Published online Apr 16, 2015. doi: 10.12998/wjcc.v3.i4.360
Peer-review started: October 30, 2014
First decision: December 26, 2014
Revised: January 28, 2015
Accepted: February 10, 2015
Article in press: February 12, 2015
Published online: April 16, 2015
Processing time: 165 Days and 9.5 Hours
AIM: To evaluate the effects of two different doses of sugammadex after maintenance anesthesia with sevofluorane and remifentanil and deep rocuronium-induced neuromuscular blockade (NMB).
METHODS: Patients between 20 and 65 years of age, with American Society of Anesthesiologists physical status classification I-II, undergoing gynecological surgery were included in a prospective, comparative and randomized study. NMB was induced with an injection of 0.6 mg/kg of rocuronium followed by continuous infusion of 0.3-0.6 mg/kg per hour to maintain a deep block. Anesthesia was maintained with sevofluorane and remifentanil. Finally, when surgery was finished, a bolus of 2 mg/kg (group A) or 4 mg/kg (group B) of sugammadex was applied when the NMB first response in the train-of-four was reached. The primary clinical endpoint was time to recovery to a train-of-four ratio of 0.9. Other variables recorded were the time until recovery of train-of-four ratio of 0.7, 0.8, hemodynamic variables (arterial blood pressure and heart rate at baseline, starting sugammadex, and minutes 2, 5 and 10) and adverse events were presented after one hour in the post-anesthesia care unit.
RESULTS: Thirty-two patients were included in the study: 16 patients in group A and 16 patients in group B. Only 14 patients each group were recorded because arterial pressure values were lost in two patients from each group in minute 10. The two groups were comparable. Median recovery time from starting of sugammadex administration to a train-of-four ratio of 0.9 in group A and B was 129 and 110 s, respectively. The estimated difference in recovery time between groups was 24 s (95%CI: 0 to 45 s, Hodges-Lehmann estimator), entirely within the predefined equivalence interval. Times to recovery to train-of-four ratios of 0.8 (group A: 101 s; group B: 82.5 s) and 0.7 (group A: 90 s; group B: 65 s) from start of sugammadex administration were not equivalent between groups. There was not a significant variation in the arterial pressure and heart rate values between the two groups and none of the patients showed any clinical evidence of residual or recurrent NMB.
CONCLUSION: A dose of 2 mg/kg of sugammadex after continuous rocuronium infusion is enough to reverse the NMB when first response in the Train-Of-Four is reached.
Core tip: The release of sugammadex in recent times has been a global shift in the strategy of the reversal of neuromuscular blockade (NMB) induced by aminosteroid neuromuscular blocking. The use of this drug has been increasing slowly, and consequently, we receive more and more questions in regards to its efficacy and safety. In this study we compared the dose of 2 mg/kg to 4 mg/kg sugammadex to reverse the NMB when first response in the train-of-four is reached after continuous infusion of rocuronium. Both doses have been shown to be effective for recovery from NMB.
- Citation: Mesa DS, Fayad MF, Arviza LP, Ruiz VDV, Carreño FC, Tamargo LA, Díaz MA, Montes SFP. Efficacy of different doses of sugammadex after continuous infusion of rocuronium. World J Clin Cases 2015; 3(4): 360-367
- URL: https://www.wjgnet.com/2307-8960/full/v3/i4/360.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v3.i4.360
Neuromuscular blockade (NMB) is an important technique in modern anesthesia because it improves surgical conditions by suppressing voluntary movements or muscular reflexes. The use of neuromuscular blockers is highly beneficial in determined types of surgery, such as laparoscopy, as it improves the surgical access and the visual field[1]. However, the extended use of NMB is associated with increased postoperative morbimortality due to the risk of residual neuromuscular paralysis or recurarization and the development of subsequent complications[2,3]. Such complications can be reduced by objective monitoring of muscle relaxation and NMB reversal after the surgical procedure.
The release of sugammadex [Bridion®, merck sharp and dohme, Oss, The Netherlands] triggers a change in the way of reversing aminosteroid neuromuscular blocking drugs. Sugammadex is a gamma-cyclodextrin with a lipophilic cavity that traps aminosteroid neuromuscular blocker molecules to form an inactive complex, thus preventing their union with nicotinic receptors and reversing their effects[4,5]. Clinical data suggests that sugammadex has a favorable efficacy and safety profile[4,6,7], allowing a safer and faster recovery-even from deep NMB[8]-than the commonly used combination of acetylcholinesterase inhibitors and anticholinergic agents.
