Published online Aug 16, 2015. doi: 10.12998/wjcc.v3.i8.705
Peer-review started: July 27, 2014
First decision: August 14, 2014
Revised: February 22, 2015
Accepted: June 4, 2015
Article in press: June 8, 2015
Published online: August 16, 2015
Processing time: 392 Days and 3 Hours
Malignant cardiac arrhythmias which result in sudden cardiac death may be present in individuals apparently healthy or be associated with other medical conditions. The way to predict their appearance represents a challenge for the medical community due to the tragic outcomes in most cases. In the last two decades some ventricular repolarization (VR) markers have been found to be useful to predict malignant cardiac arrhythmias in several clinical conditions. The corrected QT, QT dispersion, Tpeak-Tend, Tpeak-Tend dispersion and Tp-e/QT have been studied and implemented in clinical practice for this purpose. These markers are obtained from 12 lead surface electrocardiogram. In this review we discuss how these markers have demonstrated to be effective to predict malignant arrhythmias in medical conditions such as long and short QT syndromes, Brugada syndrome, early repolarization syndrome, acute myocardial ischemia, heart failure, hypertension, diabetes mellitus, obesity and highly trained athletes. Also the main pathophysiological mechanisms that explain the arrhythmogenic predisposition in these diseases and the basis for the VR markers are discussed. However, the same results have not been found in all conditions. Further studies are needed to reach a global consensus in order to incorporate these VR parameters in risk stratification of these patients.
Core tip: Malignant ventricular arrhythmias are a common cause of sudden cardiac death in clinical practice. They may present in individuals apparently healthy or be associated with other medical conditions. It is possible to predict the appearances of ventricular arrhythmias by analysing ventricular repolarization markers through surface 12 leads electrocardiogram. It may represent a tool to evaluate the risk stratification and for achieving a better medical management of our patients.
- Citation: Castro-Torres Y, Carmona-Puerta R, Katholi RE. Ventricular repolarization markers for predicting malignant arrhythmias in clinical practice. World J Clin Cases 2015; 3(8): 705-720
- URL: https://www.wjgnet.com/2307-8960/full/v3/i8/705.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v3.i8.705
An electrocardiogram (ECG) represents one of the most common medical tools used by physicians in clinical practice. Its adequate interpretation gives the possibility for diagnosing and predicting multiple cardiac diseases. These features accompanied by its relative low cost allow that ECG interpretation needs to be known by medical doctors of several specialities including internists, cardiologists, anaesthesiologists, family physicians, and all that have a direct contact with patients.
Sudden cardiac death (SCD) causes approximately 800000 deaths each year in the world[1]. It is often produced by malignant ventricular arrhythmias (MVA). In most cases it is derived from ventricular fibrillation, or less frequently, by monomorphic or polymorphic ventricular tachycardia and Torsades de Pointes[2]. MVA which may result in SCD frequently occur in sick hearts but around 15%-20% occur in healthy hearts[3].
Many people that develop MVA have a previous disease that may be the cause of this condition. By ECG analysis it is often possible to predict the development of ventricular cardiac arrhythmias in these patients. That is feasible by analysing several ventricular repolarization (VR) markers. Some of the most explored predictors in clinical practice are: QT interval and its correction by heart rate (QTc)[4], QT interval dispersion (QTd)[5] and other recently published markers like Tpeak-Tend (Tp-e)[6], Tp-e dispersion (Tp-ed)[7] and Tp-e/QT ratio[8]. These markers have demonstrated a high usefulness to indicate patients with a high risk to develop cardiac arrhythmias in multiple clinical conditions (Table 1).
| Long QT syndrome | Heart failure |
| Short QT syndrome | Hypertension |
| Brugada syndrome | Diabetes mellitus |
| Early repolarization syndrome | Overweight/obesity |
| Acute myocardial ischemia | Highly trained athletes |
| Kawasaki disease | Duchenne muscular dystrophy |
| Systemic lupus erythematosus | Liver transplantiation |
| Rheumatoid arthritis | Rheumatoid arthritis |
| Chronic renal failure | Rheumatic fever |
| Scleroderma | Chagas disease |
| Ankylosing spondylitis | Chronic hepatitis B |
| Obstructive sleep apnea | β-thalassemia |
| Spinal injury | Polycythemia vera |
The methods to measure these VR markers are not difficult. These VR markers are useful tools for evaluation of patients’ risk to develop MVA. They help us make better medical decisions in the management of patients with various medical conditions.
The aim of this manuscript is to review the usefulness of electrocardiographic VR markers to predict SCD in several conditions such as long and short QT syndromes, Brugada syndrome, early repolarization syndrome, acute myocardial ischemia, heart failure, hypertension, diabetes mellitus, obesity and high trained athletes and the possible mechanisms involved in order to encourage their clinical use to improve patients’ risk evaluation.
Measurement of the QT interval from surface 12 lead ECG was proposed at the beginning of last century[9]. It was primarily used to identify patients with arrhythmogenic syndromes which are common causes of SCD. These syndromes are long and short QT syndromes. However, its usefulness has been extended in multiple clinical conditions.
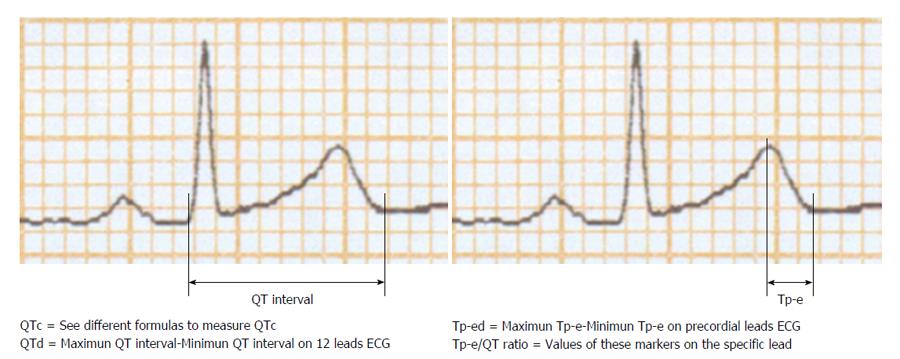
The QT interval is measured from the onset of the QRS complex to the end of the T wave (Figure 1). It should be recorded in II or V5 leads where it has demonstrated the most predictive capacity. The QT interval may be modified by heart rate. Because of this, formulas have been proposed with the aim to reduce the heart rate influence. QT interval corrected by heart rate is known as QTc. Around 20 formulas to measure the QTc have been suggested. However, the most commonly used in clinical practice are the QTc by Bazzet’s method [QTc = QT/(R-R)1/2] and by Fridericia’s method [QTc = QT/(R-R)1/2] both expressed in seconds (Table 2)[10]. Also it is recommended to consider gender in QRS prolongation. Abnormal proposed values of QTcfor adults are ≥ 450 ms in men and ≥ 460 ms in women, while a QTc ≤ 390 ms is considered short[11]. Proposed normal values of QTcin children between 1 and 15 years are < 440 ms, with a range between 440 to 460 ms and abnormal values > 460 ms[12]. However, other authors have established that the 98th percentile limit for rate-adjusted QT is approximately 450 ms in children younger than 12 years of age[11].
| Name | Formula |
| Bazzet modificated by Taran and Szilagyi | QTc = QT/(R-R)1/2 |
| Fridericia | QTc = QT/(R-R)1/3 |
| Framingham | QTc = QT + 0.154 × (1 - RR) |
| Hodges | QTc = QT + 1.75 × (HR - 60) |
| Sarma | QTc = QT - B1 Exp(-k1 × RR) |
| QTc = QT [1 - Exp(-k2 × RR)] | |
| QTc = QT (RR)1/2 + B3 | |
| QTc = QT (RR)1/2 | |
| Ecuación de fuerza | QTc = 453.65 × RR1/3.02 (R2 = 0.41) |
| Van de Water | QTc = QT - 0.087 (RR - 1000) |
| Matsunaga | QTc = log(600) QT/(logRR) |
| Kawataki | QTc = QT/RR × 0.25 |
| Mayeda | QTc = QT/RR × 0.604 |
| Larsen y Skulason | QTc = QT + 0.125 × (1 - RR) |
| Schlamowitz | QTc = QT + 0.205 × (1 - RR) |
| Wohlfart | QTc = QT + 1.23 × (HR - 60) |
| Boudolas | QTc = QT + 2.0 × (HR - 60) |
| Sagie | QTc = QT + 0.154 × (1 - RR) |
| Malik | QTc = QT/RR × 0.371 |
| Lecocq | QTc = QT/RR (0.314) |
Although QT interval and its correction (QTc) have been satisfactorily used to predict cardiac arrhythmias, another marker which evaluates its dispersion has been created and implemented in clinical practice. This marker is the QTd, which is defined as the difference between the maximum and minimum QT interval in surface 12 lead ECG[13]. QTd represents the heterogeneity state of VR. An increase in ventricular heterogeneity augments the vulnerable period time in the heart and predisposes to ventricular arrhythmias[14]. It was implemented in clinical practice by Day et al[15], and currently has been suitably evaluated as a marker to predict MVA. The values of QTd in normal subjects and general population are controversial. Reports from several studies reveal values of 33.4 ± 20.0 ms, with a range from 10.5 ± 10.0 ms to 71 ± 7.0 ms and a median in 37 ms. There are not statically significant differences between the genders while by other studies were found age-related differences < 10 ms[5]. Recently published data show that in healthy individuals a QTd > 58 ms increase 3.2-fold the risk of cardiovascular mortality[16] and those with a QTd ≥ 80 ms have 4-fold risk for cardiac death compared to patients with QTd values < 30 ms[4].
An increase in dispersion of repolarization and modifications in normal pattern of ventricular recovery are mechanisms associated with predisposition to develop cardiac arrhythmias[17]. Tp-e is the interval between the peak of the T wave and the end of the T wave (Figure 1). Commonly it is considered a reflection of the transmural cardiac repolarization expressed through surface 12 lead ECG. It has been proposed to indicate patients at an increased risk for ventricular arrhythmias[18,19]. Recent investigation examined Tp-e interval using a computer model of the rabbit heart ventricles. The authors concluded that Tp-e corresponds with global dispersion of repolarization[20].
Tp-e should be measured in precordial leads where it has been demonstrated to be more specific[21]. It has been found that in healthy men subjects Tp-e has a mean value in V5 lead of 94 ± 10 ms in men and 92 ± 11 ms in women[22]. However, there is not a consensus about Tp-e normal values and further investigations are needed to define them. Tp-e has been evaluated in several clinical conditions. It has been considered more useful to predict cardiac arrhythmias than QTc and its dispersion in some clinical conditions[23].
Tp-ed was proposed by Castro Hevia et al[7]. Using this marker, they found an increased risk for ventricular arrhythmias in patients with Brugada syndrome than healthy controls. It has been examined in other diseases demonstrating its usefulness to predict malignant arrhythmias and SCD. However, limited information has been published about it and it should be studied in further investigations.
Tp-e/QT ratio is a novel index to predict cardiac arrhythmias[8]. It includes the values of transmural dispersion (Tp-e) and spatial dispersion (QT) of VR (Figure 1). It has a substantial advantage than other markers because does not need to be corrected by heart rate. Tp-e/QT ratio almost has no variations between 60-100 beats/min. Tp-e/QT measured in healthy populations in precordial lead V6 which best reflects the trasmural axis of left ventricle has a mean value of 0.21 ± 0.03 and a range of value from 0.15 to 0.25[24].
