therapy[89]. VK2 plus ACR synergistically inhibited the growth of Huh7 cells[49]. Currently sorafenib is the only drug approved for the systemic treatment of HCC[90]. It has been shown to extend the survival period of end-stage HCC patients for several months. However, the effect of sorafenib on HCC is not yet satisfactory. Many novel-developed anti-cancer reagents that specifically target the signal transduction pathway of HCC cells have been tested clinically, but most of trials failed to demonstrate their non-inferiority to sorafenib[91,92]. A combination treatment with sorafenib and VK2 was examined in vitro and in vivo animal models and the studies showed that VK2 enhanced the tumor-suppressive effects of sorafenib[93-95].
Another way to enhance the effects of VK would be to develop a new VK derivative, which may be achieved by modifying the side chains of VK. Several approaches to develop novel VK analogs have been conducted. Since some of the effects of VK are considered to be mediated by SXR transcription factor as a ligand of VK2[34,56], Suhara et al[96,97] screened a series of chemically synthesized VK analogs by measuring the SXR-mediated transcriptional activity and found that the modification of the side chain of VK affects the SXR-mediated transcriptional activity. Setoguchi et al[98] synthesized a prodrug of an active form of menaquione-4 that is effectively delivered to HCC cells and which showed the enhanced anti-tumor effects on HCC cell growth.
Recently the repositioning (repurposing) of pre-existing drugs that have been safely used for long-term treatment in a clinical setting has been performed with many drugs such as aspirin and metformin[99,100]. Some of these drugs have begun to be used for chemoprevention and/or for the therapeutic purpose of enhancing anti-cancer effects. VKs seem to be one of the successful examples of repositioned drugs. After the discovery of VK as a cofactor of functional coagulation factor production, it has been shown to be beneficial for the maintenance of bone physiology and the prevention of cardiovascular diseases. Beyond these effects, the novel function of VK as an anti-tumor agent has been applied to the prevention and treatment of HCC, however, the beneficial effects of VK on HCC were found to be limited. The recent progress of novel technologies, such as a genome wide association studies and computational analysis, has been the first step to the repositioning of drugs[101,102]. These approaches will lead to novel applications of VKs and the development of novel VK-based reagents, and may be applied to the treatment of HCC in the future.
| 2. | Dam H, Schønheyder F. The occurrence and chemical nature of vitamin K. Biochem J. 1936;30:897-901. [PubMed] |
| 3. | Booth SL, Suttie JW. Dietary intake and adequacy of vitamin K. J Nutr. 1998;128:785-788. [PubMed] |
| 4. | Collins MD, Jones D. Distribution of isoprenoid quinone structural types in bacteria and their taxonomic implication. Microbiol Rev. 1981;45:316-354. [PubMed] |
| 5. | Thane CW, Paul AA, Bates CJ, Bolton-Smith C, Prentice A, Shearer MJ. Intake and sources of phylloquinone (vitamin K1): variation with socio-demographic and lifestyle factors in a national sample of British elderly people. Br J Nutr. 2002;87:605-613. [PubMed] |
| 6. | Shearer MJ, Newman P. Metabolism and cell biology of vitamin K. Thromb Haemost. 2008;100:530-547. [PubMed] |
| 7. | Nakagawa K, Hirota Y, Sawada N, Yuge N, Watanabe M, Uchino Y, Okuda N, Shimomura Y, Suhara Y, Okano T. Identification of UBIAD1 as a novel human menaquinone-4 biosynthetic enzyme. Nature. 2010;468:117-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 216] [Cited by in RCA: 259] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 8. | Thijssen HH, Drittij-Reijnders MJ, Fischer MA. Phylloquinone and menaquinone-4 distribution in rats: synthesis rather than uptake determines menaquinone-4 organ concentrations. J Nutr. 1996;126:537-543. [PubMed] |
