Published online Apr 16, 2015. doi: 10.12998/wjcc.v3.i4.385
Peer-review started: September 28, 2014
First decision: December 17, 2014
Revised: January 20, 2015
Accepted: February 4, 2015
Article in press: February 9, 2015
Published online: April 16, 2015
Processing time: 198 Days and 14.4 Hours
Authors describe a 53-year-old woman who presented to their diabetes clinic with a three week history of multiple painful and swollen joints. She had been diagnosed with type 2 diabetes 5 years back. On examination, both knee joints and left ankle were swollen. A soft tissue swelling appeared over the medial end of the left clavicle few days later. Rheumatoid arthritis, collagen vascular diseases and other common causes of polyarthritis were ruled out by appropriate investigations. Non steroidal anti-inflammatory drugs failed to give satisfactory pain relief and the arthritis persisted. Conventional cultures of synovial fluid samples including cultures for tuberculosis were negative. Computed tomography showed a space occupying lesion involving the left sternoclavicular joint. Fine needle aspiration from the lesion was performed and acid-fast bacilli were demonstrated in the smear using Ziehl-Neelsen stain. The explanation of her arthritis was therefore tuberculous arthritis in left sternoclavicular joint and reactive arthritis in the rest of the joints. A diagnosis of Poncet’s disease was considered in her case. We treated her with standard anti-tuberculosis drugs and the arthritis resolved within a few days. She remained symptom-free at her 2 years’ follow-up.
Core tip: Poncet’s disease (PD) is a form of reactive arthritis that develops in patients with active tuberculosis (TB). It is a rare, non-destructive parainfective symmetric polyarthritis. In cases of unexplained atypical arthritis associated with non-articular TB, PD should be considered. PD remains a clinical challenge and is essentially a diagnosis of exclusion and requires a high degree of clinical suspicion. Correct identification of this rare complication of TB is required to avoid delayed initiation of appropriate treatment. The dramatic response of arthritis in PD on starting anti-tubercular treatment substantiates the diagnosis. Further studies are required for better understanding of the pathogenesis underlying PD.
- Citation: Chakraborty PP, Ray S, Selvan C, Bhattacharjee R, Mandal SK. Poncet’s disease: An unusual presentation of tuberculosis in a diabetic lady. World J Clin Cases 2015; 3(4): 385-388
- URL: https://www.wjgnet.com/2307-8960/full/v3/i4/385.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v3.i4.385
The association between of diabetes mellitus (DM) and tuberculosis (TB) is becoming more prominent in developing countries where TB remains endemic and the burden of DM is increasing. Atypical presentations are increasingly being recognized in diabetic patients. The diagnosis of joint TB poses a challenge to the clinicians. Although, septic monoarthritis is a well-known complication of tuberculous infection; active TB may be complicated by a sterile reactive arthritis (ReA), known as Poncet’s disease (PD), which is not so common and therefore frequently missed. We report here a diabetic patient who initially presented with oligoarthritis, and later on ended with a diagnosis of PD. A review of the literature on diagnostic and therapeutic aspects involved in PD is also included. This case highlights the need for increased awareness among physicians regarding this rare complication of a common disease to avoid delay in diagnosis and starting the appropriate treatment.
A 53-year-old lady presented with a three week history of multiple painful and swollen joints. She had been diagnosed with type 2 diabetes in March 2009. Her diabetes was well controlled and she was on insulin and metformin. Her presentation to the clinic had been prompted by her inability to walk in the previous five days. She had been diagnosed with undifferentiated polyarthritis by a family physician. The joint pains started gradually over three weeks involving left ankle and knee joints. Family history for rheumatic or autoimmune diseases was negative. There was no history of mouth ulcers, eye symptoms, skin rash or genito-urinary symptoms. She denied weight loss, coughing, night sweats but mentioned about low grade fever for few weeks.
On examination, she was in pain and was unable to walk. Her blood pressure was 130/80, pulse rate 90 per minute, respiratory rate 24 per minute and a temperature of 36.80 °C. The patient’s ankle and knee joints were swollen and tender on palpation with limitation of movement. A soft, boggy swelling appeared at the medial end of the left clavicle during her hospital stay clinically resembling a cold abscess. She had no erythema nodosum on examination. No adventitious sound was detected on chest auscultation. There was no lymphadenopathy or hepatosplenomegaly. There was no muscle atrophy. She had no signs of peripheral neuropathy. Fundoscopic examination was unremarkable.