Heterogeneity of VR is associated with MVA[25]. QT interval is an index of VR and its variations represents its heterogeneity, commonly measured by QTd. An increase in VR dispersion increased the vulnerable period in the heart and predisposes to MVA. Regional differences in the potential action (inhomogeneity) may be found in several heart parts, but the transmural (ventricular wall) is the most important[14]. Ventricular myocardium is comprised by endocardial, epicardial and myocardial (M) cells. These cells are structurally similar, but have different electrophysiological properties. M cells have a longer action potential than cells located in epicardium and endocardium[26,27]. This property is noted in response to slowing of heart rate or agents than prolong the action potential. The prolonged action potential of M cells is due to a delay or blockage of K+ and a rise in Na+ currents[14]. In normal conditions, the physiological differences among these cells are minimized by electronic influences from well coupled myocytes[28]. The interplay between these opposing transmural forces among these cells determines the height and width of the T wave[29]. A rise in transmural repolarization determines a prolongation of T wave and subsequently the Tp-e. Multiple conditions determine pathophysiological changes with alteration in normal pattern of repolarization, increasing heart heterogeneity and the risk of MVA.
Application of QT, Tp-e, its dispersions and Tp-e/QT ratio to predict MVA in some diseases is discussed below.
Long QT syndrome is an arrhythmogenic channelopathy characterized by severe alterations in VR. Long QT syndrome can be congenital or acquired.
The congenital long QT syndrome is caused by hereditary defects of molecular structure in ion channel proteins. Mutations in genes that codify to sodium and potassium channels cause a prolongation of VR. These alterations predispose to the development of Torsade de Pointes. This arrhythmia can result in ventricular fibrillation and SCD[30,31]. Long QT syndrome is often an autosomal dominant disease. It has been found mutations in 13 genes in long QT syndrome, determining 13 different types of this condition. However, the most known are long QT syndrome 1, long QT syndrome 2 and long QT syndrome 3, which are related with KCNQ1, KCNH2 and SCN5A genes respectively[32].
The acquired form of long QT syndrome is a prolongation of QT interval that also predisposes to the patient to develop Torsade de Point and SCD. It may be caused by drugs[33], electrolyte abnormalities[34], hypothermia[35], toxic substances[36] and central nervous system injury[37].
Patients with QTc ≥ 500 ms have a high risk for developing MVA. Sauer et al[38] studied patients with long QT syndrome and the utility of QTc to predict SCD in these cases. Those with QTc duration between 500 and 549 ms were associated with a greater risk. In this same work, a QTc ≥ 550 ms was related with a 6.3-fold increase in the risk. Another investigation showed that patients with QTc duration ≥ 530 ms had a higher predisposition to develop cardiac arrest and SCD than those with shorter QTc values[39]. Recently, the risk for life-threating cardiac events was evaluated in 403 patients with long QT syndrome. Patients with multiple gene mutations had longer QTc than those with a single mutation (506 ± 72 ms vs 480 ± 56 ms respectively; P = 0.003) and a higher rate of life-threating cardiac events[40]. This study demonstrates the complexity of this syndrome, and how the gene mutations may modify the values of QTc and maybe other markers. The phenotypes derived from the gene mutations may express several clinical forms of this condition and make the interpretation of the results difficult.
In these studies, higher QTc values were associated with SCD. However, it has been demonstrated that the risk stratification in patients with congenital long QT syndrome is beyond QTc values. Also, it has been demonstrated that it is necessary to evaluate other variables as the gender, specific gene mutation/s and family history[41].
QTd is another marker evaluated in these cases. It has been found prolonged in patients with idiopathic long QT syndrome and represents a marker for therapeutic efficacy[42]. Yamaguchi et al[43] evaluated 27 patients with long QT syndrome. These patients were divided into two groups. The group A (n = 27) were patients with Torsades de Pointes and group B (n = 15) without this. The measurement of QTd in the first group was 112 ± 64 ms and in the second 70 ± 40 ms; P = 0.0456. These authors also evaluated the Tp-e. Values of Tp-e in V5 were 185 ± 46 ms in group A and 84 ± 18 ms in group B; P < 0.0001. Tp-e showed more significant differences than QTd between patients with and without Torsades de Pointes. Moreover, Tp-e has been found to be prolonged and to be an arrhythmogenic index in patients with long QT syndrome in other studies[44,45]. In this same investigation by Yamaguchi et al[43], Tp-e/QT ratio in V5exceeding 0.28 was also associated with the risk to develop Torsades de Pointes.
Congenital short QT syndrome is a rare chanelopathy which increases the incidence of paroxysmal atrial fibrillation, ventricular tachycardia and/or fibrillation[46-48].The diagnostic criteria are in debate and not yet been established. Most investigators use a grey area for the QTc between 370 and 330 ms[49]. Recently data have been published which reveal an estimated prevalence of 0.7 per 100000 persons using a QTc ≤ 300 ms as diagnostic criteria[50]. It has been proposed values of QTc values of 350 ms for men and 360 ms for women derived considering a cutoff values of ≤ 2 SD from the mean value of general population[51]. Currently a QTc < 320 ms is accepted as an abnormal QTc value[52]. However, the risk to develop MVA in patients with abnormal short QTc values is undetermined. In patients with a prior history of atrial or ventricular fibrillation a QT interval less than 340 ms or a QTc less than 345 ms is usually sufficient[53]. Due to the non-existence of an international consensus of QT values for the diagnosis of short QT syndrome it has been proposed diagnostic criteria. These include the values of QTc, personal clinical history, family history and genotype[49].
Several mutations in potassium channels (KCNH2, KCNQ1 and KCNJ2) and calcium channels (CACNA1C, CACNB2B, CACNA2D1) have been identified in these patients[54].
Anttonen et al[55] studied patients with symptomatic short QT syndrome and evaluated some VR markers. They found that in the group of patients with short QT syndrome values of Tp-e/QT ratio were more prolonged than in the control group (0.30 ± 0.04 vs 0.24 ± 0.04, P = 0.001). Tp-e/QT ratio in the normal population is around 0.21. In this case both patients’ groups had values over this point, but it seems that there is a cut off value which increases the risk of malignant arrhythmias which is unknown and should be explored by researchers and physicians. Similar results were found in another study. In this case after a follow-up with a 24-h ECG recording patients with short QT syndrome the Tp-e/QT ratio was 0.28 ± 0.03 and 0.21 ± 0.02 in control subjects, P = 0.01[56]. Also Tp-e has been demonstrated to be prolonged and correlated with transmural dispersion repolarization in an experimental model of short QT syndrome[57].
Short QT syndrome should be studied further like other conditions which are associated with genetic mutations and several clinical presentation forms. There are limited studies to conclude about the usefulness of VR markers to evaluate the risk of patients with short QT syndrome to develop MVA. There are points which should be analysed such as the absence of a consensus about diagnostic criteria, the low prevalence of this condition, the genetic heterogeneity and possible differences among age, gender and races. An advance in this field has been with the exploration of Tp-e/QT ratio with encouraging results.
An alteration of VR markers has been observed in other clinical conditions. Brugada syndrome was established in 1992. It is defined by a J point and ST-segment elevation 2 mm or greater followed by a negative T wave in the ECG right precordial leads[58]. Recently, a consensus report from the International Society for Holter and Noninvasive Electrophysiology has established 2 Brugada patterns[59]. They combined the type 2 and 3 patterns outlined in a previous report[60]. This syndrome has been linked to SCN5A gene mutations which affect sodium channel function[61,62]. Its prevalence is around 1 in 2000 people[63] and may explain 20% of SCD in patients without structural cardiac disease[60]. Patients with Brugada syndrome have an increased risk to develop malignant cardiac arrhythmias. They have a high incidence of ventricular tachycardia and/or ventricular fibrillation in structurally normal hearts[64-67] and are asymptomatic in most cases[68].
A prolongation of QTc in right precordial lead has been found in patients with Brugada syndrome[69]. A mechanism to explain QTc prolongation in patients with Brugada syndrome may be by the rise of the action potential notch in the right than in the left ventricular epicardium observed in these subjects.
Castro Hevia et al[7] studied 29 patients with Brugada syndrome and 29 healthy controls. Patients were followed by a mean of 42.65 ± 24.42 mo. QTc > 460 ms in V2 lead was a risk factor for arrhythmias recurrence. Maximum Tp-e (measured in precordial leads) and Tp-ed were associated with more recurrence of life-threatening cardiac events in Brugada syndrome patients compared with controls. Also, all Brugada syndrome patients with Tp-e values ≥ 100 ms or Tp-ed values > 20 ms had events during 60 mo of follow-up. As was established by the authors, this work gives a novel way to analyze the risk stratification in Brugada syndrome.
Recently 23 individuals (spontaneous n = 10 or drug-induced n = 13) with type 1 ECG pattern of Brugada syndrome received a programmed ventricular stimulation. Tp-e in leads V2 and Tp-e/QT ratio in leads V6 were significantly increased in patients who developed ventricular tachycardia/ventricular fibrillation. However, QTc (by Bazzet’s method) and QTd did not show significant differences[70].
Several mechanisms have been formulated to explain arrhythmogenic risk in patients with Brugada syndrome. One of the most accepted establishes that the decrease of the depolarizing in sodium channels presented in this condition leads to a functional predominance of the repolarizing Ito current. These differences result in an increase in epicardial action potential notch. These changes produce an increase in the transmural heterogeneity of repolarization and a predisposition to MVA[71-73]. It has been proved that phase 2 reentry might appear under these conditions[74]. Another theory is based on depolarization abnormalities. It has been observed that there are regional differences between activation of the cells located in right ventricular tract outflow myocardium and the rest of right ventricle. These alterations modify cell interactions and increase the risk of reentry arrhythmias[72,73,75]. Also it has been proposed discontinuous conduction and malfunctioning channels as hypotheses to explain arrhythmogenic risk in these patients[72].
Brugada syndrome is a very complex condition with several genetic subtypes, arrhythmic pathophysiological mechanisms, electrocardiographic patterns and clinical presentations. Thus, it is possible to understand the multiple variants described in clinical practice. These characteristics also may influence the ability of VR markers to detect patients at high risk of malignant cardiac arrhythmias in this condition. In order to elevate clinical sensitivity of these markers in these cases investigators should be take into account these factors and to design clinical trials to achieve better conclusions.
Early repolarization pattern consists in a J wave or J point elevation, a notch or slur of the terminal part of the QRS and an ST-segment elevation on surface 12 lead ECG[76-78]. It has prevalence from 6% to 24% in the general population[79] and is higher in trained athletes[80]. Early repolarization pattern was primarily considered a benign electrocardiographic finding[81,82]. However, recent studies have demonstrated an increased risk for malignant cardiac arrhythmias in these patients[83-85]. Early repolarization pattern adopts the name early repolarization syndrome when it is associated with cardiac arrhythmias and SCD[86]. Current studies have determinated three patterns of early repolarization and the risk to develop arrhythmic events of each of them[87].
The predisposition to have malignant ventricular arrhythmia in these patients was evaluated by Letsas et al[88]. They found increased values of Tp-e in leads V2, Tp-ed in precordial leads and Tp-e/QT ratio in leads V2 in patients with early repolarization pattern compared with those without it. There were not significant differences regarding markers studied in localization of early repolarization pattern (lateral vs infero-lateral). Previously, another investigation demonstrated that QTc was useful to predict primary end points in these individuals[89]. The use of these markers to indicate patients at risk to suffer SCD by MVA in this setting should be explored further.
