INTRODUCTION
Asthma is common in childhood. This respiratory disease is characterized by persistent inflammation of the airways, which is clinically manifested in the form of recurring cough, shortness of breath, wheezing and chest retraction. These episodes are associated with the obstruction of airflow, which is partially reversible and occurs mainly in the morning and at night[1,2].
Controlling the disease is the main objective of asthma management. Such control refers to the most recent clinical manifestations (previous four weeks) with regard to symptoms, limitations to physical activity, the need for a b2 agonist and the intensity of airflow limitation as well as the reduction of future risks. Asthma is therefore classified as controlled, partially controlled or uncontrolled. Addressing future risks regards reducing the instability of asthma and exacerbations, the loss of lung function and the adverse effects of treatment[2,3].
The incidence of asthma has doubled in the last 20 years due to the actual increase in the number of cases as well as better recognition of the disease on the part of the medical community. The difficulty in comparing epidemiological data from different countries or even different regions within the same country motivated the International Study of Asthma and Allergies in Childhood, which employed a simple, validated, self-administered questionnaire with a small number of items. The authors of the study evaluated 304796 children aged six to seven years from 42 countries and 463801 adolescents aged 13 to 14 years at 155 centers in 56 countries and distinguished three categories of countries based on the prevalence rates of asthma: weak (less than 5%), medium (5% to 6%) and strong (greater than 10%). Brazil was classified in eight place, with a prevalence rate of 20%[4,5].
Despite the difficulties in diagnosing asthma in children, there is evidence that half of all cases are diagnosed by three years of age and 80% are diagnosed by six years of age. Moreover, one third of the initial symptoms begin before the child has completed one year of life. Although 50% of children with asthma in Latin America exhibit daily symptoms and arousals from sleep, only 10% regularly use medication to control asthma and only treat attacks with an inhaler, while only 13% employ preventive measures and the control of exacerbations[6].
The aim or the present review was to describe important neural, inflammatory and functional mechanisms that affect children with asthma.
PHYSIOPATHOLOGY AND INFLAMMATION IN ASTHMA
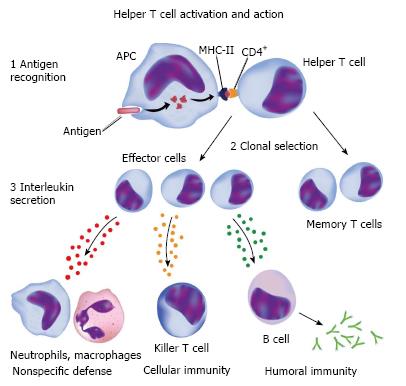
Asthma is an inflammatory disease involving the participation of mastocytes, eosinophils, T cells and dendritic cells. Among the different phenotypes, atopic asthma is the most common and is characterized by an increase in eosinophils and total immunoglobulin E (IgE), with the considerable participation of mastocytes and their products in events during the acute phase. These cells also participate in the chronic inflammatory process and bronchial hyperresponsiveness, along with macrophages, eosinophils and T lymphocytes[2]. Macrophages can either increase or diminish the inflammatory process, depending on the stimulus. Alveolar macrophages normally suppress lymphocyte function, but this function may be altered in individuals with asthma following exposure to a triggering factor[7] (Figure 1).
Figure 1 Cellular and humoral response to an antigen.
© Can Stock Photo Inc./[Alila].
Eosinophil infiltrate is a physiopathological characteristic of the airways in individuals with asthma and contributes to the differentiation of this disease from other inflammatory conditions. Eosinophils are seen as beneficial in asthma due to their function in inactivating histamine and leukotrienes. However, eosinophils are also involved in injurious processes of the airways tissues, contributing to the development of bronchial hyperresponsiveness.