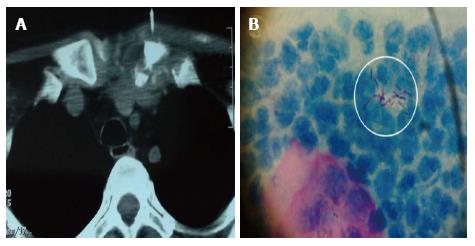
Her initial investigation results showed hemoglobin of 11.9 g/dL, a white cell count of 7.6 × 109/L, platelets of 130 × 109/L and an erythrocyte sedimentation rate (ESR) of 88 mm/h. Urine dipstick showed no white cells, protein was trace. Laboratory tests results for antinuclear antigen, rheumatoid factor and anti-cyclic citrullinated peptide antibodies were negative. The patient was nonreactive for HIV 1 and 2 by ELISA. The radiographs of the both knees and left ankle were normal. The intradermal skin test for tuberculosis reading was 17 mm, which was considered strongly positive. Synovial fluid was aspirated from the left ankle and both knees and analysis revealed leucocytes between 4.0 and 8.2 × 109/L, no crystals and the smears were negative for Gram stain. On bacterial culture of the synovial fluid, no growth was found and synovial fluid cultures for TB were also negative. The plausible explanation of her arthritis would therefore be a form of a reactive arthritis. Since no diagnosis could be made, we went for exploring the neck swelling. Computed tomography scan of the neck was performed which showed a space occupying lesion (SOL) around the medial end of clavicle (Figure 1A). Fine needle aspiration from the lesion showed epithelioid cell collection and multinucleated giant cells on the background of necrotic tissue. AFB stain was positive (Figure 1B).
Treatment with non steroidal anti-inflammatory drugs only gives partial relief. Since there was strong evidences suggesting active joint tuberculosis, she was put on four-drug antituberculosis treatment, including rifampicin, isoniazid, pyrazinamide, and ethambutol. Her joint symptoms showed a remarkable improvement within two weeks of initiation of therapy.
Following treatment with standard anti-tubercular treatment, the arthritis had completely resolved in three weeks period. The consistent association of arthritis with presence of active tuberculosis, the lack of evidence of any other known rheumatic disease and the resolution of symptoms on anti-tuberculosis therapy were all consistent with the diagnosis of PD in our patient. She had remained asymptomatic at 2 years’ follow-up.
PD or tubercular rheumatism is a form of reactive polyarthritis related with active TB in which no mycobacterial involvement can be found in the affected bones or joints, and there is absence of other detectable causes of polyarthritis[1-3]. TB reactive arthritis was first described by Poncet[4] in 1897 and named after him as PD. PD is considered a ReA, but the clinical presentation of PD is different from the classical pattern of ReA[5]. Unlike ReA, the onset of symptoms in PD prior to the start of arthritis is much longer than only a few weeks, whereas arthritis resolution upon starting of anti-tubercular therapy is generally within a few weeks. Chronic arthritis is not encountered in PD. Although the clinical presentation is somewhat different, the pathogenetic mechanism is considered to be similar. It is thought to occur due to a hypersensitive immune cell mediated response to the tuberculoprotein, resulting in an inflammatory reaction in the joint spaces[2,6]. Due to the rarity of PD despite the frequency of TB, a genetic predisposition has been suggested in the pathogenesis, with links to the HLA DR3 and HLA DR4 haplotypes[7].
Clinically the disease is different to the well recognized TB monoarthritis; a septic mycobacterium infection of a joint leading to its destruction. From the cases previously described, Poncet’s arthritis is non-destructive and resolves completely following TB treatment. The arthritis mainly affects the larger joints, with the knee being the most common, followed by the ankle and wrist joints. The axial skeleton tends not to be involved. It is described as a symmetrical polyarthritis but many studies[8] have suggested it to be pauciarticular arthritis, mainly of the larger joints as was the case with our patient. Other common symptoms include lymphadenopathy (mainly cervical and axillary), grumbling fevers (which may be present many weeks prior to developing the arthritis) and skin changes; classically erythema nodosum[9]. In the review of 50 cases report on PD by Kroot et al[1] erythema nodosum was present only in 6% of the patients.
The diagnosis is usually one of exclusion and should be considered in all patients with a symmetrical arthritis in TB prevalent regions. Extra-pulmonary TB, particularly lymph node TB, is traditionally thought to be the main culprit[10,11]. The complete resolution of rheumatic symptoms on anti-tuberculosis therapy further confirms the diagnosis. Resolution of the arthritis of PD with anti-tubercular drugs ranged from a week to few months.