Halogenated anesthetics, such as sevoflurane, increase the effect and duration of rocuronium[9], and this effect is clinically most significant when using a continuous infusion of rocuronium[10]. However, such do not appear to alter the efficacy or safety of sugammadex[11-13]. We hypothesize that a dose of sugammadex could result in a suitable recovery time although it depends on the individual redistribution and elimination of rocuronium as well[14]. The provider has not defined what the ideal dose of sugammadex for reversal the NMB when first response in the train-of-four (TOF) is reached. So, we have designed a study based upon on this hypothesis: after a surgical procedure, a dose of 2 mg/kg sugammadex is comparable to a dose of 4 mg/kg for reversal the NMB induced by a continuous infusion of rocuronium administered when first response in the TOF (T1) is reached.
A prospective, randomized and comparative study was designed to include patients undergoing a gynecological surgery, and took place over one year. The study was approved by the Regional Research Ethics Board of Principality of Asturias (Ref 118/2013; approved in August, 2013) and, after being given a verbal explanation, all patients gave their written informed consent. Applicable regulations and good clinical practice guidelines concerning NMB were followed in all cases[15].
The study included patients between 20 and 65 years of age, with American Society of Anesthesiologists (ASA) physical status classification I-II, who were scheduled for elective gynecological laparoscopy procedures under general anesthesia with sevoflurane requiring NMB with a minimum duration of 1 h, and carried out by the same surgical team.
The sample size was calculated on the basis of data for previous recovery time from NMB to first response in the TOF after sevoflurane anesthesia followed by 4 mg/kg sugammadex[14]. A 50% increase in recovery time was considered to be clinically relevant. To obtain statistically significant results with a probability of type I error (α = 0.05), probability of type II error (β = 0.10), and a statistical power of 90%, a total of 22 patients were required. Therefore, 32 patients were recruited to compensate for any possible losses.
Patients were randomized to receive a dose of 2 mg/kg (group A) or 4 mg/kg (group B) after surgical procedure by the responsible anesthesiologist as previously had been determined. A manual randomization method was performed.
Exclusion criteria were as follows: previous known neuromuscular disease, obesity [defined as a body mass index (BMI) ≥ 30 kg/m2], allergy to any drug used in the general anesthesia, history of malignant hyperthermia, liver or kidney insufficiency, predicted difficult airways or a previous history of difficult intubation, use of drugs that affect the neuromuscular system (for example: magnesium, anticonvulsants, aminoglycosides), pregnancy or lactation, or any other medical condition which could affect level of consciousness.
All patients received intramuscular 2 mg midazolam as premedication. Standard monitoring was performed once the patients were in the operating room (pulseoximetry, capnography, electrocardiography and noninvasive arterial pressure). Patients were preoxygenated with FIO2 of 1.0 for 3 min before induction of anesthesia with intravenous propofol (1.5-2.5 mg/kg) and fentanyl (1-2 mcg/kg).
Neuromuscular function was monitored through kinemyography (KMG) in form of the Mechanosensor-Neuromuscular Module Transmission (M-NMT®) (GE Healthcare, Helsinki, Finland) integrated in the Datex-Ohmeda anesthesia machine. The right arm was placed at an angle of 90° to the longitudinal axis of the body and the electrodes were placed on cleaned skin 3-6 cm apart over the ulnar nerve at the wrist. M-NMT was placed on the adductor pollicis muscle. Physical means were used to maintain the peripheral temperature above 35 °C.
Once the induction of anesthesia was finished and before the administration of rocuronium, the M-NMT monitor was calibrated using 200 μs pulses at a rate of 2 Hz, starting at 5 mA with increments of 5 mA. The maximal current was increased by 15%, yielding the supramaximal stimulation. The 0.6 mg/kg of rocuronium bolus was then injected provided that a first 2 Hz TOF stimulation for 1.5 s yielded four equal responses within 15% of the calibration. When there was no measurable response to TOF stimulation, the patients were intubated and mechanical ventilation was initiated. This initial dose was followed by a continuous infusion of 0.3-0.6 mg/kg per hour of rocuronium which was adjusted to maintain a deep block with a TOF response of zero and PTCs less than 10 for the duration of the procedure. TOF stimulations were repeated every 15 s throughout the study. A PTC mode was initially applied 5 min after obtaining complete NMB and repeated every 6 min. Anesthesia was maintained with sevoflurane 1%-3% end-tidal. In both groups analgesia was provided by remifentanil with a dose of 0.05-0.5 mcg/kg per minute.