The arrhythmogenic substrate of early repolarization syndrome has been proposed to be the outward current of Ito. Ito current is more prominent in epicardium than endocardium. This gradient voltage has been associated with the beginning of cardiac arrhythmias[90,91]. Furthermore, an investigational group demonstrated that there are abnormal large spatial repolarizations gradients in early repolarization that predispose to reentry cardiac arrhythmias[77]. Previous investigations demonstrated a high incidence of malignant arrhythmias in patients with early repolarization pattern in inferior/inferiolateral leads or in inferior, lateral and right precordial leads than patients with only in the lateral precordial leads[87]. These differences provide additional data and increase the complexity of this condition. As the arrhythmogenic index may be variable depending of ECG topography, VR markers should be explored independently to reach trusty results.
Cardiovascular diseases are the most common cause of mortality in developed countries[92]. Acute coronary syndrome is often associated with SCD[93]. In patients with acute coronary syndrome an increased risk for MVA with a worse prognosis has been observed[94]. QT interval has been used to estimate the arrhythmogenic risk after an acute coronary syndrome. Schwartz et al[95] demonstrated the clinical value of QT interval many years ago. They showed that patients with Q wave acute myocardial infarction and prolonged values of QT interval had a high probability to develop SCD. Another study documented that exercise after an acute myocardial infarction may induce changes in QTc. In this case, the prolongation of QTc interval predisposes to develop SCD and was useful to differentiate patients at high risk for SCD and those at low risk for it[96]. These results show the benefit of using the QTc for identifying patients with exercise-induced ischemia who are at risk of SCD. In addition, a correlation between QTc prolongation and SCD in patients with non-ST elevation acute coronary syndrome has been observed[97,98]. These studies support previous results about the utility of QTc in acute coronary syndrome and additionally provide new elements from QTc applicability in risk stratification for SCD.
Other VR markers have been explored with the purpose to increase the sensibility to predict SCD in patients with acute myocardial ischemia. Ciolli et al[99] studied 101 patients with acute myocardial infarction and a control group of 97 healthy patients. After 10 d follow-up, QTd was significantly more prolonged in subjects who developed severe ventricular arrhythmias than control (125.8 ± 68.5 vs 80.8 ± 38.9, P < 0.0005).Similar results were found by other researchers. In this case, QTd was found to be increased in patients with ventricular tachycardia or ventricular fibrillation compared those with only ventricular premature beats (96.25 ± 15.97 ms vs 80 ± 15.04 ms, P < 0.01)[100]. Moreover, QTd represented a predictor to death by ventricular arrhythmias. It was a very interesting work about QTd utility in patients with acute myocardial infarction. As can been seen, it was not only prolonged in patients with ventricular arrhythmias but was a predictor of death. This study provides relevant data to continue our investigation about QTd importance in this field.
Tp-e also has been found to be useful to predict cardiac arrhythmias in patients after myocardial infarction. Seventy-six patients with previous myocardial infarction were followed during 23 ± 19 mo. Tp-e was longer in patients who developed ventricular arrhythmias than those without (116 ± 26 ms vs 102 ± 20 ms, P = 0.01). Additionally, Tp-e was found to be an independent predictor of ventricular arrhythmias when adjusted for age, ejection fraction and QRS duration[101]. Also, QTc has been found to be prolonged in patients with unstable angina with an increased risk for cardiac arrhythmias[102].
Underlying mechanisms to explain modification of these indicators in acute myocardial ischemia include an expression of M cells properties. Activation of M cells determines an increase in the action potential in the heart and subsequently a QT interval and Tp-eprolongation[14,26-29]. Other proposed mechanisms are the reduction in epicardium temperature[103], acidosis[104] and changes in sodium and potassium currents[105]. These processes increase myocardium heterogeneity and predispose to malignant cardiac arrhythmias.
Reperfusion therapies (fibrinolysis and primary percutaneous coronary intervention) are the primary goal in ST-elevation myocardial infarction for improving clinical outcomes. They have demonstrated to be useful reducing the incidence of cardiac arrhythmias, including ventricular tachycardia and/or ventricular fibrillation[106,107]. There are studies demonstrating the usefulness of VR markers to predict malignant cardiac arrhythmias in this setting. QTc[108], QTd[109-111], Tp-e[112,113] and Tp-e/QT[114] have been found to be useful to indicate effectiveness of thrombolytic therapy and primary percutaneous coronary intervention in patients with acute ST-elevation myocardial infarction. These results indicate patients with reduced risk to develop MVA giving the physician another tool to evaluate the patients’ risk in this setting. However, these markers need further study in other situation commonly present in patients treated with reperfusion methods. Patients with ST-elevation acute myocardial infarction develop with relative frequency a transient ST-elevation on ECG when undergoing treatment with fibrinolysis or primary percutaneous coronary intervention. This phenomenon is named “reperfusion peak” and it is followed by a complete ST-resolution[115]. The real significance of this event is not clear. In some studies it has been related with negative cardiac outcomes[116,117]. Application of VR markers in this setting may be important to detect patients at an increased risk for malignant arrhythmias and SCD when presenting with this phenomenon.
Patients with heart failure frequently have ventricular arrhythmias. Ventricular tachycardia and premature ventricular beats are seen with increased incidence in individuals with a dilated left ventricle and reduced ejection fraction[118]. Taking this into account, several studies have been designed to explore arrhythmia markers and to determine the arrhythmogenic risk in these patients.
Davey et al[119] found that QTc was significantly longer in heart failure patients than in controls or in subjects with left ventricular hypertrophy (471 ± 10 ms, 421 ± 6 ms, 420 ± 6 ms, P < 0.05 respectively). Tp-e also has been found to be useful in this setting. Its prolongation in V1 lead has correlated with increased incidence of SCD in chronic heart failure patients[120]. Tp-e has been evaluated in patients with left ventricular ejection fraction ≤ 35% and therapy with implantable cardioverter-defibrillator. It demonstrated to be effective predicting ventricular tachycardia and overall mortality[121]. Heart failure is defined as a syndrome derived from multiple cardiac and non-cardiac conditions. It has a complex pathophysiology and mechanisms proposed for the development of MVA are diverse and include: ischaemia, infarction, cardiomyopathy, myocarditis, hypokalaemia, hypomagnesaemia and digitalis overdose[118]. All of these processes may increase the ventricular heterogeneity and predispose to MVA. Some factors which could be associated are age, aetiology, drugs and comorbidities. As heart failure may be caused by multiple conditions, to analyse the risk for MVA can be very difficult.
Hypertension is a very common condition worldwide. Its prevalence is around 30%-45% in the general population[122]. Hypertensive patients have an increased risk for cardiac arrhythmias due to an increase in VR dispersion[123]. Left ventricular hypertrophy often develops in hypertension and it increases the risk of developing cardiac arrhythmias[124,125]. Carmona Puerta et al[126] observed that QTd and Tp-e were more prolonged in patients with left ventricular hypertrophy than those without it. Also, a linear correlation among QTd, Tp-e and duration of hypertension was observed (r = 0.453, P = 0.001 and r = 0.306, P = 0.034 respectively). Mozos et al[127] support these findings with similar results. In another investigation, QTc and Tp-e were associated with systolic blood pressure, body mass index, and left ventricular mass in resistant hypertensive patients[128]. The prognosis of patients with resistant hypertension is probably worse compared with those that have easily controlled hypertension. However, it has not been specifically evaluated. It may be influenced by some risk factors or associated conditions[129]. This investigation may give us a new alternative to evaluate the prognoses of resistant hypertensive patients by the analyses of VR markers. However, prospective studies would be required to achieve definite conclusions.
Recently, an investigational group demonstrated that in hypertensive patients without ischemic heart disease the global cardiovascular risk is related to some electrocardiographic markers for cardiac arrhythmias. QTc and Tp-e showed the most significant correlation (P = 0.010 and P = 0.000 respectively)[130]. Global cardiovascular risk may be examined by several methods. In this study the risk score proposed in the 2007 European Guidelines for the Management of Hypertension was used. The use of global cardiovascular risk scores associated with VR markers could help to identify patients at high risk of malignant cardiac arrhythmias. It could represent a novel tool for physicians in the future for better patients’ management.
Further studies should be conducted to explore other arrhythmias markers and global cardiovascular risk scores.
There are several pathophysiological mechanisms to explain the predisposition to develop MVA in hypertensive patients. Left ventricular hypertrophy is found often in these cases and is recognized as the most important factor. It correlates with malignant arrhythmias and SCD[131]. Left ventricular hypertrophy causes early after-depolarization and favors the occurrence of ventricular arrhythmias[132]. It may also cause myocardial ischemia by an unbalance between blood supply to the myocardium and oxygen consumption. Moreover, in this condition it may cause an increase in subendocardial ischemia due to a reduction in diastolic blood flow to this region[133]. These processes may be more intense in long-term hypertensive patients’ in which left ventricular hypertrophy is more frequent. In fact, QTd has been found to be increased in elderly hypertensive individuals with the presence of left ventricular hypertrophy and myocardial ischaemia on ECG[134]. Myocardial ischemia increases cardiac heterogeneity and the predisposition to SCD.
Other changes in the heart of hypertensive patients include arise of collagen deposits as part of ventricular remodeling. Ventricular remodeling favors ventricular heterogeneity and ventricular re-entry arrhythmias[135]. In hypertensive patients there is often an increase in sympathetic nervous system activity, and activation of this system has been associated with increased cardiac arrhythmias[136]. Additionally, alterations in gap-junctions in myocardial cells and an elevated risk to develop ventricular arrhythmias in hypertensive animal models with hypokalaemia has been observed[137].
Diabetes mellitus had a prevalence of 360 million people worldwide in 2011. This prevalence will increase to 552 million people with diabetes by 2030[138]. SCD is often observed in diabetic patients and may be associated with ventricular arrhythmias[139,140]. Ventricular arrhythmias predictors in these patients have been evaluated in several studies.
Patients with type 1 diabetes mellitus and autonomic dysfunction have shown significantly higher values of QTd than patients without autonomic dysfunction and controls. After a follow-up by 24 h holter monitoring, individuals with autonomic dysfunction and a prolonged QTd had a higher incidence of ventricular arrhythmias[141].
Clemente et al[142] designed an investigation with the aim to study the effects of diabetes mellitus on VR markers. A group of 110 diabetic patients and a group of 110 controls were selected. Maximum QTc was significantly greater in diabetic patients than in controls (413.70 ± 28.10 ms vs 395.31 ± 16.28 ms, P < 0.001). Diabetics had a significantly higher mean QTd than controls (27.49 ± 10.10 ms vs 15.73 ± 4.18 ms, P < 0.001). Similar results were found with the measurement of QTd corrected by heart rate (29.92 ± 10.57 ms vs 16.68 ± 4.48 ms, P < 0.001).
More recently an investigational group found that QTc and QTd are prolonged in newborns of diabetic mothers. QTd prolongation was associated with interventricular septal thickness at end diastole (r = 0.514, P = 0.042). The authors concluded that elevated values of these markers represent risk factors for the development of arrhythmias in these patients[143].
The results of these studies are encouraging, but further studies are needed to know with more precision the risk of diabetic patients to develop malignant cardiac arrhythmias. It should be important to evaluate other markers to know whether they may be added to the risk estimation in these patients.
The proposed mechanisms explain predisposition to develop cardiac arrhythmias in diabetic patients are diverse. They have a high incidence of coronary atherosclerosis, microvascular disease and autonomic neuropathy. The arrhythmogenic substrate, may be in part, due to compensatory hypertrophy in non-infarcted myocardium, progressive ventricular remodeling and neurohormonal abnormalities[144,145]. These processes increase VR heterogeneity and elevate the risk of re-entry arrhythmias.