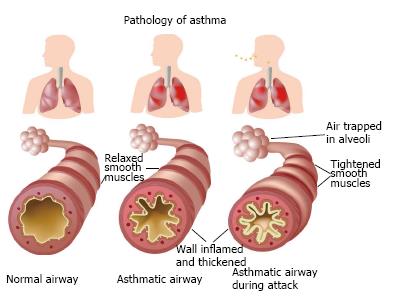
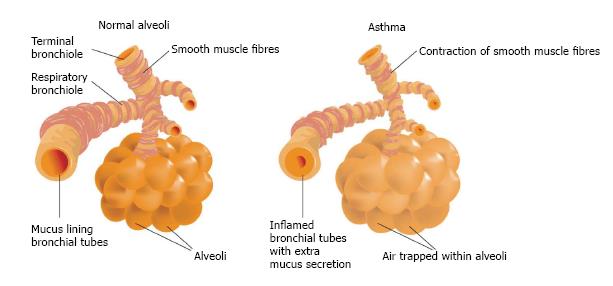
Chronic inflammation results in the obstruction of distal airways due to the accumulation of secretion and cell debris, the contraction of bronchial smooth muscles, thickening of the epithelial basement membrane and bronchial wall edema[8]. The most striking characteristic of asthma is persistent inflammation of the airways even when the child is not in the throes of an attack. The degree of inflammation is associated with bronchial hyperresponsiveness and symptoms. Chronic inflammation is caused by an imbalance between pro-inflammatory and anti-inflammatory mechanisms[9]. The persistence of this inflammatory condition over the years combined with frequent acute attacks can exert a negative influence on lung function (Figure 2).
Figure 2 Bronchi in normal conditions, asthma and during asthma attack.
© Can Stock Photo Inc./[Alila].
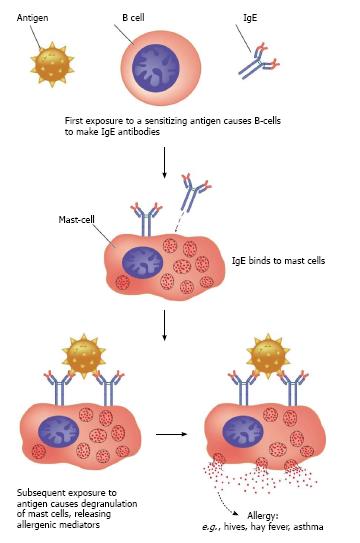
Inflammation has been the focus of treatment for asthma in the last 20 years. Prior to the 1980s, asthma was seen as recurrent wheezing that responded to b2 agonists. The inflammatory reaction begins with contact between an allergen (pollutant or virus) and dendritic cells, which activate mastocytes that, in turn, release IgE specific to given allergens. This initial process leads to the release of different mediators. The consequence of this initial phase (four to six hours) of the inflammatory cascade is clinically manifested as bronchospasms[10]. In the late phase of the inflammatory reaction, granulocytes are released by bone marrow and migrate to the target organ (lungs), causing tissue injury and the release of toxins[11,12]. Inflammatory cytokines stimulate the formation of inducible nitric oxide synthase (iNOS). At high concentrations, NO is the major contributor to the inflammatory process in asthma[13] (Figure 3).
Figure 3 Activation of mast cells and release of IgE.
© Can Stock Photo Inc./[Blambs].
Cell apoptosis is maintained activated at all moments of the inflammatory cascade, possibly reducing the reserve of cells that could cease this process. Asthma is characterized by a reduction in apoptotic potential and consequent perpetuation of the inflammatory process. Moreover, the molecules involved in the tissue repair process seem to be relatively ineffective. These events results in chronic inflammation and the structural remodeling of the airways. All these factors seem to be the basis for bronchial hyperreactiveness and other symptoms[10-12].
Differences between childhood and adult asthma
Childhood is the period of greatest incidence of asthma, however the identification of these children still problematic. The clinical syndrome that is recognized as asthma fails to develops in all children and infants with wheezing (in less than half as already stated earlier).
Another difficulty in the identification of asthma in childhood is related to pulmonary function test as well as the documentation of the obstruction and its reversibility objectively and quantitatively in small children. The challenge is to detect by means of non-invasive biomarkers in infants and pre school children who can become asthmatic by facilitating in this way the clinical control and reducing the morbidity of the disease in adulthood.
What is known so far that insults occurred postnatally as aspiration, viral infections or allergic sensitization may alter the airway neural control for long periods[13].
Respiratory infection and asthma in childhood
Various pathogens may cause wheezing in childhood, in particular, respiratory syncytial virus (RSV). Bronchiolitis from RSV increases the risk for recurrent wheezing (more than 3 episodes of wheezing per year) and not persistent (up to 3 episodes of wheezing per year); However, the risk gradually decreases with age and becomes not significant to 13 years of age[14]. These data indicate that RSV infections may contribute substantially to the risk of recurrent wheezing and possibly asthma in childhood and also suggest that other co-factors (for example: genetic, environmental and development) contribute to the onset or severity of asthma over time.