Upon completion of the surgery, the administration of sevoflurane, remifentanilo and rocuronium ended. At the reappearance of the T1, every patient received a dose of sugammadex according to the group in which they had been randomized (2 mg/kg in group A, or 4 mg/kg in group B), and they were awoken once complete NMB reversal (TOF ratio ≥ 0.9) was reached. Neuromuscular monitoring was continued until patients were extubated. Once recovered, they were transferred to the post-anesthesia care unit.
After one hour in the post-anesthesia care unit, a member of the team who was blind to the sugammadex dose that the patient had received, evaluated in each patient the presence of any residual paralysis through neuromuscular monitoring and performed a clinical assessment by signs of muscular weakness and clinical tests (lifting the head for more than 5 s, holding a tongue depressor between the teeth and generalized muscular weakness). The post-anesthesia oxygen saturation, breathing rate and any possible hemodynamic instability as well as the appearance of any adverse effect was also recorded. The same post-surgical analgesia protocol was applied to all patients.
Patient baseline quantitative variables in the two groups were compared by two-sided Student t-test if they followed a normal distribution. Categorical variables were analyzed by Pearson χ2 test (or Fisher Exact test if expected count less than 5). Odds ratio (OR) and its CI was calculated if necessary.
The primary efficacy variable was the time (in seconds) between commencing sugammadex administration and reaching recovery of the TOF ratio to 0.9. The time until recovery of TOF ratios to 0.7 and 0.8 were studied too. We used the statistical approaching method described by Rex et al[14]. The CI approach was used to demonstrate equivalence in recovery of the TOF ratios between the two treatment groups. Non statistical signification was established if the two-sided 95%CI for the estimated difference of median between group A and group B was within the interval ranging from 0% to 50% of the median of group B. The 95%CI was obtained by using the nonparametric methods of Hodges-Lehmann. Similarly, TOF ratio to 0.7 and 0.8 were studied.
The hemodynamic variables were the evolution of arterial blood pressure (AP) and the heart rate (HR) after sugammadex injection. AP and HR were recorded every 5 min throughout the intervention: previously, during the start of sugammadex, and 2, 5 and 10 min after initiating administration of the drug. Any possible secondary effect associated to its administration was also recorded.
Data for AP and HR were analyzed by repeated measure analysis of variance (ANOVA-RM). The within-subjects terms were the AP and HR values for each patient, and the repeated term was the time point (baseline, starting, and minute 2, 5 and 10). Pillai’s Trace[16] is calculated for AP and HR and their interactions with sugammadex doses. They were corrected with epsilon multipliers if the assumption of circularity had been violated following Mauchly’s test[17]. Lower bound was elected to be the most conservative. Post-hoc analyses were executed. The P-values < 0.05 were considered significant. All tests were 2-sided. Data was analyzed using SPSS 17.0 for Windows (SPSS Inc., Chicago, United States).
A total of 32 patients were included in the study, 16 patients in group A and 16 patients in group B. All descriptive variables are summarized in Table 1. However, AP was not taken in the 10th minute in two patients in each group. Because AP in the 10th minute is a related sample within temporal evolution (the others are AP baseline, pre-sugammadex, minute 2th and minute 5th), only 14 patients from each group were computed (another two were excluded). So, all results were analyzed by per-protocol; however, AP values were lost in two patients in each group for the 10th minute.
| Sugammadex (dose) | Group A (n = 16) | Group B (n = 16) | P-value | ||
| Age (yr) | 43.6 | (SD 12.01) | 47.1 | (SD 14.18) | 0.46 |
| Weight (kg) | 65.5 | (SD 11.22) | 60.9 | (SD 10.62) | 0.25 |
| Height (cm) | 163.2 | (SD 4.76) | 160.1 | (SD 5.91) | 0.12 |
| BMI (kg/m2) | 24.1 | (SD 3.56) | 23.2 | (SD 3.65) | 0.47 |
| Intervention (time-minute) | 95.2 | (SD 26.91) | 94.7 | (SD 30.02) | 0.96 |
| ASA (1-2) | 1.4 | (SD 0.51) | 1.2 | (SD 0.48) | 0.28 |
| ASA 1a | n = 9 | (56.25%) | n = 12 | (75.00%) | 0.23 |
The gynecological interventions were fourteen vaginal assisted laparoscopic hysterectomies (43.7%), eleven laparoscopic ovarian cystectomies (34.4%) and seven laparoscopic adnexectomies (21.9%). The two groups were comparable in terms of age, BMI and ASA (Table 1). Surgical time was more than 60 min in all cases.