Obesity is a common condition worldwide. It is present in developed and non-developed countries and affects peoples of all ages. People with obesity have an increased risk to develop several diseases which worsens significantly their prognosis[146,147]. Several studies have demonstrated alterations in VR markers in overweight or obese people which appear to increase risk for MVA in these cases. Mshui et al[148] evaluated maximum and minimum QTc in obese patients before and after therapeutic weight reduction. Both QTc values were significantly longer in obese patients than controls before weight reduction. With weight reduction these markers were reduced significantly in the obese group. The authors concluded that obesity is a cause of prolongation of this marker and that it may be modified by weight reduction. Positive effects on reduction in QTc values with a low calorie diet followed by a weight reduction were observed in a previous study[149] supporting the benefits of weight loss to improve the QTc. Continuing on this topic, Seyfeli et al[150] studied both QTc and QTd in obese women and controls with m (B) index = 40 ± 3 kg/m2vs 22 ± 1 kg/m2, P < 0.001 respectively. A significant correlation was found with m (B) index, maximum QTc and QTd (r = 0.410, P < 0.001 and r = 0.429, P < 0.001 respectively). Another work, found similar outcomes, but failed to find a statistically significant association among uncomplicated obesity and overweight with QTd, Tp-e, Tp-ed and Tp-e/QT[151].
Minimal myocardial dysfunction may be detected in obese patients even in the absence of any apparent symptoms[152]. These primary heart changes may increase the cardiac heterogeneity and could explain prolongation of these markers observed in uncomplicated obesity. For other hand, some conditions such as hyperinsulinemia, glucose intolerance and autonomic dysfunction may affect the values of these markers increasing the risk of ventricular arrhythmias[150]. Heterogeneity of samples involved in these studies and possible association of obesity/overweight with other comorbidities are factors which may modify and explain these results. These investigations show that QTc appears to be the most useful marker in these patients, but to achieve definite conclusions further studies should be performed.
SCD has been observed with higher incidence in athletes than the general population[153]. It is the main cause of death during exercise. MVA may play an important role in these cases. Several studies have examined the arrhythmogenic risk in these patients. QTc has been found more prolonged in athletes than non-athletes. It was observed by Lengyel et al[154]. They studied 76 professional soccer players and 76 controls with age mean 22.0 ± 0.61 and 22.0 ± 0.54 respectively. The maximum QTc by Fridericia and Hodges formulas were significantly longer in athletes than controls. Lawan et al[155] studied variations of QTd in 100 dynamic athletes, 50 static athletes and 100-matched controls. Results showed that both groups of athletes had an increased prolongation of QTd and that it significantly related to duration of physical activity. In other investigations it was found that maximum QTc, QTd and Tp-e were more prolonged inelite female water polo players than controls[156].
Currently the results derived from application of these markers to predict malignant cardiac arrhythmias in elite athletes are inconsistent. Maximum QTc has demonstrated to be shorter in professional soccer player than controls 413.9 ms vs 445.3 ms, P < 0.001 respectively)[157]. Similar results have been obtained by other researchers[158-160]. There are some points which should be analyzed in future studies and could represent possible explanations to contradictory outcomes after application of these markers in several setting of high trained physical activity: (1) Lack of identification of associated comorbidities; (2) Cardiac modifications presented in these cases (e.g., left ventricular hypertrophy; (3) Exercise type depending on each sport characteristics; (4) Duration and intensity of physical activity; and (5) Recovery after highly trained activity.
These elements may act alone or combined to explain why VR markers are prolonged in athletes in some studies but not in others. The development of left ventricular hypertrophy has been widely studied and commonly associated with no prolongation of these parameters. In this case it could be a shield factor against the appearance of malignant cardiac arrhythmias and for that these markers are reduced compared with non-athletes. It has been observed that patients with left ventricular hypertrophy induced by physical training activity have shorter values of QTd than those with pathological left ventricular hypertrophy by hypertension[159].
As was discussed above, SCD occurs with increased incidence in athletes. Many of these cases are secondary to conditions which predispose to malignant cardiac arrhythmias. For that reason and due to the usefulness of these markers in other clinical conditions there should be continued investigation in this field. Other more expensive tests could be avoided in these patients if ECG through the analysis of these markers demonstrates to be effective in these cases.
The prevalence of malignant cardiac arrhythmias and their association with SCD is relatively frequent. They may occur in healthy individuals without structural cardiac abnormalities or be found in other medical conditions. There is the potential to predict their development and subsequently design strategies to avoid adverse outcomes in our patients through the analysis and interpretation of VR markers. So far, the predictive capacities of VR markers have demonstrated to be useful in some clinical situations but not completely in others. Some of these predictors should be explored further, such as Tp-ed and Tp-e/QT which are relatively new in clinical practice. In order to increase their value, they need to be studied in healthy patients to know normal values of markers by sex, age and maybe among races. For achieve this aim it will be important to design studies with long-term follow up and many patients. However, this approach will be difficult in some conditions which have low incidence in the general population such as long and short QT syndromes and Brugada syndrome. A way to increase the sensitivity of these markers could be to combine several of them during studies in order to reduce the margin of error.
There are other medical conditions not discussed in this manuscript in which markers have demonstrated positive results[161-177] such as Kawasaki disease, systemic lupus eythematosus, rheumatoid arthritis, chronic renal failure, scleroderma, Duchenne muscular dystrophy, liver transplantation, HIV-infected patients, rheumatic fever and Chagas disease among others (Table 1). The interest in these ventricular repolarization markers has grown and has been introduced novel predictors with good results[120,178-181]. The QT variability index has been associated with SCD. A study by Piccirillo et al[120] demonstrated that patients with chronic heart failure with left ventricular ejection fraction ≤ 35% and SCD have higher values of QT variability index than in the group of left ventricular ejection fraction > 35%. The QT/RR and Tp-e/RR slopes have demonstrated to be useful to show loss of rate-dependent property in patients with Brugada syndrome with history of ventricular fibrillation[178]. Another marker commonly studied in patients with ventricular tachycardia is JT interval and its correction by heart rate. A study population of 20 patients with idiopathic ventricular tachycardia and 30 controls demonstrated that JT interval and its correction by heart rate were higher in patients with previous history of malignant arrhythmias JT (272 ± 36 ms vs 265 ± 25 ms, P = 0.01), JTc (336 ± 28 ms vs 318 ± 18 ms, P=0.01)[179]. T wave alternant have been proposed has a marker for heart heterogeneity and lead to electrical instability. These phenomenon have often found in patients with long QT syndrome that develop MVA[180]. Also, it has been assessed the value of T wave alternant in predicting SCD in patients after an acute myocardial infarction[181].
Currently there is a growing evidence to support the use of these markers to evaluate the risk of ventricular arrhythmias. However, it should be necessary to discuss some possible limitations on their use: (1) The manual determination of T wave offset is unreliable, principally when there is a low T wave amplitude and merges of T waves with U and/or P waves. In order to reduce the margin of error it has been developed automatic methods[5]. A precise calculation of some markers as Tp-e could be difficult in clinical practice and a computer method may represent a better choice; (2) For an accurate assessment of QTd is necessary all 12 leads of the ECG to be recorded simultaneously in order to avoid the effect of QT dynamicity[5]. However, not always is possible to achieve this aim due to mainly to technological deficit in non-developed countries; and (3) Although the utility of these markers for predicting malignant arrhythmias has been recognized, there are some conditions such as long and short QT syndromes, Brugada syndrome and early repolarization pattern when the patients’ risk stratification must be evaluated taking into account another variables such as gender, age, family history and gene mutations.
These markers extend our alternative for arrhythmias’ prediction. It is necessary to continue medical studies in this field with the aim to clarify some aspects discussed above and to achieve a global consensus which would result in better management of our patients.
The QTc, QTd, Tp-e, Tp-ed and Tp-e/QT have been found to be useful in predicting malignant cardiac arrhythmias in multiple medical conditions, but further long-term studies are needed. The aim should be to include them as tools to evaluate arrhythmogenic risk and as a way to improve clinical management of patients.
| 1. | Zipes DP, Wellens HJ. Sudden cardiac death. Circulation. 1998;98:2334-2351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1260] [Cited by in RCA: 1212] [Article Influence: 43.3] [Reference Citation Analysis (0)] |
| 2. | Israel CW. Mechanisms of sudden cardiac death. Indian Heart J. 2014;66 Suppl 1:S10-S17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 38] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 3. | Brugada R. La muerte súbita en el corazón sano. Rev Esp Cardiol. 2010;10:78A-84A. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 4. | Elming H, Holm E, Jun L, Torp-Pedersen C, Køber L, Kircshoff M, Malik M, Camm J. The prognostic value of the QT interval and QT interval dispersion in all-cause and cardiac mortality and morbidity in a population of Danish citizens. Eur Heart J. 1998;19:1391-1400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 222] [Cited by in RCA: 225] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 5. | Malik M, Batchvarov VN. Measurement, interpretation and clinical potential of QT dispersion. J Am Coll Cardiol. 2000;36:1749-1766. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 394] [Cited by in RCA: 415] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 6. | Wang JF, Shan QJ, Yang B, Chen ML, Zou JG, Chen C, Xu DJ, Cao KJ. [Tpeak-Tend interval and risk of cardiac events in patients with Brugada syndrome]. Zhonghua Xinxueguanbing Zazhi. 2007;35:629-632. [PubMed] |
| 7. | Castro Hevia J, Antzelevitch C, Tornés Bárzaga F, Dorantes Sánchez M, Dorticós Balea F, Zayas Molina R, Quiñones Pérez MA, Fayad Rodríguez Y. Tpeak-Tend and Tpeak-Tend dispersion as risk factors for ventricular tachycardia/ventricular fibrillation in patients with the Brugada syndrome. J Am Coll Cardiol. 2006;47:1828-1834. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 373] [Cited by in RCA: 383] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 8. | Castro-Torres Y. Tpeak-Tend/QT: un nuevo predictor electrocardiográfico de muerte súbita cardíaca. Cardiocore. 2014;49:86-87. [RCA] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 9. | Cobos Gil MA, García Rubira JC. Who was the creator of Bazett’s formula? Rev Esp Cardiol. 2008;61:896-897. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 10. | Chávez González E. El intervalo QT, su origen e importancia del conocimiento de fórmulas para su medición en diferentes circunstancias clínicas. Cor Salud. 2014;6:79-85. |