| 10. | Hassan GS. Menadione. Profiles Drug Subst Excip Relat Methodol. 2013;38:227-313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 25] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 11. | Jiang Y, Yang J, Yang C, Meng F, Zhou Y, Yu B, Khan M, Yang H. Vitamin K4 induces tumor cytotoxicity in human prostate carcinoma PC-3 cells via the mitochondria-related apoptotic pathway. Pharmazie. 2013;68:442-448. [PubMed] |
| 12. | Pratt R, Sah PP, Dufrenoy J, Pickering VL. Vitamin K(5) as an Inhibitor of the Growth of Fungi and of Fermentation by Yeast. Proc Natl Acad Sci USA. 1948;34:323-328. [PubMed] |
| 13. | Taketomi S, Fujita T, Yokono K. Insulin receptor and postbinding defects in KK mouse adipocytes and improvement by ciglitazone. Diabetes Res Clin Pract. 1988;5:125-134. [PubMed] |
| 14. | Kohlmeier M, Salomon A, Saupe J, Shearer MJ. Transport of vitamin K to bone in humans. J Nutr. 1996;126:1192S-1196S. [PubMed] |
| 15. | Takada T, Yamanashi Y, Konishi K, Yamamoto T, Toyoda Y, Masuo Y, Yamamoto H, Suzuki H. NPC1L1 is a key regulator of intestinal vitamin K absorption and a modulator of warfarin therapy. Sci Transl Med. 2015;7:275ra23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 72] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 16. | Thijssen HH, Drittij-Reijnders MJ. Vitamin K status in human tissues: tissue-specific accumulation of phylloquinone and menaquinone-4. Br J Nutr. 1996;75:121-127. [PubMed] |
| 17. | Suttie JW. Vitamin K-dependent carboxylase. Annu Rev Biochem. 1985;54:459-477. [PubMed] |
| 18. | Presnell SR, Stafford DW. The vitamin K-dependent carboxylase. Thromb Haemost. 2002;87:937-946. [PubMed] |
| 19. | Oldenburg J, Bevans CG, Müller CR, Watzka M. Vitamin K epoxide reductase complex subunit 1 (VKORC1): the key protein of the vitamin K cycle. Antioxid Redox Signal. 2006;8:347-353. [PubMed] |
| 20. | Bell RG, Matschiner JT. Warfarin and the inhibition of vitamin K activity by an oxide metabolite. Nature. 1972;237:32-33. [PubMed] |
| 21. | Whitlon DS, Sadowski JA, Suttie JW. Mechanism of coumarin action: significance of vitamin K epoxide reductase inhibition. Biochemistry. 1978;17:1371-1377. [PubMed] |
| 22. | Rost S, Fregin A, Ivaskevicius V, Conzelmann E, Hörtnagel K, Pelz HJ, Lappegard K, Seifried E, Scharrer I, Tuddenham EG. Mutations in VKORC1 cause warfarin resistance and multiple coagulation factor deficiency type 2. Nature. 2004;427:537-541. [PubMed] |
| 23. | Li T, Chang CY, Jin DY, Lin PJ, Khvorova A, Stafford DW. Identification of the gene for vitamin K epoxide reductase. Nature. 2004;427:541-544. [PubMed] |
| 24. | Spohn G, Kleinridders A, Wunderlich FT, Watzka M, Zaucke F, Blumbach K, Geisen C, Seifried E, Müller C, Paulsson M. VKORC1 deficiency in mice causes early postnatal lethality due to severe bleeding. Thromb Haemost. 2009;101:1044-1050. [PubMed] |
| 25. | Martius C. The metabolic relationships between the different K vitamins and the synthesis of the ubiquinones. Am J Clin Nutr. 1961;9:97-103. [PubMed] |
| 26. | Okano T, Shimomura Y, Yamane M, Suhara Y, Kamao M, Sugiura M, Nakagawa K. Conversion of phylloquinone (Vitamin K1) into menaquinone-4 (Vitamin K2) in mice: two possible routes for menaquinone-4 accumulation in cerebra of mice. J Biol Chem. 2008;283:11270-11279. [PubMed] |
| 27. | Shearer MJ, Newman P. Recent trends in the metabolism and cell biology of vitamin K with special reference to vitamin K cycling and MK-4 biosynthesis. J Lipid Res. 2014;55:345-362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 150] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 28. | Hauschka PV, Lian JB, Gallop PM. Direct identification of the calcium-binding amino acid, gamma-carboxyglutamate, in mineralized tissue. Proc Natl Acad Sci USA. 1975;72:3925-3929. [PubMed] |