All patients recovered to a TOF ratio of 0.9 within 3 min (maximum value 175 s). Median recovery time from starting of sugammadex administration to a TOF ratio of 0.9 was 129 s in group A and 110 s in group B. The estimated difference in recovery time between the two groups was 24 s (95%CI: 0 to 45 s, Hodges-Lehmann estimator). This CI was entirely within the predefined equivalence interval (for a median of 110 s in group B = 0 to 52.5 s), so equivalence was assumed. Times to recovery to TOF ratios of 0.8 and 0.7 from start of sugammadex administration were not equivalent between groups. Median time to recovery to a TOF ratio of 0.8 was 101 s in group A and 82.5 s in group B, with an estimated difference of 18 (95%CI: -5 to 39 s, Hodges-Lehmann estimator). 95%CI was out of predefined equivalence interval of 0 to 43.7 s. Median time to recovery to a TOF ratio of 0.7 was 90 s in group A and 65 s in group B, with an estimated difference of 10 (95%CI: -10 to 35, Hodges-Lehmann estimator). So, 95%CI was out of predefined equivalence interval of 0 to 32.5 s. Equivalences were not assumed for TOF ratio 0.8 and TOF ratio 0.7 (Table 2).
| Group A (n = 14) | Group B (n = 14) | |||||||
| Mean | Median | Mean | Median | Assumed calculated interval (increased of 0% to 50% of median in group B) | Estimated difference median by Hodges-Lehmann estimator | 95%CI | ||
| TOF ratio 0.9 | 118.8 | 129 | 96.6 | 105 | 0 to 52.5 | 24 | 0 to 45 | Differences not assumed |
| TOF ratio 0.8 | 96.7 | 101 | 80.1 | 82.5 | 0 to 43.7 | 18 | -5 to 39 | Assumed |
| TOF ratio 0.7 | 78.4 | 90 | 66.3 | 65 | 0 to 32.5 | 10 | -10 to 35 | Assumed |
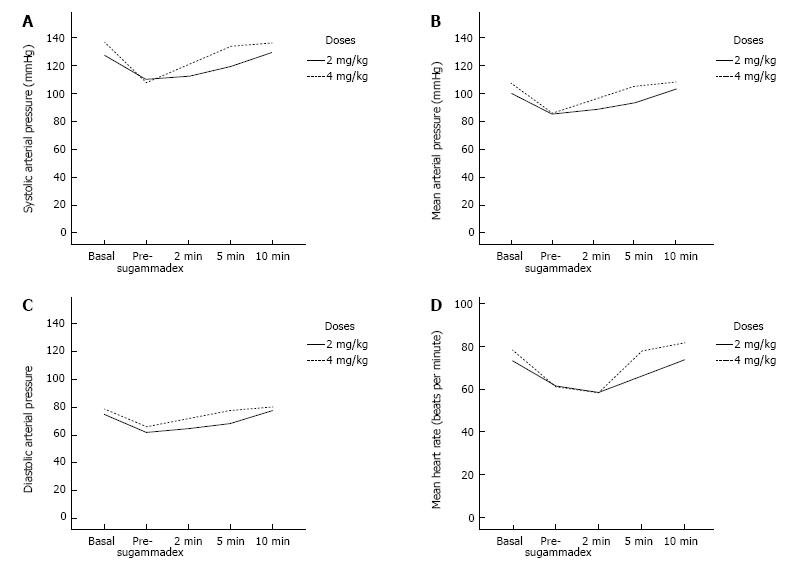
There was no significant variation in the AP and HR between the two groups. Although both of them maintained AP and HR within normal ranges the entire time, there was a logical increment of AP and HR as time passed until the effect of anesthetic drugs disappeared. So, post-hoc analyses were statistically significant across the 2nd, 5th and 10th minute within each group (Figure 1).