To conclude, active tuberculosis needs to be considered in the differential diagnosis of patients presenting with fever and polyarthritis of unclear cause, particularly in regions where the prevalence of tuberculosis is high. The diagnosis of this clinical entity remains a clinical challenge and demands a high index of suspicion. Since not all clinicians are aware of PD, this entity is probably underdiagnosed.
A 53-year-old diabetic woman presented with multiple painful and swollen joints.
The patient’s ankle and knee joints were swollen and tender on palpation with limitation of movement. A soft, boggy swelling appeared at the medial end of the left clavicle during her hospital stay clinically resembling a cold abscess.
Septic arthritis, reactive arthritis, rheumatoid arthritis.
Her investigation results showed hemoglobin of 11.9 g/dL, a white cell count of 7.6 × 109/L, platelets of 130 × 109/L and an erythrocyte sedimentation rate of 88 mm/h. Results for antinuclear antigen, rheumatoid factor and anti-cyclic citrullinated peptide antibodies were negative. The intradermal skin test for tuberculosis reading was 17 mm, which is considered a positive test.
The radiographs of the both knees and left ankle were normal. Computed tomography scan of the neck showed a space occupying lesion (SOL) around the medial end of clavicle.
Fine needle aspiration from supraclavicular SOL/of the left supraclavicular node showed epithelioid cell collection and multinucleated giant cells on the background of necrotic tissue. AFB stain was positive.
The patient was put on four drug anti-tuberculosis (TB) therapy since the evidence was strongly suggestive of active joint tuberculosis.
Reviewing the literature, more than 50 case reports were found. In most reports “Poncet’s disease” was described as an aseptic polyarthritis, presumably reactive arthritis arthritis developing in the presence of active TB elsewhere.
Poncet’s disease is a reactive polyarthritis associated with active TB in which no mycobacterial involvement can be found in the affected bones or joints.
The diagnosis of Poncet’s disease remains clinical and is established on excluding other potential causes of arthritis in a patient with active tuberculosis. In cases with unexplained atypical arthritis and non-articular TB, Poncet’s disease should be considered.
It is a case report describing Poncet’s disease which goes unnoticed and underdiagnosed. The manuscript is scientifically sound and based on the discovery of facts. Data presented are duly supported by appropriate figures.
| 1. | Kroot EJ, Hazes JM, Colin EM, Dolhain RJ. Poncet’s disease: reactive arthritis accompanying tuberculosis. Two case reports and a review of the literature. Rheumatology (Oxford). 2007;46:484-489. [PubMed] |
| 2. | Dall L, Long L, Stanford J. Poncet’s disease: tuberculous rheumatism. Rev Infect Dis. 1989;11:105-107. [PubMed] |
| 3. | Hameed K, Karim M, Islam N, Gibson T. The diagnosis of Poncet’s disease. Br J Rheumatol. 1993;32:824-826. [PubMed] |
| 4. | Poncet A. De la polyarthrite tuberculeuse deformante ou pseudorheumatisme chronique tuberculeux. Congres Francaise de Chirurgie. 1887;1:732-739. |
| 5. | Flores D, Marquez J, Garza M, Espinoza LR. Reactive arthritis: newer developments. Rheum Dis Clin North Am. 2003;29:37-59, vi. [PubMed] |
| 6. | Malaviya AN, Kotwal PP. Arthritis associated with tuberculosis. Best Pract Res Clin Rheumatol. 2003;17:319-343. [PubMed] |
| 7. | Ames PR, Capasso G, Testa V, Maffulli N, Tortora M, Gaeta GB. Chronic tuberculous rheumatism (Poncet’s disease) in a gymnast. Br J Rheumatol. 1990;29:72-74. [PubMed] |
| 8. | Arora VK, Verma V. Tuberculous rheumatism (Poncet’s disease): three case reports. Ind J Tuberculosis. 1991;38:229. |
| 9. | Segula D, Mahmood N, Allain TJ. Ward round: A 43-year-old diabetic man with multiple joint pains. Malawi Med J. 2012;24:84-86. [PubMed] |
| 10. | Kawsar M, D’Cruz D, Nathan M, Murphy M. Poncet’s disease in a patient with AIDS. Rheumatology (Oxford). 2001;40:346-347. [PubMed] |
| 11. | Wollheim FA. Enteropathic arthritis. Textbook of Rheumatology. 5th ed. Phildelphia: WB Saunders 1997; 1006-1014. |
P- Reviewer: Bisen P, Espinoza LR, Gedela K S- Editor: Ji FF L- Editor: A E- Editor: Lu YJ
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/