| 11. | Rautaharju PM, Surawicz B, Gettes LS, Bailey JJ, Childers R, Deal BJ, Gorgels A, Hancock EW, Josephson M, Kligfield P. AHA/ACCF/HRS recommendations for the standardization and interpretation of the electrocardiogram: part IV: the ST segment, T and U waves, and the QT interval: a scientific statement from the American Heart Association Electrocardiography and Arrhythmias Committee, Council on Clinical Cardiology; the American College of Cardiology Foundation; and the Heart Rhythm Society. Endorsed by the International Society for Computerized Electrocardiology. J Am Coll Cardiol. 2009;53:982-991. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 532] [Cited by in RCA: 625] [Article Influence: 36.8] [Reference Citation Analysis (0)] |
| 13. | Macfarlane PW. Measurement of QT dispersion. Heart. 1998;80:421-423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 14. | Antzelevitch C, Shimizu W, Yan GX, Sicouri S. Cellular basis for QT dispersion. J Electrocardiol. 1998;30 Suppl:168-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 171] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 15. | Day CP, McComb JM, Campbell RW. QT dispersion: an indication of arrhythmia risk in patients with long QT intervals. Br Heart J. 1990;63:342-344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 793] [Cited by in RCA: 792] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 16. | Okin PM, Devereux RB, Howard BV, Fabsitz RR, Lee ET, Welty TK. Assessment of QT interval and QT dispersion for prediction of all-cause and cardiovascular mortality in American Indians: The Strong Heart Study. Circulation. 2000;101:61-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 260] [Cited by in RCA: 272] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 17. | Kors JA, Ritsema van Eck HJ, van Herpen G. The meaning of the Tp-Te interval and its diagnostic value. J Electrocardiol. 2008;41:575-580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 202] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 18. | Antzelevitch C, Shimizu W, Yan GX, Sicouri S, Weissenburger J, Nesterenko VV, Burashnikov A, Di Diego J, Saffitz J, Thomas GP. The M cell: its contribution to the ECG and to normal and abnormal electrical function of the heart. J Cardiovasc Electrophysiol. 1999;10:1124-1152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 376] [Cited by in RCA: 333] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 19. | Xia Y, Liang Y, Kongstad O, Holm M, Olsson B, Yuan S. Tpeak-Tend interval as an index of global dispersion of ventricular repolarization: evaluations using monophasic action potential mapping of the epi- and endocardium in swine. J Interv Card Electrophysiol. 2005;14:79-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 97] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 20. | Arteyeva NV, Goshka SL, Sedova KA, Bernikova OG, Azarov JE. What does the T(peak)-T(end) interval reflect? An experimental and model study. J Electrocardiol. 2013;46:296.e1-296.e8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 40] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 21. | Antzelevitch C, Oliva A. Amplification of spatial dispersion of repolarization underlies sudden cardiac death associated with catecholaminergic polymorphic VT, long QT, short QT and Brugada syndromes. J Intern Med. 2006;259:48-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 93] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 22. | Haarmark C, Graff C, Andersen MP, Hardahl T, Struijk JJ, Toft E, Xue J, Rowlandson GI, Hansen PR, Kanters JK. Reference values of electrocardiogram repolarization variables in a healthy population. J Electrocardiol. 2010;43:31-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 46] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 23. | Antzelevitch C, Shimizu W. Cellular mechanisms underlying the long QT syndrome. Curr Opin Cardiol. 2002;17:43-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 182] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 24. | Gupta P, Patel C, Patel H, Narayanaswamy S, Malhotra B, Green JT, Yan GX. T(p-e)/QT ratio as an index of arrhythmogenesis. J Electrocardiol. 2008;41:567-574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 348] [Cited by in RCA: 424] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 25. | Mirvis DM. Spatial variation of QT intervals in normal persons and patients with acute myocardial infarction. J Am Coll Cardiol. 1985;5:625-631. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 155] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 26. | Patel C, Burke JF, Patel H, Gupta P, Kowey PR, Antzelevitch C, Yan GX. Is there a significant transmural gradient in repolarization time in the intact heart? Cellular basis of the T wave: a century of controversy. Circ Arrhythm Electrophysiol. 2009;2:80-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 72] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 27. | Anyukhovsky EP, Sosunov EA, Rosen MR. Regional differences in electrophysiological properties of epicardium, midmyocardium, and endocardium. In vitro and in vivo correlations. Circulation. 1996;94:1981-1988. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 135] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 28. | Anyukhovsky EP, Sosunov EA, Gainullin RZ, Rosen MR. The controversial M cell. J Cardiovasc Electrophysiol. 1999;10:244-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 88] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 29. | Yan GX, Antzelevitch C. Cellular basis for the normal T wave and the electrocardiographic manifestations of the long-QT syndrome. Circulation. 1998;98:1928-1936. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 698] [Cited by in RCA: 661] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 30. | Antzelevitch C, Sicouri S, Di Diego JM, Burashnikov A, Viskin S, Shimizu W, Yan GX, Kowey P, Zhang L. Does Tpeak-Tend provide an index of transmural dispersion of repolarization? Heart Rhythm. 2007;4:1114-1116; author reply 1116-1119. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 213] [Cited by in RCA: 218] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 31. | Kaufman ES. Arrhythmic risk in congenital long QT syndrome. J Electrocardiol. 2011;44:645-649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 32. | Bhuiyan ZA, Al-Shahrani S, Al-Aama J, Wilde AA, Momenah TS. Congenital Long QT Syndrome: An Update and Present Perspective in Saudi Arabia. Front Pediatr. 2013;1:39. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 33. | Mahida S, Hogarth AJ, Cowan C, Tayebjee MH, Graham LN, Pepper CB. Genetics of congenital and drug-induced long QT syndromes: current evidence and future research perspectives. J Interv Card Electrophysiol. 2013;37:9-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 43] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 34. | Ayangade-Johnson G, Villafane J. Long QT interval resembling long QT syndrome in a newborn with electrolyte dysbalance. J Ky Med Assoc. 2001;99:285-287. [PubMed] |
| 35. | Carmona Puerta R, Rodríguez Álvarez JM, Castro Torres Y. Desarrollo progresivo de onda J gigante y prolongación extrema del intervalo QT en la hipotermia inducida. Cor Salud. 2013;5:308-310. |
| 36. | Hill GE, Ogunnaike B, Nasir D. Patients presenting with acute toxin ingestion. Anesthesiol Clin. 2010;28:117-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 37. | Collier BR, Miller SL, Kramer GS, Balon JA, Gonzalez LS. Traumatic subarachnoid hemorrhage and QTc prolongation. J Neurosurg Anesthesiol. 2004;16:196-200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 17] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 38. | Sauer AJ, Moss AJ, McNitt S, Peterson DR, Zareba W, Robinson JL, Qi M, Goldenberg I, Hobbs JB, Ackerman MJ. Long QT syndrome in adults. J Am Coll Cardiol. 2007;49:329-337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 299] [Cited by in RCA: 272] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 39. | Hobbs JB, Peterson DR, Moss AJ, McNitt S, Zareba W, Goldenberg I, Qi M, Robinson JL, Sauer AJ, Ackerman MJ. Risk of aborted cardiac arrest or sudden cardiac death during adolescence in the long-QT syndrome. JAMA. 2006;296:1249-1254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 229] [Cited by in RCA: 208] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 40. | Mullally J, Goldenberg I, Moss AJ, Lopes CM, Ackerman MJ, Zareba W, McNitt S, Robinson JL, Benhorin J, Kaufman ES. Risk of life-threatening cardiac events among patients with long QT syndrome and multiple mutations. Heart Rhythm. 2013;10:378-382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 35] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 41. | Priori SG, Schwartz PJ, Napolitano C, Bloise R, Ronchetti E, Grillo M, Vicentini A, Spazzolini C, Nastoli J, Bottelli G. Risk stratification in the long-QT syndrome. N Engl J Med. 2003;348:1866-1874. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1045] [Cited by in RCA: 957] [Article Influence: 41.6] [Reference Citation Analysis (0)] |
| 42. | Priori SG, Napolitano C, Diehl L, Schwartz PJ. Dispersion of the QT interval. A marker of therapeutic efficacy in the idiopathic long QT syndrome. Circulation. 1994;89:1681-1689. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 259] [Cited by in RCA: 248] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 43. | Yamaguchi M, Shimizu M, Ino H, Terai H, Uchiyama K, Oe K, Mabuchi T, Konno T, Kaneda T, Mabuchi H. T wave peak-to-end interval and QT dispersion in acquired long QT syndrome: a new index for arrhythmogenicity. Clin Sci (Lond). 2003;105:671-676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 227] [Cited by in RCA: 222] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 44. | Lubinski A, Lewicka-Nowak E, Kempa M, Baczynska AM, Romanowska I, Swiatecka G. New insight into repolarization abnormalities in patients with congenital long QT syndrome: the increased transmural dispersion of repolarization. Pacing Clin Electrophysiol. 1998;21:172-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 101] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 45. | Takenaka K, Ai T, Shimizu W, Kobori A, Ninomiya T, Otani H, Kubota T, Takaki H, Kamakura S, Horie M. Exercise stress test amplifies genotype-phenotype correlation in the LQT1 and LQT2 forms of the long-QT syndrome. Circulation. 2003;107:838-844. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 177] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 46. | Bellocq C, van Ginneken AC, Bezzina CR, Alders M, Escande D, Mannens MM, Baró I, Wilde AA. Mutation in the KCNQ1 gene leading to the short QT-interval syndrome. Circulation. 2004;109:2394-2397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 472] [Cited by in RCA: 420] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 47. | Brugada R, Hong K, Dumaine R, Cordeiro J, Gaita F, Borggrefe M, Menendez TM, Brugada J, Pollevick GD, Wolpert C. Sudden death associated with short-QT syndrome linked to mutations in HERG. Circulation. 2004;109:30-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 601] [Cited by in RCA: 538] [Article Influence: 23.4] [Reference Citation Analysis (0)] |
| 48. | Priori SG, Pandit SV, Rivolta I, Berenfeld O, Ronchetti E, Dhamoon A, Napolitano C, Anumonwo J, di Barletta MR, Gudapakkam S. A novel form of short QT syndrome (SQT3) is caused by a mutation in the KCNJ2 gene. Circ Res. 2005;96:800-807. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 478] [Cited by in RCA: 421] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 49. | Gollob MH, Redpath CJ, Roberts JD. The short QT syndrome: proposed diagnostic criteria. J Am Coll Cardiol. 2011;57:802-812. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 220] [Cited by in RCA: 209] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 50. | Iribarren C, Round AD, Peng JA, Lu M, Klatsky AL, Zaroff JG, Holve TJ, Prasad A, Stang P. Short QT in a cohort of 1.7 million persons: prevalence, correlates, and prognosis. Ann Noninvasive Electrocardiol. 2014;19:490-500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 31] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 51. | Giudicessi JR, Ackerman MJ. Determinants of incomplete penetrance and variable expressivity in heritable cardiac arrhythmia syndromes. Transl Res. 2013;161:1-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 139] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 52. | Rautaharju PM, Zhou SH, Wong S, Calhoun HP, Berenson GS, Prineas R, Davignon A. Sex differences in the evolution of the electrocardiographic QT interval with age. Can J Cardiol. 1992;8:690-695. [PubMed] |