Based on neuromuscular monitoring and clinical signs, none of the patients showed any clinical evidence of residual or recurrent NMB. Although group B had more adverse events than group A, there was no statistical difference between them (group A: 12.5% vs group B: 18.7%, OR = 1.62; 95%CI: 0.23-11.26, P = 0.99). There were no severe adverse effects, even with an increased dosage of sugammadex. As a consequence, in the immediate post-operatory period in group A, there was one case of nausea and another case of pain, while in group B, there was one case of nausea, one case of pain and one patient suffered tremors in lower limbs (Table 3). Habitual symptomatic treatments were adopted and they were effective without any more clinical relevance.
| Adverse events | Group A | Group B |
| Arterial hypertension | 0% | 0% |
| Arterial hypotension | 0% | 0% |
| Bradycardia | 0% | 0% |
| Cough | 0% | 0% |
| Headache | 0% | 0% |
| Nausea | 6.20% | 6.20% |
| Pain | 6.20% | 6.20% |
| Residual neuromuscular blocking | 0% | 0% |
| Vomiting | 0% | 0% |
| Others | 0% | 6.20% |
Our study suggests that a dose of 2 mg/kg sugammadex is enough for the recovery of NMB induced by a continuous infusion of rocuronium in patients who kept anaesthetized with sevoflurane. This lower dose did not have any clinically relevant recovery time augmentation or increased risk of residual recurarization. We have not been able to observe other adverse events in our patients.
The main limitation in our study was the lack of rocuronium and sugammadex plasma concentration determinations at different moments of the study. Although previous studies have shown a similar rocuronium pharmacokinetic profile when compared continuous infusion vs intravenous bolus dose[18], significant variations in plasma concentrations of rocuronium were also observed in those continuously infused with this drug (highly variable, up to 30% for some patients)[14]. For this reason, neuromuscular transmission monitoring suggested a better option in patients who received continuous infusions of rocuronium as a more realistic approach to the global effect of the drug. This is not routinely used in current daily monitoring in clinical practice[19,20] and a study published in the United Kingdom in 2007 reported that 62% of anesthetists surveyed had never used monitors to evaluate the effect of NMB[20].
Another point of interest was the use of a dose of 4 mg/kg of sugammadex. It has been demonstrated as preferable in the reversion of deep NMB[8]. The provider recommends a dose of 4 mg/kg if recovery has reached at least 1-2 PTCs, and a dose of 2 mg/kg sugammadex when spontaneous recovery has occurred up to least the reappearance of second response in the TOF[21]. Other authors consider in clinical practice that the appropriate dose of sugammadex for reversing a moderate block (TOF-count 1-3) is 2 mg/kg of sugammadex[22].
A TOF ratio ≥ 0.9 was used as the main desirable objective variable because a postoperative residual curarization TOF ratio < 0.9 is associated with increased morbidity and extended stay in the post-anesthesia care room[23]. It has been published that with 4 mg/kg of sugammadex, the time to recover a TOF ratio of 0.9 from 1-2 PTCs (induced by a bolus of rocuronium under anesthesia with sevoflurane) was 1.7 min compared to 3.2 min with a dose of 2 mg/kg of sugammadex[12]. However, studies comparing the efficacy of sugammadex in surgical patients when NMB was induced through the infusion of rocuronium are very scarce. Rex et al[14] demonstrated that just one dose (4 mg/kg) of sugammadex administered at a NMB to T1, after continuous infusion of rocuronium, was sufficient and safe with both sevoflurane and propofol. This use of continuous infusion of rocuronium has been shown to lengthen the NMB recovery time compared with one single bolus[24], thereby providing a more stable drug concentrations with a constant degree of paralysis. In our series, we find that difference between the means of the TOF 0.9 of both groups is lower than previously described: approximately an increase of only 23% vs the estimated published of 88%[12]. This difference can be attributed to different time of reversal of NMB and different procedures.
A limitation of our study is the age of the patients and the kind of surgical intervention (young and gynecological patients). In contrast, these patients were elected because they were attended by the same surgical team; hence similar laparoscopic conditions were expected in all cases. We decided to limit the age to 65 years because, even though reversal from profound block with sugammadex can be performed safely and effectively, there have been reports regarding older patients who recover more slowly than younger ones[25,26]. This slower recovery could be due to age-related decreased cardiac output and muscular blood flow[26].
Another possible bias in our study could be that surgical procedures lasted 60 min. They may be classified as insufficient. Nonetheless, it has been seen that a dose of 2 or 4 mg/kg of sugammadex is sufficient for reversion of NMB, even when deep NMB (1-2 PTCs) is maintained for 2 h or more, with reversal being performed when the second TOF response occurs[8,27].