| 29. | Kidd PM. Vitamins D and K as pleiotropic nutrients: clinical importance to the skeletal and cardiovascular systems and preliminary evidence for synergy. Altern Med Rev. 2010;15:199-222. [PubMed] |
| 30. | Chlebowski RT, Dietrich M, Akman S, Block JB. Vitamin K3 inhibition of malignant murine cell growth and human tumor colony formation. Cancer Treat Rep. 1985;69:527-532. [PubMed] |
| 31. | Wu FY, Liao WC, Chang HM. Comparison of antitumor activity of vitamins K1, K2 and K3 on human tumor cells by two (MTT and SRB) cell viability assays. Life Sci. 1993;52:1797-1804. [PubMed] |
| 32. | Sakai I, Hashimoto S, Yoda M, Hida T, Ohsawa S, Nakajo S, Nakaya K. Novel role of vitamin K2: a potent inducer of differentiation of various human myeloid leukemia cell lines. Biochem Biophys Res Commun. 1994;205:1305-1310. [PubMed] |
| 33. | Wang Z, Wang M, Finn F, Carr BI. The growth inhibitory effects of vitamins K and their actions on gene expression. Hepatology. 1995;22:876-882. [PubMed] |
| 34. | Tabb MM, Sun A, Zhou C, Grün F, Errandi J, Romero K, Pham H, Inoue S, Mallick S, Lin M. Vitamin K2 regulation of bone homeostasis is mediated by the steroid and xenobiotic receptor SXR. J Biol Chem. 2003;278:43919-43927. [PubMed] |
| 35. | Zhou C, Tabb MM, Nelson EL, Grün F, Verma S, Sadatrafiei A, Lin M, Mallick S, Forman BM, Thummel KE. Mutual repression between steroid and xenobiotic receptor and NF-kappaB signaling pathways links xenobiotic metabolism and inflammation. J Clin Invest. 2006;116:2280-2289. [PubMed] |
| 36. | Otsuka M, Kato N, Shao RX, Hoshida Y, Ijichi H, Koike Y, Taniguchi H, Moriyama M, Shiratori Y, Kawabe T. Vitamin K2 inhibits the growth and invasiveness of hepatocellular carcinoma cells via protein kinase A activation. Hepatology. 2004;40:243-251. [PubMed] |
| 37. | Ozaki I, Zhang H, Mizuta T, Ide Y, Eguchi Y, Yasutake T, Sakamaki T, Pestell RG, Yamamoto K. Menatetrenone, a vitamin K2 analogue, inhibits hepatocellular carcinoma cell growth by suppressing cyclin D1 expression through inhibition of nuclear factor kappaB activation. Clin Cancer Res. 2007;13:2236-2245. [PubMed] |
| 38. | Ohsaki Y, Shirakawa H, Hiwatashi K, Furukawa Y, Mizutani T, Komai M. Vitamin K suppresses lipopolysaccharide-induced inflammation in the rat. Biosci Biotechnol Biochem. 2006;70:926-932. [PubMed] |
| 39. | Takeuchi Y, Suzawa M, Fukumoto S, Fujita T. Vitamin K(2) inhibits adipogenesis, osteoclastogenesis, and ODF/RANK ligand expression in murine bone marrow cell cultures. Bone. 2000;27:769-776. [PubMed] |
| 40. | Yamaguchi M, Weitzmann MN. Vitamin K2 stimulates osteoblastogenesis and suppresses osteoclastogenesis by suppressing NF-κB activation. Int J Mol Med. 2011;27:3-14. [PubMed] |
| 41. | Ide Y, Zhang H, Hamajima H, Kawaguchi Y, Eguchi Y, Mizuta T, Yamamoto K, Fujimoto K, Ozaki I. Inhibition of matrix metalloproteinase expression by menatetrenone, a vitamin K2 analogue. Oncol Rep. 2009;22:599-604. [PubMed] |
| 42. | Zhang H, Ozaki I, Hamajima H, Iwane S, Takahashi H, Kawaguchi Y, Eguchi Y, Yamamoto K, Mizuta T. Vitamin K2 augments 5-fluorouracil-induced growth inhibition of human hepatocellular carcinoma cells by inhibiting NF-κB activation. Oncol Rep. 2011;25:159-166. [PubMed] |
| 43. | Xia J, Matsuhashi S, Hamajima H, Iwane S, Takahashi H, Eguchi Y, Mizuta T, Fujimoto K, Kuroda S, Ozaki I. The role of PKC isoforms in the inhibition of NF-κB activation by vitamin K2 in human hepatocellular carcinoma cells. J Nutr Biochem. 2012;23:1668-1675. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 29] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 44. | Xia J, Ozaki I, Matsuhashi S, Qi J, Iwane S, Takahashi H, Eguchi Y, Mizuta T, Anzai K. Mechanisms of PKC-mediated enhancement of HIF-1 activity and its inhibition by vitamin K2 in hepatocellular carcinoma cells. Hepatology. 2014;59 Suppl:817A. |
| 45. | Matsumoto K, Okano J, Nagahara T, Murawaki Y. Apoptosis of liver cancer cells by vitamin K2 and enhancement by MEK inhibition. Int J Oncol. 2006;29:1501-1508. [PubMed] |