Respiratory infections and asthma are closely related and children are very susceptible to these infections, but less than half of them develop recurrent wheezing.
Some types of respiratory infection in infancy can stimulate Th1 cells and as regards the pathophysiology of childhood asthma in the balance and the way in which this balance Th1/Th2 is reached in this age group still generate doubts. What is known is that the non atopic child have a reduction of Th2 in the first year of life, already in atopic child Th2 response is associated with the production of IFN-γ in the neonatal period[14].
Atopic asthma is characterized by this imbalance Th1/Th2. Th2 cells when stimulated produces interleukin 4 (IL4) and IL13 that induce the production of IgE by B cells. The IL9 is also produced by Th2 stimulates the proliferation of mast cells which in turn starts the production of histamine, leukotrienes and prostaglandins that lead to an additional activation of eosinophils, basophils and Th2[15].
The endothelial lesion caused by viral infection leads to increased airway permeability facilitating the exposure of allergens to cells in the nerve endings promoting a neurogenic inflammation. Severe respiratory infections increase the recruitment of eosinophils to the airways bringing the risk of persistent asthma in childhood[13].
Fraction of exhaled nitric oxide
Gustafsson et al[16] (1991) were the first researchers to isolate the nitric oxide (NO) molecule. According to the authors, NO is formed by the action of NO synthase on the semi-essential amino acid L-arginine. Two basic isoforms are produced: constitutive and inducible[17]. NO synthase performs a number of biological functions, such as neurotransmission, vasodilatation and bronchodilatation as well as playing a role in the immune system. The type I constitutive isoform (epithelial) promotes vasodilatation and the type II constitutive isoform (neuronal) is responsible for the transmission of stimuli from the central nervous system and autonomic nervous system through the non-adrenergic, non-cholinergic pathway[17,18]. The inducible isoform (iNOS) is stimulated with the perpetuation of the inflammatory process and contributes to this process due to its pro-inflammatory activity. When activated, this isoform promotes an increase in the production of secretion in the airways and a large number of inflammatory cells, favoring necrosis of the ciliated tissue[7,19]. The constitutive isoforms are produced in small amounts that are not detectable in exhaled breath and perform their biological and physiological roles, whereas iNOS is produced in large amounts, has cytotoxic effects and is detectable in exhaled breath (FeNO)[20,21].
FUNCTIONAL CAPACITY
Fear of shortness of breath often makes children with asthma avoid physical exercise, leading to a sedentary lifestyle, psychological and postural problems as well as a poor quality of life. Studies have demonstrated that children with moderate to severe uncontrolled asthma exhibit chronic physical deconditioning and reduced cardiopulmonary capacity[22,23]. Villa et al[24] (2011) evaluated children with persistent moderate to severe asthma and found that those with severe asthma exhibited diminished lower limb endurance. Thus, controlled physical activities are needed to prevent the impairment of cardiopulmonary capacity and physical fitness.
Repeated physical activities with varying intensity that last only a few seconds and are intercalated with short rest periods are more appropriate for children. Besides preferring spontaneous activities with a recreational components and considerable variety, children explore the anabolic effects of physical exercise more[25]. Thus, physically-based play activities are indicated for children with asthma. While 40% to 90% of children with asthma exhibited exercise-induced bronchospasms, the regular practice of physical activity is able to improve this symptom, often with no direct impact on lung function[2].
Advances in technology have facilitated the performance of movements and physical activity[26-28]. Interactive video games in the last ten years have contributed to energy expenditure among otherwise sedentary children, with the possible applications regarding lung rehabilitation among children with asthma. However, previous studies have only employed this resource for the training of children without lung diseases[29]. Thus, there is a need for scientific evidence of the benefits of interactive video games for the improvement of physical fitness in this population.
Inflammation and physical exercise
A persistent inflammatory state is a common trait of chronic diseases. Inflammation is indicated by the high concentration of inflammatory markers, such as IL6, tumor necrosis factor alpha (TNFα) and C-reactive protein in the blood plasma[30]. Physical exercise has an anti-inflammatory effect and regular practice seem to offer protection against the development and aggravation of chronic diseases. While children with asthma tend to avoid physical exertion, the practice of physical exercise can be beneficial to this population. However, there are no specific guidelines with regard to the type, intensity, duration and frequency of training[31].