We also observed the safety of using sugammadex. Adverse events related to the administration of sugammadex have been reported in the literature with an incidence of 14%, the most common being nausea, vomiting, bradycardia, hypertension and hypotension, oliguria, vertigo, headache, cough, dry mouth and intraoperative movements[28]. However, these adverse effects were not related with the use of sugammadex[11,12] or the dose administered. In our series, we found a similar occurrence in the two groups and there was no statistical difference between them. We consider that they were expectable, without direct relationship with the studied drug and not clinically relevant.
It could be supposed that the use of sugammadex would lead to a reduction of adverse events in the immediate postoperative period. They require additional resources and a longer recovery time. So, sugammadex could improve efficiency and reduce the costs related to surgical activities[29,30]. Nevertheless, the reduction of the sugammadex dose to save costs could be a mistake which may lead to other complications, such as the recurrence of NMB after an apparently successful recovery[31]. In this study we do not analyze the economic implications of the lower dose. We think that the group size is too small to establish conclusions, because they were selected and calculated to observe the effect on TOF 0.9 of sugammadex in two different doses. A dose of 2 mg/kg is evidently the half of cost of 4 mg/kg but it is only in respect to a simple drug expenditure and we cannot apply it to the complete surgical procedure and its multiple non-contemplated influent variables.
Only future investigation will make enable us to consider readjusting the currently recommended doses in specific circumstances, without an increase in the risks[32]. So, more studies are necessary in different surgical sceneries to understand all possibilities of sugammadex.
In conclusion, in our study, a dose of 2 mg/kg sugammadex was found to be efficient and safe for reversing the NMB when first response in the TOF is reached, after a continuous infusion of rocuronium without increasing the risk of residual recurarization. Future studies are required to determine any possible readjustments of doses and the consequent risks that lower doses of sugammadex may cause in the reversal of NMB. In the future, with the absence of plasma level of drugs, neuromuscular monitoring will be essential in the daily anesthetic practice, especially when rocuronium is given as a continuous infusion for the immediacy of its results.
The authors wish to thank Martin Hadley-Adams, Alyssa Verret and Carmen López Soto for translating the manuscript.
The introduction of sugammadex to antagonize non-depolarising neuromuscular blockade (NMB) has led to significant changes in anaesthesia practice. Residual effects of neuromuscular block can have significant clinical consequences and can cause critical respiratory events. The superiority of sugammadex (vs neostigmine) for reversing neuromuscular block has now been well established.
Sugammadex should be dosed according to the prescriber information issued by the manufacturer. The provider recommends a dose of 4 mg/kg if recovery has reached at least 1-2 PTCs, and a dose of 2 mg/kg sugammadex when spontaneous recovery has occurred up to at least the reappearance of second response in the train-of-four (TOF), but they don´t define the ideal dose of sugammadex for reversal the NMB when first response in the TOF is reached.
This study suggests that a dose of 2 mg/kg sugammadex (vs 4 mg/kg) is enough for the recovery of NMB induced by a continuous infusion of rocuronium in patients who kept anaesthetized with sevoflurane when first response in the TOF is reached. This lower dose did not have any clinically relevant recovery time augmentation or increased risk of residual recurarization. The authors have not been able to observe more adverse events in the patients.
A dose of 2 mg/kg sugammadex was found to be efficient and safe for reversing the NMB when first response in the TOF is reached without increasing the risk of residual recurarization. Future studies are required to determine any possible readjustments of doses and the consequent risks that lower doses of sugammadex may cause in the reversal of NMB. In the future, quantitative neuromuscular monitoring is mandatory and increased postoperative vigilance is required in order to identify the problems of incomplete reversal.
After injection of a nondepolarizing neuromuscular blocking drug in a dose sufficient for smooth tracheal intubation, TOF recording demonstrates three phases of NMB: intense, moderate or surgical blockade, and recovery. Intense NMB is also called the period of no response because no response to TOF or single-twitch stimulation occurs. Although this phase it is not possible to determinate exactly how long intense NMB will last, correlation does exist between PTC stimulation and the time to reappearance of the first response to TOF stimulation. Moderate blockade begins when the first response to TOF stimulation appears. This phase is characterized by a gradual return of the four responses to TOF stimulation. The return of the fourth response in the TOF heralds the recovery phase. Satisfactory recovery from NMB has not occurred until the TOF ratio is > 0.9.
This is a study comparing the efficacy and safety of two different doses of sugammadex. The paper is well written and designed.