| 53. | Bjerregaard P, Collier JL, Gussak I. Upper limits of QT/QTc intervals in the short QT syndrome. Review of the world-wide short QT syndrome population and 3 new USA families. Heart Rhythm. 2008;5:AB43. |
| 54. | Brugada J, Gussak I, Brugada P. Short QT syndrome: a predictable story. Cardiology. 2014;128:231-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 20] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 55. | Anttonen O, Junttila MJ, Maury P, Schimpf R, Wolpert C, Borggrefe M, Giustetto C, Gaita F, Sacher F, Haïssaguerre M. Differences in twelve-lead electrocardiogram between symptomatic and asymptomatic subjects with short QT interval. Heart Rhythm. 2009;6:267-271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 29] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 56. | Anttonen O, Väänänen H, Junttila J, Huikuri HV, Viitasalo M. Electrocardiographic transmural dispersion of repolarization in patients with inherited short QT syndrome. Ann Noninvasive Electrocardiol. 2008;13:295-300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 31] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 57. | Extramiana F, Antzelevitch C. Amplified transmural dispersion of repolarization as the basis for arrhythmogenesis in a canine ventricular-wedge model of short-QT syndrome. Circulation. 2004;110:3661-3666. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 142] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 58. | Brugada P, Brugada J. Right bundle branch block, persistent ST segment elevation and sudden cardiac death: a distinct clinical and electrocardiographic syndrome. A multicenter report. J Am Coll Cardiol. 1992;20:1391-1396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2298] [Cited by in RCA: 2163] [Article Influence: 63.6] [Reference Citation Analysis (0)] |
| 59. | Bayés de Luna A, Brugada J, Baranchuk A, Borggrefe M, Breithardt G, Goldwasser D, Lambiase P, Riera AP, Garcia-Niebla J, Pastore C. Current electrocardiographic criteria for diagnosis of Brugada pattern: a consensus report. J Electrocardiol. 2012;45:433-442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 293] [Cited by in RCA: 262] [Article Influence: 18.7] [Reference Citation Analysis (1)] |
| 60. | Antzelevitch C, Brugada P, Borggrefe M, Brugada J, Brugada R, Corrado D, Gussak I, LeMarec H, Nademanee K, Perez Riera AR. Brugada syndrome: report of the second consensus conference: endorsed by the Heart Rhythm Society and the European Heart Rhythm Association. Circulation. 2005;111:659-670. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1288] [Cited by in RCA: 1205] [Article Influence: 57.4] [Reference Citation Analysis (0)] |
| 61. | Brugada J, Brugada R, Brugada P. Right bundle-branch block and ST-segment elevation in leads V1 through V3: a marker for sudden death in patients without demonstrable structural heart disease. Circulation. 1998;97:457-460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 454] [Cited by in RCA: 404] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 62. | Kapplinger JD, Tester DJ, Alders M, Benito B, Berthet M, Brugada J, Brugada P, Fressart V, Guerchicoff A, Harris-Kerr C. An international compendium of mutations in the SCN5A-encoded cardiac sodium channel in patients referred for Brugada syndrome genetic testing. Heart Rhythm. 2010;7:33-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 509] [Cited by in RCA: 577] [Article Influence: 33.9] [Reference Citation Analysis (0)] |
| 63. | Postema PG. About Brugada syndrome and its prevalence. Europace. 2012;14:925-928. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 49] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 64. | Giustetto C, Drago S, Demarchi PG, Dalmasso P, Bianchi F, Masi AS, Carvalho P, Occhetta E, Rossetti G, Riccardi R. Risk stratification of the patients with Brugada type electrocardiogram: a community-based prospective study. Europace. 2009;11:507-513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 89] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 65. | Benito B, Brugada J, Brugada R, Brugada P. Brugada syndrome. Rev Esp Cardiol. 2009;62:1297-1315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 71] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 66. | Probst V, Veltmann C, Eckardt L, Meregalli PG, Gaita F, Tan HL, Babuty D, Sacher F, Giustetto C, Schulze-Bahr E. Long-term prognosis of patients diagnosed with Brugada syndrome: Results from the FINGER Brugada Syndrome Registry. Circulation. 2010;121:635-643. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 595] [Cited by in RCA: 629] [Article Influence: 39.3] [Reference Citation Analysis (0)] |
| 67. | Nademanee K, Veerakul G, Chandanamattha P, Chaothawee L, Ariyachaipanich A, Jirasirirojanakorn K, Likittanasombat K, Bhuripanyo K, Ngarmukos T. Prevention of ventricular fibrillation episodes in Brugada syndrome by catheter ablation over the anterior right ventricular outflow tract epicardium. Circulation. 2011;123:1270-1279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 532] [Cited by in RCA: 565] [Article Influence: 37.7] [Reference Citation Analysis (0)] |
| 68. | Eckardt L, Probst V, Smits JP, Bahr ES, Wolpert C, Schimpf R, Wichter T, Boisseau P, Heinecke A, Breithardt G. Long-term prognosis of individuals with right precordial ST-segment-elevation Brugada syndrome. Circulation. 2005;111:257-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 317] [Cited by in RCA: 284] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 69. | Pitzalis MV, Anaclerio M, Iacoviello M, Forleo C, Guida P, Troccoli R, Massari F, Mastropasqua F, Sorrentino S, Manghisi A. QT-interval prolongation in right precordial leads: an additional electrocardiographic hallmark of Brugada syndrome. J Am Coll Cardiol. 2003;42:1632-1637. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 57] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 70. | Letsas KP, Weber R, Astheimer K, Kalusche D, Arentz T. Tpeak-Tend interval and Tpeak-Tend/QT ratio as markers of ventricular tachycardia inducibility in subjects with Brugada ECG phenotype. Europace. 2010;12:271-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 90] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 71. | Yan GX, Antzelevitch C. Cellular basis for the Brugada syndrome and other mechanisms of arrhythmogenesis associated with ST-segment elevation. Circulation. 1999;100:1660-1666. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 770] [Cited by in RCA: 728] [Article Influence: 27.0] [Reference Citation Analysis (0)] |
| 72. | Bébarová M. Arrhythmogenesis in Brugada syndrome: impact and constrains of current concepts. Int J Cardiol. 2013;167:1760-1771. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 73. | Jellins J, Milanovic M, Taitz DJ, Wan SH, Yam PW. Brugada syndrome. Hong Kong Med J. 2013;19:159-167. [PubMed] |
| 74. | Bloch Thomsen PE, Joergensen RM, Kanters JK, Jensen TJ, Haarbo J, Hagemann A, Vestergaard A, Saermark K. Phase 2 reentry in man. Heart Rhythm. 2005;2:797-803. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 23] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 75. | Meregalli PG, Wilde AA, Tan HL. Pathophysiological mechanisms of Brugada syndrome: depolarization disorder, repolarization disorder, or more? Cardiovasc Res. 2005;67:367-378. [PubMed] |
| 76. | Adhikarla C, Boga M, Wood AD, Froelicher VF. Natural history of the electrocardiographic pattern of early repolarization in ambulatory patients. Am J Cardiol. 2011;108:1831-1835. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 77. | Antzelevitch C, Yan GX. J-wave syndromes. from cell to bedside. J Electrocardiol. 2011;44:656-661. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 33] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 78. | Littmann L, Tenczer J. Widening spectrum of the J-wave syndromes. J Electrocardiol. 2012;45:23-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 79. | Rosso R, Glikson E, Belhassen B, Katz A, Halkin A, Steinvil A, Viskin S. Distinguishing “benign” from “malignant early repolarization”: the value of the ST-segment morphology. Heart Rhythm. 2012;9:225-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 161] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 80. | Corrado D, Biffi A, Basso C, Pelliccia A, Thiene G. 12-lead ECG in the athlete: physiological versus pathological abnormalities. Br J Sports Med. 2009;43:669-676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 63] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 81. | Myers GB, Klein HA. Normal variations in multiple precordial leads. Am Heart J. 1947;34:785-808. [PubMed] |
| 82. | Klatsky AL, Oehm R, Cooper RA, Udaltsova N, Armstrong MA. The early repolarization normal variant electrocardiogram: correlates and consequences. Am J Med. 2003;115:171-177. [PubMed] |
| 83. | Haïssaguerre M, Derval N, Sacher F, Jesel L, Deisenhofer I, de Roy L, Pasquié JL, Nogami A, Babuty D, Yli-Mayry S. Sudden cardiac arrest associated with early repolarization. N Engl J Med. 2008;358:2016-2023. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1100] [Cited by in RCA: 1002] [Article Influence: 55.7] [Reference Citation Analysis (0)] |
| 84. | Nam GB, Kim YH, Antzelevitch C. Augmentation of J waves and electrical storms in patients with early repolarization. N Engl J Med. 2008;358:2078-2079. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 248] [Cited by in RCA: 214] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 85. | Rosso R, Kogan E, Belhassen B, Rozovski U, Scheinman MM, Zeltser D, Halkin A, Steinvil A, Heller K, Glikson M. J-point elevation in survivors of primary ventricular fibrillation and matched control subjects: incidence and clinical significance. J Am Coll Cardiol. 2008;52:1231-1238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 456] [Cited by in RCA: 423] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 86. | Rezus C, Floria M, Moga VD, Sirbu O, Dima N, Ionescu SD, Ambarus V. Early repolarization syndrome: electrocardiographic signs and clinical implications. Ann Noninvasive Electrocardiol. 2014;19:15-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 87. | Antzelevitch C, Yan GX. J wave syndromes. Heart Rhythm. 2010;7:549-558. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 496] [Cited by in RCA: 395] [Article Influence: 24.7] [Reference Citation Analysis (0)] |
| 88. | Letsas KP, Charalampous C, Korantzopoulos P, Tsikrikas S, Bramos D, Kollias G, Efremidis M, Sideris A. Novel indexes of heterogeneity of ventricular repolarization in subjects with early repolarization pattern. Europace. 2012;14:877-881. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 1.8] [Reference Citation Analysis (1)] |
| 89. | Tikkanen JT, Anttonen O, Junttila MJ, Aro AL, Kerola T, Rissanen HA, Reunanen A, Huikuri HV. Long-term outcome associated with early repolarization on electrocardiography. N Engl J Med. 2009;361:2529-2537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 641] [Cited by in RCA: 588] [Article Influence: 34.6] [Reference Citation Analysis (1)] |
| 90. | Shu J, Zhu T, Yang L, Cui C, Yan GX. ST-segment elevation in the early repolarization syndrome, idiopathic ventricular fibrillation, and the Brugada syndrome: cellular and clinical linkage. J Electrocardiol. 2005;38:26-32. [PubMed] |
| 91. | Ghosh S, Cooper DH, Vijayakumar R, Zhang J, Pollak S, Haïssaguerre M, Rudy Y. Early repolarization associated with sudden death: insights from noninvasive electrocardiographic imaging. Heart Rhythm. 2010;7:534-537. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 55] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 92. | Orozco-Beltran D, Cooper RS, Gil-Guillen V, Bertomeu-Martinez V, Pita-Fernandez S, Durazo-Arvizu R, Carratala-Munuera C, Cea-Calvo L, Bertomeu-Gonzalez V, Seoane-Pillado T. Trends in mortality from myocardial infarction. A comparative study between Spain and the United States: 1990-2006. Rev Esp Cardiol (Engl Ed). 2012;65:1079-1085. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 93. | Bayés de Luna A, Elosua R. Sudden death. Rev Esp Cardiol (Engl Ed). 2012;65:1039-1052. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 36] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 94. | O’Gara PT, Kushner FG, Ascheim DD, Casey DE, Chung MK, de Lemos JA, Ettinger SM, Fang JC, Fesmire FM, Franklin BA. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;61:e78-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1953] [Cited by in RCA: 2319] [Article Influence: 165.6] [Reference Citation Analysis (0)] |