| 46. | Li L, Qi Z, Qian J, Bi F, Lv J, Xu L, Zhang L, Chen H, Jia R. Induction of apoptosis in hepatocellular carcinoma Smmc-7721 cells by vitamin K(2) is associated with p53 and independent of the intrinsic apoptotic pathway. Mol Cell Biochem. 2010;342:125-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 15] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 47. | Karasawa S, Azuma M, Kasama T, Sakamoto S, Kabe Y, Imai T, Yamaguchi Y, Miyazawa K, Handa H. Vitamin K2 covalently binds to Bak and induces Bak-mediated apoptosis. Mol Pharmacol. 2013;83:613-620. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 39] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 48. | Yaguchi M, Miyazawa K, Katagiri T, Nishimaki J, Kizaki M, Tohyama K, Toyama K. Vitamin K2 and its derivatives induce apoptosis in leukemia cells and enhance the effect of all-trans retinoic acid. Leukemia. 1997;11:779-787. [PubMed] |
| 49. | Kanamori T, Shimizu M, Okuno M, Matsushima-Nishiwaki R, Tsurumi H, Kojima S, Moriwaki H. Synergistic growth inhibition by acyclic retinoid and vitamin K2 in human hepatocellular carcinoma cells. Cancer Sci. 2007;98:431-437. [PubMed] |
| 50. | Suzuki M, Shiraha H, Fujikawa T, Takaoka N, Ueda N, Nakanishi Y, Koike K, Takaki A, Shiratori Y. Des-gamma-carboxy prothrombin is a potential autologous growth factor for hepatocellular carcinoma. J Biol Chem. 2005;280:6409-6415. [PubMed] |
| 51. | Ueda N, Shiraha H, Fujikawa T, Takaoka N, Nakanishi Y, Suzuki M, Matsuo N, Tanaka S, Nishina S, Uemura M. Exon 2 deletion splice variant of gamma-glutamyl carboxylase causes des-gamma-carboxy prothrombin production in hepatocellular carcinoma cell lines. Mol Oncol. 2008;2:241-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 52. | Ma M, Qu XJ, Mu GY, Chen MH, Cheng YN, Kokudo N, Tang W, Cui SX. Vitamin K2 inhibits the growth of hepatocellular carcinoma via decrease of des-gamma-carboxy prothrombin. Chemotherapy. 2009;55:28-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 53. | McGarvey TW, Nguyen T, Puthiyaveettil R, Tomaszewski JE, Malkowicz SB. TERE1, a novel gene affecting growth regulation in prostate carcinoma. Prostate. 2003;54:144-155. [PubMed] |
| 54. | Fredericks WJ, McGarvey T, Wang H, Zheng Y, Fredericks NJ, Yin H, Wang LP, Hsiao W, Lee R, Weiss JS. The TERE1 protein interacts with mitochondrial TBL2: regulation of trans-membrane potential, ROS/RNS and SXR target genes. J Cell Biochem. 2013;114:2170-2187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 55. | Fredericks WJ, Sepulveda J, Lai P, Tomaszewski JE, Lin MF, McGarvey T, Rauscher FJ, Malkowicz SB. The tumor suppressor TERE1 (UBIAD1) prenyltransferase regulates the elevated cholesterol phenotype in castration resistant prostate cancer by controlling a program of ligand dependent SXR target genes. Oncotarget. 2013;4:1075-1092. [PubMed] |
| 56. | Azuma K, Urano T, Ouchi Y, Inoue S. Vitamin K2 suppresses proliferation and motility of hepatocellular carcinoma cells by activating steroid and xenobiotic receptor. Endocr J. 2009;56:843-849. [PubMed] |
| 57. | Azuma K, Urano T, Watabe T, Ouchi Y, Inoue S. PROX1 suppresses vitamin K-induced transcriptional activity of Steroid and Xenobiotic Receptor. Genes Cells. 2011;16:1063-1070. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 58. | Nakagawa K, Sawada N, Hirota Y, Uchino Y, Suhara Y, Hasegawa T, Amizuka N, Okamoto T, Tsugawa N, Kamao M. Vitamin K2 biosynthetic enzyme, UBIAD1 is essential for embryonic development of mice. PLoS One. 2014;9:e104078. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 38] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 59. | Avior Y, Levy G, Zimerman M, Kitsberg D, Schwartz R, Sadeh R, Moussaieff A, Cohen M, Itskovitz-Eldor J, Nahmias Y. Microbial-derived lithocholic acid and vitamin K2 drive the metabolic maturation of pluripotent stem cells-derived and fetal hepatocytes. Hepatology. 2015;62:265-278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 68] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 60. | Saxena SP, Fan T, Li M, Israels ED, Israels LG. A novel role for vitamin K1 in a tyrosine phosphorylation cascade during chick embryogenesis. J Clin Invest. 1997;99:602-607. [PubMed] |