P- Reviewer: Cosmo GD, Higa K, Kvolik S, Luchetti M S- Editor: Ji FF L- Editor: A E- Editor: Lu YJ
| 1. | Geldner G, Niskanen M, Laurila P, Mizikov V, Hübler M, Beck G, Rietbergen H, Nicolayenko E. A randomised controlled trial comparing sugammadex and neostigmine at different depths of neuromuscular blockade in patients undergoing laparoscopic surgery. Anaesthesia. 2012;67:991-998. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 106] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 2. | Berg H, Roed J, Viby-Mogensen J, Mortensen CR, Engbaek J, Skovgaard LT, Krintel JJ. Residual neuromuscular block is a risk factor for postoperative pulmonary complications. A prospective, randomised, and blinded study of postoperative pulmonary complications after atracurium, vecuronium and pancuronium. Acta Anaesthesiol Scand. 1997;41:1095-1103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 476] [Cited by in RCA: 404] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 3. | Murphy GS, Brull SJ. Residual neuromuscular block: lessons unlearned. Part I: definitions, incidence, and adverse physiologic effects of residual neuromuscular block. Anesth Analg. 2010;111:120-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 288] [Cited by in RCA: 307] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 4. | Bom A, Bradley M, Cameron K, Clark JK, Van Egmond J, Feilden H, MacLean EJ, Muir AW, Palin R, Rees DC. A novel concept of reversing neuromuscular block: chemical encapsulation of rocuronium bromide by a cyclodextrin-based synthetic host. Angew Chem Int Ed Engl. 2002;41:266-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 5. | Epemolu O, Bom A, Hope F, Mason R. Reversal of neuromuscular blockade and simultaneous increase in plasma rocuronium concentration after the intravenous infusion of the novel reversal agent Org 25969. Anesthesiology. 2003;99:632-637; discussion 6A. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 107] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 6. | Sacan O, White PF, Tufanogullari B, Klein K. Sugammadex reversal of rocuronium-induced neuromuscular blockade: a comparison with neostigmine-glycopyrrolate and edrophonium-atropine. Anesth Analg. 2007;104:569-574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 148] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 7. | Blobner M, Eriksson LI, Scholz J, Motsch J, Della Rocca G, Prins ME. Reversal of rocuronium-induced neuromuscular blockade with sugammadex compared with neostigmine during sevoflurane anaesthesia: results of a randomised, controlled trial. Eur J Anaesthesiol. 2010;27:874-881. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 170] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 8. | Mirakhur RK. Sugammadex in clinical practice. Anaesthesia. 2009;64 Suppl 1:45-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 75] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 9. | Lowry DW, Mirakhur RK, McCarthy GJ, Carroll MT, McCourt KC. Neuromuscular effects of rocuronium during sevoflurane, isoflurane, and intravenous anesthesia. Anesth Analg. 1998;87:936-940. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 26] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 10. | Pühringer FK, Gordon M, Demeyer I, Sparr HJ, Ingimarsson J, Klarin B, van Duijnhoven W, Heeringa M. Sugammadex rapidly reverses moderate rocuronium- or vecuronium-induced neuromuscular block during sevoflurane anaesthesia: a dose-response relationship. Br J Anaesth. 2010;105:610-619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 57] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 11. | Duvaldestin P, Kuizenga K, Saldien V, Claudius C, Servin F, Klein J, Debaene B, Heeringa M. A randomized, dose-response study of sugammadex given for the reversal of deep rocuronium- or vecuronium-induced neuromuscular blockade under sevoflurane anesthesia. Anesth Analg. 2010;110:74-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 112] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 12. | Veiga-Ruiz G, Domínguez N, Orozco J, Janda M, Hofmockel R, Alvarez-Gómez JA. [Efficacy of sugammadex in the reversal of neuromuscular blockade induced by rocuronium in long-duration surgery: under inhaled vs. intravenous anesthesia]. Rev Esp Anestesiol Reanim. 2009;56:349-354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 13. | Viby-Mogensen J. Dose-response relationship and time course of action of rocuronium bromide in perspective. Eur J Anaesthesiol Suppl. 1994;9:28-32. [PubMed] |
| 14. | Rex C, Wagner S, Spies C, Scholz J, Rietbergen H, Heeringa M, Wulf H. Reversal of neuromuscular blockade by sugammadex after continuous infusion of rocuronium in patients randomized to sevoflurane or propofol maintenance anesthesia. Anesthesiology. 2009;111:30-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 33] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 15. | Fuchs-Buder T, Claudius C, Skovgaard LT, Eriksson LI, Mirakhur RK, Viby-Mogensen J. Good clinical research practice in pharmacodynamic studies of neuromuscular blocking agents II: the Stockholm revision. Acta Anaesthesiol Scand. 2007;51:789-808. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 452] [Cited by in RCA: 473] [Article Influence: 24.9] [Reference Citation Analysis (0)] |