| 95. | Schwartz PJ, Wolf S. QT interval prolongation as predictor of sudden death in patients with myocardial infarction. Circulation. 1978;57:1074-1077. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 564] [Cited by in RCA: 534] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 96. | Yi G, Crook R, Guo XH, Staunton A, Camm AJ, Malik M. Exercise-induced changes in the QT interval duration and dispersion in patients with sudden cardiac death after myocardial infarction. Int J Cardiol. 1998;63:271-279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 97. | Gadaleta FL, Llois SC, Sinisi VA, Quiles J, Avanzas P, Kaski JC. [Corrected QT interval prolongation: a new predictor of cardiovascular risk in patients with non-ST-elevation acute coronary syndrome]. Rev Esp Cardiol. 2008;61:572-578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 98. | Karwatowska-Prokopczuk E, Wang W, Cheng ML, Zeng D, Schwartz PJ, Belardinelli L. The risk of sudden cardiac death in patients with non-ST elevation acute coronary syndrome and prolonged QTc interval: effect of ranolazine. Europace. 2013;15:429-436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 99. | Ciolli A, Di Lorenzo M, Bevilacqua U, Lo Sardo G, Tripi M, Fidati R, Palamara A. QT dispersion and early arrhythmic risk during acute myocardial infarction. G Ital Cardiol. 1999;29:1438-1444. [PubMed] |
| 100. | Mulay DV, Quadri SM. QT dispersion and early arrhythmic risk in acute myocardial infarction. Indian Heart J. 2004;56:636-641. [PubMed] |
| 101. | Hetland M, Haugaa KH, Sarvari SI, Erikssen G, Kongsgaard E, Edvardsen T. A novel ECG-index for prediction of ventricular arrhythmias in patients after myocardial infarction. Ann Noninvasive Electrocardiol. 2014;19:330-337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 37] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 102. | Shawl FA, Velasco CE, Goldbaum TS, Forman MB. Effect of coronary angioplasty on electrocardiographic changes in patients with unstable angina secondary to left anterior descending coronary artery disease. J Am Coll Cardiol. 1990;16:325-331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 25] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 103. | Hale SL, Kloner RA. Elevated body temperature during myocardial ischemia/reperfusion exacerbates necrosis and worsens no-reflow. Coron Artery Dis. 2002;13:177-181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 104. | Bethell HW, Vandenberg JI, Smith GA, Grace AA. Changes in ventricular repolarization during acidosis and low-flow ischemia. Am J Physiol. 1998;275:H551-H561. [PubMed] |
| 105. | Shivkumar K, Deutsch NA, Lamp ST, Khuu K, Goldhaber JI, Weiss JN. Mechanism of hypoxic K loss in rabbit ventricle. J Clin Invest. 1997;100:1782-1788. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 45] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 106. | Steg PG, James SK, Atar D, Badano LP, Blömstrom-Lundqvist C, Borger MA, Di Mario C, Dickstein K, Ducrocq G, Fernandez-Aviles F. ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation. Eur Heart J. 2012;33:2569-2619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3540] [Cited by in RCA: 3736] [Article Influence: 266.9] [Reference Citation Analysis (0)] |
| 107. | Wijns W, Kolh P, Danchin N, Di Mario C, Falk V, Folliguet T, Garg S, Huber K, James S, Knuuti J. Guidelines on myocardial revascularization. Eur Heart J. 2010;31:2501-2555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1660] [Cited by in RCA: 1726] [Article Influence: 107.9] [Reference Citation Analysis (0)] |
| 108. | Xiao WT, Wang XP, Gao CY, Yan JJ, Li MW, Zhang Y, Liu JJ. [Predictive value of corrected QT interval, corrected Tp-e interval and Tp-e/QT ratio on malignant arrhythmia events in acute ST-segment elevation myocardial infarction patients undergoing thrombolysis]. Zhonghua Xinxueguanbing Zazhi. 2012;40:473-476. [PubMed] |
| 109. | Ornek E, Duran M, Ornek D, Demirçelik BM, Murat S, Kurtul A, Çiçekçioğlu H, Çetin M, Kahveci K, Doger C. The effect of thrombolytic therapy on QT dispersion in acute myocardial infarction and its role in the prediction of reperfusion arrhythmias. Niger J Clin Pract. 2014;17:183-187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 110. | Moreno FL, Villanueva T, Karagounis LA, Anderson JL. Reduction in QT interval dispersion by successful thrombolytic therapy in acute myocardial infarction. TEAM-2 Study Investigators. Circulation. 1994;90:94-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 156] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 111. | Fukushima N, Tsurumi Y, Jujo K, Fukushima K, Sekiguchi H, Honda A, Yumino D, Kawana M, Hagiwara N. Impact of myocardial reperfusion status on QT dispersion after successful recanalization of the infarct-related artery in acute myocardial infarction. J Interv Cardiol. 2014;27:252-259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 112. | Haarmark C, Hansen PR, Vedel-Larsen E, Pedersen SH, Graff C, Andersen MP, Toft E, Wang F, Struijk JJ, Kanters JK. The prognostic value of the Tpeak-Tend interval in patients undergoing primary percutaneous coronary intervention for ST-segment elevation myocardial infarction. J Electrocardiol. 2009;42:555-560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 110] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 113. | Szydło K, Wita K, Trusz-Gluza M, Tabor Z. Late phase of repolarization (TpeakTend) as a prognostic marker of left ventricle remodeling in patients with anterior myocardial infarction treated with primary coronary intervention. Cardiol J. 2010;17:244-248. [PubMed] |
| 114. | Zhao X, Xie Z, Chu Y, Yang L, Xu W, Yang X, Liu X, Tian L. Association between Tp-e/QT ratio and prognosis in patients undergoing primary percutaneous coronary intervention for ST-segment elevation myocardial infarction. Clin Cardiol. 2012;35:559-564. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 105] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 115. | Castro Torres Y, Fleites Pérez A, Dawson M. The reperfusion ST-peak in acute myocardial infarction: a topic where the current knowledge is insufficient. Afr J Emerg Med. 2013;3:141. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 116. | Kobayashi N, Ohmura N, Nakada I, Yasu T, Iwanaka H, Kubo N, Katsuki T, Fujii M, Yaginuma T, Saito M. Further ST elevation at reperfusion by direct percutaneous transluminal coronary angioplasty predicts poor recovery of left ventricular systolic function in anterior wall AMI. Am J Cardiol. 1997;79:862-866. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 32] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 117. | Lønborg J, Kelbæk H, Holmvang L, Vejlstrup N, Jørgensen E, Helqvist S, Saunamäki K, Dridi NP, Ahtarovski KA, Terkelsen CJ. ST peak during primary percutaneous coronary intervention predicts final infarct size, left ventricular function, and clinical outcome. J Electrocardiol. 2012;45:708-716. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 118. | McMurray JJ, Adamopoulos S, Anker SD, Auricchio A, Böhm M, Dickstein K, Falk V, Filippatos G, Fonseca C, Gomez-Sanchez MA. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2012;33:1787-1847. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3411] [Cited by in RCA: 3593] [Article Influence: 256.6] [Reference Citation Analysis (0)] |
| 119. | Davey PP, Barlow C, Hart G. Prolongation of the QT interval in heart failure occurs at low but not at high heart rates. Clin Sci (Lond). 2000;98:603-610. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 0.4] [Reference Citation Analysis (1)] |
| 120. | Piccirillo G, Rossi P, Mitra M, Quaglione R, Dell’Armi A, Di Barba D, Maisto D, Lizio A, Barillà F, Magrì D. Indexes of temporal myocardial repolarization dispersion and sudden cardiac death in heart failure: any difference? Ann Noninvasive Electrocardiol. 2013;18:130-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 29] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 121. | Morin DP, Saad MN, Shams OF, Owen JS, Xue JQ, Abi-Samra FM, Khatib S, Nelson-Twakor OS, Milani RV. Relationships between the T-peak to T-end interval, ventricular tachyarrhythmia, and death in left ventricular systolic dysfunction. Europace. 2012;14:1172-1179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 50] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 122. | Mancia G, Fagard R, Narkiewicz K, Redon J, Zanchetti A, Böhm M, Christiaens T, Cifkova R, De Backer G, Dominiczak A. 2013 ESH/ESC Guidelines for the management of hypertension: the Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Eur Heart J. 2013;34:2159-2219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3156] [Cited by in RCA: 3284] [Article Influence: 252.6] [Reference Citation Analysis (0)] |
| 123. | Arenal Maíz A, Castel MA, López Gil M, Merino Llorens JL. [Update on arrhythmias and cardiac electrophysiology]. Rev Esp Cardiol. 2009;62 Suppl 1:67-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 124. | Katholi RE, Couri DM. Left ventricular hypertrophy: major risk factor in patients with hypertension: update and practical clinical applications. Int J Hypertens. 2011;2011:495349. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 111] [Cited by in RCA: 130] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 125. | Zhao Z, Yuan Z, Ji Y, Wu Y, Qi Y. Left ventricular hypertrophy amplifies the QT, and Tp-e intervals and the Tp-e/ QT ratio of left chest ECG. J Biomed Res. 2010;24:69-72. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 126. | Carmona Puerta R, León Aliz E, Morales Salinas A. Vulnerabilidad arrítmica incrementada y su relación con la hipertensión arterial. Relampa. 2011;24:96-105. |
| 127. | Mozos I, Serban C. The relation between QT interval and T-wave variables in hypertensive patients. J Pharm Bioallied Sci. 2011;3:339-344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 25] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 128. | Salles GF, Cardoso CR, Leocadio SM, Muxfeldt ES. Recent ventricular repolarization markers in resistant hypertension: are they different from the traditional QT interval? Am J Hypertens. 2008;21:47-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 129. | Calhoun DA, Jones D, Textor S, Goff DC, Murphy TP, Toto RD, White A, Cushman WC, White W, Sica D. Resistant hypertension: diagnosis, evaluation, and treatment. A scientific statement from the American Heart Association Professional Education Committee of the Council for High Blood Pressure Research. Hypertension. 2008;51:1403-1419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1103] [Cited by in RCA: 1081] [Article Influence: 60.1] [Reference Citation Analysis (0)] |
| 130. | Morales Salinas A, León Aliz E, Carmona Puerta R, Villanueva Ramos Y, Poveda Rodríguez R, López Machado R, Vázquez Subit C, Castro Torres Y. Riesgo cardiovascular y marcadores electrocardiográficos de arritmias en pacientes hipertensos sin cardiopatía isquémica. Rev Fed Arg Cardiol. 2013;42:189-194. |
| 131. | De Ambroggi L, Francia P, De Ambroggi G. Repolarization abnormalities and arrhythmogenesis in hypertrophic myocardium. Anadolu Kardiyol Derg. 2007;7 Suppl 1:71-72. [PubMed] |
| 132. | Hart G. Cellular electrophysiology in cardiac hypertrophy and failure. Cardiovasc Res. 1994;28:933-946. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 126] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 133. | Ohtsuka S, Kakihana M, Watanabe H, Enomoto T, Ajisaka R, Sugishita Y. Alterations in left ventricular wall stress and coronary circulation in patients with isolated systolic hypertension. J Hypertens. 1996;14:1349-1355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 21] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 134. | Gryglewska B, Grodzicki T, Czarnecka D, Kawecka-Jaszcz K, Kocemba J. QT dispersion and hypertensive heart disease in the elderly. J Hypertens. 2000;18:461-464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 19] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 135. | Müller-Brunotte R, Kahan T, López B, Edner M, González A, Díez J, Malmqvist K. Myocardial fibrosis and diastolic dysfunction in patients with hypertension: results from the Swedish Irbesartan Left Ventricular Hypertrophy Investigation versus Atenolol (SILVHIA). J Hypertens. 2007;25:1958-1966. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 79] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 136. | Barron HV, Lesh MD. Autonomic nervous system and sudden cardiac death. J Am Coll Cardiol. 1996;27:1053-1060. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 156] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 137. | Tribulova N, Okruhlicova L, Novakova S, Pancza D, Bernatova I, Pechanova O, Weismann P, Manoach M, Seki S, Mochizuki S. Hypertension-related intermyocyte junction remodelling is associated with a higher incidence of low-K(+)-induced lethal arrhythmias in isolated rat heart. Exp Physiol. 2002;87:195-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 138. | Rydén L, Grant PJ, Anker SD, Berne C, Cosentino F, Danchin N, Deaton C, Escaned J, Hammes HP, Huikuri H. ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD: the Task Force on diabetes, pre-diabetes, and cardiovascular diseases of the European Society of Cardiology (ESC) and developed in collaboration with the European Association for the Study of Diabetes (EASD). Eur Heart J. 2013;34:3035-3087. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1378] [Cited by in RCA: 1466] [Article Influence: 112.8] [Reference Citation Analysis (5)] |