Three mechanisms seem to explain the anti-inflammatory effect of physical exercise. The first is the reduction in visceral fat, as excess fat contributes to the production of pro-inflammatory adipokines, such as TNF and leptin, as well as the reduction in adiponectin. The second mechanism is the increase in the production and release of anti-inflammatory cytokines, such as IL6, stemming from muscle contractions caused by myosin, which induces different metabolic effects, such as lypolysis and the oxidation of fat, as well as contributing to the homeostasis of glucose during physical exercise. The third mechanism is the reduction in the expression of receptors of monocytes and macrophages, which have a lower inflammatory response to endotoxins in physically active individuals[30-33].
The effect of myokines (IL6 released by the contraction of skeletal muscle) regulates the release of TNFa, resulting in a protective effect[34] (Tilg et al[34] 1997). Moreover, IL6 stimulates the release of the anti-inflammatory interleukins 10 and 1ra. IL10 inhibits the production of interleukins 1a and 1b as well as the induction of iNOS[35]. This mechanism may explain the likely effect of physical exercise on changes in the concentration of FeNO.
AUTONOMIC NERVOUS SYSTEM AND CONNECTIONS WITH RESPIRATORY AND CARDIOVASCULAR SYSTEMS
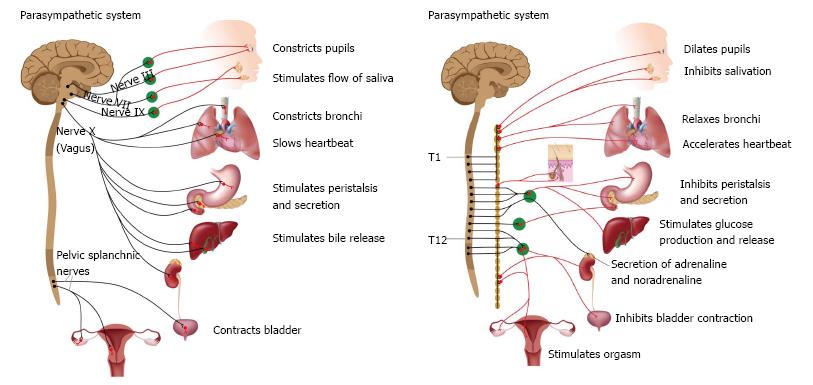
The respiratory and cardiovascular systems are intrinsically linked and the autonomic nervous system (ANS) is one of the pathways that connect these systems[36] (Figure 4).
Figure 4 Autonomic nervous system subdivision.
© Can Stock Photo Inc./[Alila].
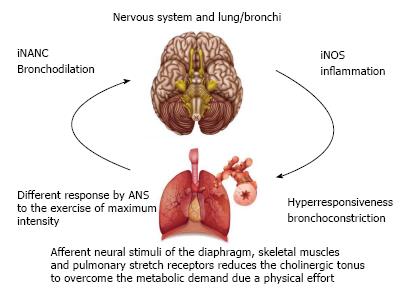
Moreover, the ANS exerts an important influence on the pathogenesis and control of asthma[37]. Afferent pulmonary nerve pathways regulate cholinergic tone, which is increased when the respiratory rate is increased. This mechanism occurs in response to physiological and physiopathological stimuli. A physiological response occurs when the peripheral respiratory muscles and pulmonary stretch receptors send afferent stimuli to the central nervous system during physical exertion, which results in the reduction in cholinergic tone as well as bronchial dilatation to compensate for the increase in ventilation demand. This mechanism is altered in individuals with asthma[37].
Understanding the relationships between the ANS and respiratory system may help clarify the causes of cardiovascular disorders with a pulmonary origin. From the neuroanatomic standpoint, afferent and efferent neural pathways of the respiratory and cardiovascular systems converge in common regions. Afferent pathways converge in the solitary tract nucleus and efferent pathways converge in the nucleus ambiguous, which is responsible for the generation of the respiratory rate and heart rate[36].
In individuals with asthma, the inflammatory process is increased during periods of exacerbation. The increase in vagal tone during stable periods of the disease may be explained by an attempt to maintain inflammatory control. The ANS exerts an influence on the relaxation and constriction of the smooth muscles of the bronchioles. Relaxation occurs through either the activation of beta receptors of the sympathetic pathway or the activation of the non-adrenergic, non-cholinergic and intestinal peptide pathways. Constriction is mediated by either sympathetic receptors or the vagal cholinergic pathway[37].