| 16. | Mauchly JW. Significance Test for Sphericity of a Normal n-Variate Distribution. Ann Math Statist. 1940;11:204-209. [RCA] [DOI] [Full Text] [Cited by in Crossref: 619] [Cited by in RCA: 617] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 17. | Pillai KCS. Some New Test Criteria in Multivariate Analysis. Ann Math Statist. 1955;26:117-121. [RCA] [DOI] [Full Text] [Cited by in Crossref: 230] [Cited by in RCA: 232] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 18. | McCoy EP, Mirakhur RK, Maddineni VR, Wierda JM, Proost JH. Pharmacokinetics of rocuronium after bolus and continuous infusion during halothane anaesthesia. Br J Anaesth. 1996;76:29-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.0] [Reference Citation Analysis (0)] |
| 19. | Naguib M, Kopman AF, Lien CA, Hunter JM, Lopez A, Brull SJ. A survey of current management of neuromuscular block in the United States and Europe. Anesth Analg. 2010;111:110-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 225] [Cited by in RCA: 233] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 20. | Grayling M, Sweeney BP. Recovery from neuromuscular blockade: a survey of practice. Anaesthesia. 2007;62:806-809. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 85] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 21. | Merck Sharp and Dohme (MSD). Bridion® Sugammadex. Labelling. Available from: http: //www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000885/WC500052310.pdf. |
| 22. | Fuchs-Buder T, Meistelman C, Raft J. Sugammadex: clinical development and practical use. Korean J Anesthesiol. 2013;65:495-500. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 50] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 23. | Plaud B, Debaene B, Donati F, Marty J. Residual paralysis after emergence from anesthesia. Anesthesiology. 2010;112:1013-1022. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 152] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 24. | Jellish WS, Brody M, Sawicki K, Slogoff S. Recovery from neuromuscular blockade after either bolus and prolonged infusions of cisatracurium or rocuronium using either isoflurane or propofol-based anesthetics. Anesth Analg. 2000;91:1250-1255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.0] [Reference Citation Analysis (1)] |
| 25. | McDonagh DL, Benedict PE, Kovac AL, Drover DR, Brister NW, Morte JB, Monk TG. Efficacy, safety, and pharmacokinetics of sugammadex for the reversal of rocuronium-induced neuromuscular blockade in elderly patients. Anesthesiology. 2011;114:318-329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 87] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 26. | Suzuki T, Kitajima O, Ueda K, Kondo Y, Kato J, Ogawa S. Reversibility of rocuronium-induced profound neuromuscular block with sugammadex in younger and older patients. Br J Anaesth. 2011;106:823-826. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 36] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 27. | Shields M, Giovannelli M, Mirakhur RK, Moppett I, Adams J, Hermens Y. Org 25969 (sugammadex), a selective relaxant binding agent for antagonism of prolonged rocuronium-induced neuromuscular block. Br J Anaesth. 2006;96:36-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 125] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 28. | Pühringer FK, Rex C, Sielenkämper AW, Claudius C, Larsen PB, Prins ME, Eikermann M, Khuenl-Brady KS. Reversal of profound, high-dose rocuronium-induced neuromuscular blockade by sugammadex at two different time points: an international, multicenter, randomized, dose-finding, safety assessor-blinded, phase II trial. Anesthesiology. 2008;109:188-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 165] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 29. | Chambers D, Paulden M, Paton F, Heirs M, Duffy S, Craig D, Hunter J, Wilson J, Sculpher M, Woolacott N. Sugammadex for the reversal of muscle relaxation in general anaesthesia: a systematic review and economic assessment. Health Technol Assess. 2010;14:1-211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 54] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 30. | Fuchs-Buder T, Meistelman C, Schreiber JU. Is sugammadex economically viable for routine use. Curr Opin Anaesthesiol. 2012;25:217-220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 31. | Eleveld DJ, Kuizenga K, Proost JH, Wierda JM. A temporary decrease in twitch response during reversal of rocuronium-induced muscle relaxation with a small dose of sugammadex. Anesth Analg. 2007;104:582-584. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 99] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 32. | Fuchs-Buder T. Less is not always more: sugammadex and the risk of under-dosing. Eur J Anaesthesiol. 2010;27:849-850. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 1.0] [Reference Citation Analysis (0)] |