| 61. | Townsend CW. The hemorrhagic disease of the new-born. Arch Pediatr. 1894;11:559-565. |
| 62. | Sutor AH, von Kries R, Cornelissen EA, McNinch AW, Andrew M. Vitamin K deficiency bleeding (VKDB) in infancy. ISTH Pediatric/Perinatal Subcommittee. International Society on Thrombosis and Haemostasis. Thromb Haemost. 1999;81:456-461. [PubMed] |
| 63. | Shearer MJ. Vitamin K deficiency bleeding (VKDB) in early infancy. Blood Rev. 2009;23:49-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 167] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 64. | Greer FR, Marshall S, Cherry J, Suttie JW. Vitamin K status of lactating mothers, human milk, and breast-feeding infants. Pediatrics. 1991;88:751-756. [PubMed] |
| 65. | Greer FR. Vitamin K the basics--what’s new? Early Hum Dev. 2010;86 Suppl 1:43-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 31] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 66. | Shearer MJ, Fu X, Booth SL. Vitamin K nutrition, metabolism, and requirements: current concepts and future research. Adv Nutr. 2012;3:182-195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 176] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 67. | Iwamoto J. Vitamin K2 therapy for postmenopausal osteoporosis. Nutrients. 2014;6:1971-1980. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 65] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 68. | Iwamoto I, Kosha S, Noguchi S, Murakami M, Fujino T, Douchi T, Nagata Y. A longitudinal study of the effect of vitamin K2 on bone mineral density in postmenopausal women a comparative study with vitamin D3 and estrogen-progestin therapy. Maturitas. 1999;31:161-164. [PubMed] |
| 69. | Guralp O, Erel CT. Effects of vitamin K in postmenopausal women: mini review. Maturitas. 2014;77:294-299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 70. | Beulens JW, Booth SL, van den Heuvel EG, Stoecklin E, Baka A, Vermeer C. The role of menaquinones (vitamin K2) in human health. Br J Nutr. 2013;110:1357-1368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 224] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 71. | Geleijnse JM, Vermeer C, Grobbee DE, Schurgers LJ, Knapen MH, van der Meer IM, Hofman A, Witteman JC. Dietary intake of menaquinone is associated with a reduced risk of coronary heart disease: the Rotterdam Study. J Nutr. 2004;134:3100-3105. [PubMed] |
| 72. | El Asmar MS, Naoum JJ, Arbid EJ. Vitamin k dependent proteins and the role of vitamin k2 in the modulation of vascular calcification: a review. Oman Med J. 2014;29:172-177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 63] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 73. | Hegarty JM, Yang H, Chi NC. UBIAD1-mediated vitamin K2 synthesis is required for vascular endothelial cell survival and development. Development. 2013;140:1713-1719. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 45] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 74. | Nimptsch K, Rohrmann S, Kaaks R, Linseisen J. Dietary vitamin K intake in relation to cancer incidence and mortality: results from the Heidelberg cohort of the European Prospective Investigation into Cancer and Nutrition (EPIC-Heidelberg). Am J Clin Nutr. 2010;91:1348-1358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 83] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 75. | Juanola-Falgarona M, Salas-Salvadó J, Ibarrola-Jurado N, Rabassa-Soler A, Díaz-López A, Guasch-Ferré M, Hernández-Alonso P, Balanza R, Bulló M. Effect of the glycemic index of the diet on weight loss, modulation of satiety, inflammation, and other metabolic risk factors: a randomized controlled trial. Am J Clin Nutr. 2014;100:27-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 113] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 76. | Liebman HA, Furie BC, Tong MJ, Blanchard RA, Lo KJ, Lee SD, Coleman MS, Furie B. Des-gamma-carboxy (abnormal) prothrombin as a serum marker of primary hepatocellular carcinoma. N Engl J Med. 1984;310:1427-1431. [PubMed] |