| 139. | Balkau B, Jouven X, Ducimetière P, Eschwège E. Diabetes as a risk factor for sudden death. Lancet. 1999;354:1968-1969. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 98] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 140. | Jouven X, Lemaître RN, Rea TD, Sotoodehnia N, Empana JP, Siscovick DS. Diabetes, glucose level, and risk of sudden cardiac death. Eur Heart J. 2005;26:2142-2147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 201] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 141. | Aytemir K, Aksöyek S, Ozer N, Gürlek A, Oto A. QT dispersion and autonomic nervous system function in patients with type 1 diabetes. Int J Cardiol. 1998;65:45-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 28] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 142. | Clemente D, Pereira T, Ribeiro S. Ventricular repolarization in diabetic patients: characterization and clinical implications. Arq Bras Cardiol. 2012;99:1015-1022. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 18] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 143. | Arslan D, Guvenc O, Cimen D, Ulu H, Oran B. Prolonged QT dispersion in the infants of diabetic mothers. Pediatr Cardiol. 2014;35:1052-1056. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 144. | Siscovick DS, Sotoodehnia N, Rea TD, Raghunathan TE, Jouven X, Lemaitre RN. Type 2 diabetes mellitus and the risk of sudden cardiac arrest in the community. Rev Endocr Metab Disord. 2010;11:53-59. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 80] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 145. | Castro Torres Y, Fleites Pérez A, Prado Castro D. A recent evaluation of ventricular repolarization in diabetic patients. Arq Bras Cardiol. 2013;100:387. [PubMed] |
| 146. | Lau DC, Douketis JD, Morrison KM, Hramiak IM, Sharma AM, Ur E. 2006 Canadian clinical practice guidelines on the management and prevention of obesity in adults and children [summary]. CMAJ. 2007;176:S1-S13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 646] [Cited by in RCA: 682] [Article Influence: 35.9] [Reference Citation Analysis (0)] |
| 147. | Tsigos C, Hainer V, Basdevant A, Finer N, Fried M, Mathus-Vliegen E, Micic D, Maislos M, Roman G, Schutz Y. Management of obesity in adults: European clinical practice guidelines. Obes Facts. 2008;1:106-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 257] [Cited by in RCA: 241] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 148. | Mshui ME, Saikawa T, Ito K, Hara M, Sakata T. QT interval and QT dispersion before and after diet therapy in patients with simple obesity. Proc Soc Exp Biol Med. 1999;220:133-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 34] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 149. | Pietrobelli A, Rothacker D, Gallagher D, Heymsfield SB. Electrocardiographic QTC interval: short-term weight loss effects. Int J Obes Relat Metab Disord. 1997;21:110-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 23] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 150. | Seyfeli E, Duru M, Kuvandik G, Kaya H, Yalcin F. Effect of obesity on P-wave dispersion and QT dispersion in women. Int J Obes (Lond). 2006;30:957-961. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 54] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 151. | Braschi A, Abrignani MG, Francavilla VC, Francavilla G. Novel electrocardiographic parameters of altered repolarization in uncomplicated overweight and obesity. Obesity (Silver Spring). 2011;19:875-881. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 25] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 152. | Zarich SW, Kowalchuk GJ, McGuire MP, Benotti PN, Mascioli EA, Nesto RW. Left ventricular filling abnormalities in asymptomatic morbid obesity. Am J Cardiol. 1991;68:377-381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 80] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 153. | Corrado D, Basso C, Rizzoli G, Schiavon M, Thiene G. Does sports activity enhance the risk of sudden death in adolescents and young adults? J Am Coll Cardiol. 2003;42:1959-1963. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 844] [Cited by in RCA: 809] [Article Influence: 36.8] [Reference Citation Analysis (1)] |
| 154. | Lengyel C, Orosz A, Hegyi P, Komka Z, Udvardy A, Bosnyák E, Trájer E, Pavlik G, Tóth M, Wittmann T. Increased short-term variability of the QT interval in professional soccer players: possible implications for arrhythmia prediction. PLoS One. 2011;6:e18751. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 155. | Lawan A, Ali MA, Dan-Bauchi SS. QT dispersion in dynamic and static group of athletes. Niger J Physiol Sci. 2006;21:5-8. [PubMed] |
| 156. | Carmona Puerta R, Fernández Arbolaez V, Rabassa López-Calleja MA, Ramos Ramírez R, Padrón Pe-a G, Chávez González E. Análisis de los intervalos QT, JT y Tpico-Tfinal y sus dispersiones en practicantes femeninas de polo acuático de élite. Rev Arg Cardiol. 2012;80:231-235. |
| 157. | Kasikcioglu E, Kayserilioglu A, Yildiz S, Akhan H, Cuhadaroglu C. Qt dispersion in soccer players during exercise testing. Int J Sports Med. 2004;25:177-181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 158. | Mayet J, Kanagaratnam P, Shahi M, Senior R, Doherty M, Poulter NR, Sever PS, Handler CE, Thom SA, Foale RA. QT dispersion in athletic left ventricular hypertrophy. Am Heart J. 1999;137:678-681. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 13] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 159. | Lonati LM, Magnaghi G, Bizzi C, Leonetti G. Patterns of QT dispersion in athletic and hypertensive left ventricular hypertrophy. Ann Noninvasive Electrocardiol. 2004;9:252-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 160. | Turkmen M, Barutcu I, Esen AM, Ocak Y, Melek M, Kaya D, Karakaya O, Saglam M, Basaran Y. Assessment of QT interval duration and dispersion in athlete’s heart. J Int Med Res. 2004;32:626-632. [PubMed] |
| 161. | Ghelani SJ, Singh S, Manojkumar R. QT interval dispersion in North Indian children with Kawasaki disease without overt coronary artery abnormalities. Rheumatol Int. 2011;31:301-305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 162. | Acar GR, Akkoyun M, Nacar AB, Dirnak I, Yıldırım Çetin G, Nur Yıldırım M, Zencir C, Karaman K, Cetin M, Sayarlıoğlu M. Evaluation of Tp-e interval and Tp-e/QT ratio in patients with rheumatoid arthritis. Turk Kardiyol Dern Ars. 2014;42:29-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 21] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 163. | Bourré-Tessier J, Urowitz MB, Clarke AE, Bernatsky S, Krantz MJ, Huynh T, Joseph L, Belisle P, Bae SC, Hanly JG. Electrocardiographic findings in systemic lupus erythematosus: data from an international inception cohort. Arthritis Care Res (Hoboken). 2015;67:128-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 32] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 164. | Alabd MA, El-Hammady W, Shawky A, Nammas W, El-Tayeb M. QT Interval and QT Dispersion in Patients Undergoing Hemodialysis: Revisiting the Old Theory. Nephron Extra. 2011;1:1-8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 165. | Claudio Bde Q, Costa MA, Penna F, Konder MT, Celoria BM, Souza LL, Pozzan R, Schneider RS, Albuquerque FN, Albuquerque DC. Impact of psychotropic drugs on QT interval dispersion in adult patients. Arq Bras Cardiol. 2014;102:465-472. [PubMed] |
| 166. | Menon SC, Etheridge SP, Liesemer KN, Williams RV, Bardsley T, Heywood MC, Puchalski MD. Predictive value of myocardial delayed enhancement in Duchenne muscular dystrophy. Pediatr Cardiol. 2014;35:1279-1285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 72] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 167. | Zahmatkeshan M, Fallahzadeh E, Najib SS, Amoozgar H, Malekhosseini SA, Nikeghbalian S. The Relationship between QT Interval Dispersion and End-Stage Liver Disease Score in the Patients Undergoing Orthotopic Liver Transplantation. Int Cardiovasc Res J. 2013;7:135-140. [PubMed] |
| 168. | Wongcharoen W, Suaklin S, Tantisirivit N, Phrommintikul A, Chattipakorn N. QT dispersion in HIV-infected patients receiving combined antiretroviral therapy. Ann Noninvasive Electrocardiol. 2014;19:561-566. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 169. | Sgreccia A, Morelli S, Ferrante L, Perrone C, De Marzio P, De Vincentiis G, Scopinaro F. QT interval and QT dispersion in systemic sclerosis (scleroderma). J Intern Med. 1998;243:127-132. [PubMed] |
| 170. | Polat TB, Yalcin Y, Akdeniz C, Zeybek C, Erdem A, Celebi A. QT dispersion in acute rheumatic fever. Cardiol Young. 2006;16:141-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 171. | Armaganijan L, Moreira DA, Nolasco de Araújo RR, Puzzi MA, Munhoz FP, Carvalho MJ, Gallo LN, França JI, Lopes RD. The usefulness of T-wave peak to T-wave end interval in identifying malignant arrhythmias in patients with Chagas disease. Hellenic J Cardiol. 2013;54:429-434. [PubMed] |
| 172. | Acar G, Yorgun H, Inci MF, Akkoyun M, Bakan B, Nacar AB, Dirnak I, Cetin GY, Bozoglan O. Evaluation of Tp-e interval and Tp-e/QT ratio in patients with ankylosing spondylitis. Mod Rheumatol. 2014;24:327-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 20] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 173. | Demir C, Demir M. Evaluation of Tp-e interval and Tp-e/QT ratio in patients with chronic hepatitis B. Prague Med Rep. 2013;114:239-245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 174. | Kilicaslan F, Tokatli A, Ozdag F, Uzun M, Uz O, Isilak Z, Yiginer O, Yalcin M, Guney MS, Cebeci BS. Tp-e interval, Tp-e/QT ratio, and Tp-e/QTc ratio are prolonged in patients with moderate and severe obstructive sleep apnea. Pacing Clin Electrophysiol. 2012;35:966-972. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 61] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 175. | Kayrak M, Acar K, Gul EE, Ozbek O, Abdulhalikov T, Sonmez O, Alibaşiç H. The Association between Myocardial Iron Load and Ventricular Repolarization Parameters in Asymptomatic Beta-Thalassemia Patients. Adv Hematol. 2012;2012:170510. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 176. | Ravensbergen HJ, Walsh ML, Krassioukov AV, Claydon VE. Electrocardiogram-based predictors for arrhythmia after spinal cord injury. Clin Auton Res. 2012;22:265-273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 177. | Kayrak M, Acar K, Gul EE, Abdulhalikov T, Bağlıcaklıoğlu M, Sonmez O, Kaya Z, Arı H. Electrocardiographic findings in patients with polycythemia vera. Int J Med Sci. 2012;9:93-102. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 178. | Sangawa M, Morita H, Nakatsu T, Nishii N, Miura D, Miura A, Tada T, Murakami M, Hiramatsu S, Nagase S. Abnormal transmural repolarization process in patients with Brugada syndrome. Heart Rhythm. 2009;6:1163-1169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 179. | Ducceschi V, D’Andrea A, Sarubbi B, Liccardo B, Mayer MS, Salvi G, Santangelo L, Iacono A. Repolarization abnormalities in patients with idiopathic ventricular tachycardias. Can J Cardiol. 1998;14:1451-1455. [PubMed] |
| 180. | Schwartz PJ, Malliani A. Electrical alternation of the T-wave: clinical and experimental evidence of its relationship with the sympathetic nervous system and with the long Q-T syndrome. Am Heart J. 1975;89:45-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 395] [Cited by in RCA: 345] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 181. | Yu H, Pi-Hua F, Yuan W, Xiao-Feng L, Jun L, Zhi L, Sen L, Zhang S. Prediction of sudden cardiac death in patients after acute myocardial infarction using T-wave alternans: a prospective study. J Electrocardiol. 2012;45:60-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
P- Reviewer: De Ponti R, Letsas K S- Editor: Tian YL L- Editor: A E- Editor: Liu SQ
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/