| 77. | Soulier JP, Gozin D, Lefrere JJ. A new method to assay des-gamma-carboxyprothrombin. Results obtained in 75 cases of hepatocellular carcinoma. Gastroenterology. 1986;91:1258-1262. [PubMed] |
| 78. | Fujiyama S, Morishita T, Hashiguchi O, Sato T. Plasma abnormal prothrombin (des-gamma-carboxy prothrombin) as a marker of hepatocellular carcinoma. Cancer. 1988;61:1621-1628. [PubMed] |
| 79. | Lefrère JJ, Gozin D, Soulier JP, Bettan L, Mavier P, Dhumeaux D, Guillaumont M, Leclercq M. Specificity of increased des-gamma-carboxyprothrombin in hepatocellular carcinoma after vitamin K1 injection. J Hepatol. 1987;5:27-29. [PubMed] |
| 80. | Furukawa M, Nakanishi T, Okuda H, Ishida S, Obata H. Changes of plasma des-gamma-carboxy prothrombin levels in patients with hepatocellular carcinoma in response to vitamin K. Cancer. 1992;69:31-38. [PubMed] |
| 81. | Habu D, Shiomi S, Tamori A, Takeda T, Tanaka T, Kubo S, Nishiguchi S. Role of vitamin K2 in the development of hepatocellular carcinoma in women with viral cirrhosis of the liver. JAMA. 2004;292:358-361. [PubMed] |
| 82. | Mizuta T, Ozaki I, Eguchi Y, Yasutake T, Kawazoe S, Fujimoto K, Yamamoto K. The effect of menatetrenone, a vitamin K2 analog, on disease recurrence and survival in patients with hepatocellular carcinoma after curative treatment: a pilot study. Cancer. 2006;106:867-872. [PubMed] |
| 83. | Kakizaki S, Sohara N, Sato K, Suzuki H, Yanagisawa M, Nakajima H, Takagi H, Naganuma A, Otsuka T, Takahashi H. Preventive effects of vitamin K on recurrent disease in patients with hepatocellular carcinoma arising from hepatitis C viral infection. J Gastroenterol Hepatol. 2007;22:518-522. [PubMed] |
| 84. | Hotta N, Ayada M, Sato K, Ishikawa T, Okumura A, Matsumoto E, Ohashi T, Kakumu S. Effect of vitamin K2 on the recurrence in patients with hepatocellular carcinoma. Hepatogastroenterology. 2007;54:2073-2077. [PubMed] |
| 85. | Yoshiji H, Noguchi R, Toyohara M, Ikenaka Y, Kitade M, Kaji K, Yamazaki M, Yamao J, Mitoro A, Sawai M. Combination of vitamin K2 and angiotensin-converting enzyme inhibitor ameliorates cumulative recurrence of hepatocellular carcinoma. J Hepatol. 2009;51:315-321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 72] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 86. | Ishizuka M, Kubota K, Shimoda M, Kita J, Kato M, Park KH, Shiraki T. Effect of menatetrenone, a vitamin k2 analog, on recurrence of hepatocellular carcinoma after surgical resection: a prospective randomized controlled trial. Anticancer Res. 2012;32:5415-5420. [PubMed] |
| 87. | Yoshida H, Shiratori Y, Kudo M, Shiina S, Mizuta T, Kojiro M, Yamamoto K, Koike Y, Saito K, Koyanagi N. Effect of vitamin K2 on the recurrence of hepatocellular carcinoma. Hepatology. 2011;54:532-540. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 101] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 88. | Zhong JH, Mo XS, Xiang BD, Yuan WP, Jiang JF, Xie GS, Li LQ. Postoperative use of the chemopreventive vitamin K2 analog in patients with hepatocellular carcinoma. PLoS One. 2013;8:e58082. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 28] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 89. | Okita K, Izumi N, Ikeda K, Osaki Y, Numata K, Ikeda M, Kokudo N, Imanaka K, Nishiguchi S, Kondo S. Survey of survival among patients with hepatitis C virus-related hepatocellular carcinoma treated with peretinoin, an acyclic retinoid, after the completion of a randomized, placebo-controlled trial. J Gastroenterol. 2015;50:667-674. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 32] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 90. | Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9016] [Cited by in RCA: 10531] [Article Influence: 585.1] [Reference Citation Analysis (9)] |
| 91. | Chuma M, Terashita K, Sakamoto N. New molecularly targeted therapies against advanced hepatocellular carcinoma: From molecular pathogenesis to clinical trials and future directions. Hepatol Res. 2014;Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 70] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 92. | Llovet JM, Hernandez-Gea V. Hepatocellular carcinoma: reasons for phase III failure and novel perspectives on trial design. Clin Cancer Res. 2014;20:2072-2079. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 272] [Cited by in RCA: 326] [Article Influence: 27.2] [Reference Citation Analysis (0)] |
| 93. | Wei G, Wang M, Hyslop T, Wang Z, Carr BI. Vitamin K enhancement of sorafenib-mediated HCC cell growth inhibition in vitro and in vivo. Int J Cancer. 2010;127:2949-2958. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 44] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 94. | Zhang Y, Zhang B, Zhang A, Zhao Y, Zhao J, Liu J, Gao J, Fang D, Rao Z. Synergistic growth inhibition by sorafenib and vitamin K2 in human hepatocellular carcinoma cells. Clinics (Sao Paulo). 2012;67:1093-1099. [PubMed] |
| 95. | Carr BI, Wang Z, Wang M, Cavallini A, D’Alessandro R, Refolo MG. c-Met-Akt pathway-mediated enhancement of inhibitory c-Raf phosphorylation is involved in vitamin K1 and sorafenib synergy on HCC growth inhibition. Cancer Biol Ther. 2011;12:531-538. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 24] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 96. | Suhara Y, Watanabe M, Nakagawa K, Wada A, Ito Y, Takeda K, Takahashi K, Okano T. Synthesis of novel vitamin K2 analogues with modification at the ω-terminal position and their biological evaluation as potent steroid and xenobiotic receptor (SXR) agonists. J Med Chem. 2011;54:4269-4273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 97. | Suhara Y, Hanada N, Okitsu T, Sakai M, Watanabe M, Nakagawa K, Wada A, Takeda K, Takahashi K, Tokiwa H. Structure-activity relationship of novel menaquinone-4 analogues: modification of the side chain affects their biological activities. J Med Chem. 2012;55:1553-1558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 98. | Setoguchi S, Watase D, Matsunaga K, Matsubara M, Kubo Y, Kusuda M, Nagata-Akaho N, Enjoji M, Nakashima M, Takeshita M. Enhanced antitumor effects of novel intracellular delivery of an active form of menaquinone-4, menahydroquinone-4, into hepatocellular carcinoma. Cancer Prev Res (Phila). 2015;8:129-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 99. | Shim JS, Liu JO. Recent advances in drug repositioning for the discovery of new anticancer drugs. Int J Biol Sci. 2014;10:654-663. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 254] [Cited by in RCA: 277] [Article Influence: 23.1] [Reference Citation Analysis (0)] |
| 100. | Yue W, Yang CS, DiPaola RS, Tan XL. Repurposing of metformin and aspirin by targeting AMPK-mTOR and inflammation for pancreatic cancer prevention and treatment. Cancer Prev Res (Phila). 2014;7:388-397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 129] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 101. | Jiao M, Liu G, Xue Y, Ding C. Computational drug repositioning for cancer therapeutics. Curr Top Med Chem. 2015;15:767-775. [PubMed] |
| 102. | Zhang J, Jiang K, Lv L, Wang H, Shen Z, Gao Z, Wang B, Yang Y, Ye Y, Wang S. Use of genome-wide association studies for cancer research and drug repositioning. PLoS One. 2015;10:e0116477. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
P- Reviewer: Dai YC, Xiao EH, Yang L S- Editor: Ji FF L- Editor: A E- Editor: Liu SQ
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/