Heart rate variability has been used as a measure of vagal autonomic activity and neuroimmunomodulation. A number of studies have addressed the influence of respiration on heart rate variability. Chronic respiratory diseases, such as asthma, demonstrate a link between the inflammatory process and the immune reaction as well as progression with cardiovascular consequences that can contribute to the increase in illness and mortality rates[36].
Asthma and autonomic modulation
The activation of the autonomic mechanism in the respiratory system is due to the reflex response of receptors located in the airways, regulating bronchial contractility[38-41]. Autonomic dysfunction is associated with asthma[42,43], with an increase in bronchial sensitivity to cholinergic constrictors and possibly a reduction in sensitivity to adrenergic b2 bronchodilators. Besides its essential role in the cardiovascular system, the ANS plays an important role in the regulation of the contraction of bronchial smooth muscles[37].
Asthma severity is directed related to autonomic dysfunction even under conditions in which the patient is not a period of exacerbation. The functions of the ANS in children with asthma also differ in comparison to adults and healthy children[43,44]. This severity is correlated with the magnitude of vagal activation in the stable phase[43]. During periods of exacerbation, children with persistent moderate asthma experience greater action of the sympathetic nervous system, unlike what occurs in stable periods, which conflicts with the hypothesis of hyperactivity of vagal tone in the acute phase[45].
Neural mechanisms are also responsible for the regulation of inflammation. The activation of the vagus nerve is responsible for the inhibition of the activation of macrophages and the synthesis of TNFα as well as the activation of the reticuloendothelial system for the release of acetylcholine[46].
Autonomic modulation and degree of effort in asthma
As mentioned above, asthma progresses with autonomic abnormality during physical effort. The ANS responds to stimuli send by the muscles, lungs and diaphragm for the withdrawal of vagal tone, resulting in an increase in the diameter of the airways. This is a mechanical response to a neural stimulus that allows enhancing the airflow, with a consequent improvement in gas exchange in response to the increase in metabolic demand[37].
Different degrees of physical stress can trigger changes in autonomic modulation in children with asthma. During physical exertion, the heart rate and cardiac output are increased mainly due to vagal withdrawal stemming from a central command. In the transition from an intense activity involving an increase in heart rate greater than 100 beats per minute, the increase in sympathetic activity is necessary to induce tachycardia as well as increase both heart contractibility and peripheral vascular resistance[47]. During maximum physical effort, this mechanism of vagal withdrawal and sympathetic activation seems to be altered and relatively ineffective in children with asthma. Vagal withdrawal also seems to occur during sub-maximum effort[48] (Figure 5).
Figure 5 Lung and nervous system interaction.
© Can Stock Photo Inc. / [Alexilus and Bluering].
Management of shortness of breath and respiratory failure using noninvasive ventilation
During respiratory distress, children with asthma experience bronchial obstruction and hyperinflation, which predisposes such individuals to respiratory failure. Thus, the aims of the management of severe acute asthma include the correction of hypoxemia, alleviation of the airflow obstruction and the reduction of the inflammatory process through medications. The therapeutic goal is to reduce respiratory work and optimize gas exchange. Continuous positive airway pressure (CPAP), which is a form of noninvasive ventilation (NIV), is used to reduce the work of respiratory muscles imposed by hyperinflation of the lungs and produces a change in autonomic modulation stemming from the increase in intra-thoracic pressure[49,50] (Figure 6).
Figure 6 Hyperinflation.
© Can Stock Photo Inc. / [Blambs].
There is scientific evidence of the efficacy of NIV for an asthma attack. Gupta et al[51] (2010) report improved respiratory work, fast recovery of lung function as well as reductions in inhaler dose, stay in an intensive care unit and the duration of hospitalization among adults with acute asthma treated with bi-level NIV. CPAP caused changes in both alveolar and intra-thoracic pressures and the activation of pulmonary stretch receptors affects autonomic modulation[37,50]. In a study involving an experimental model, Xue et al[52] (2011) found that the increase in intra-thoracic pressure leads to a reduction in bronchial hyperresponsiveness, which persists for up to 24 h following the removal of CPAP. Busk et al[53] (2013) found a reduction in bronchial hyperresponsiveness in adults with asthma after seven days of treatment and consider CPAP to be an important non-pharmacological tool for the treatment of this condition.
Parasympathetic nerve stimulation results in both the contraction and relaxation of the bronchial musculature. The non-adrenergic, non-cholinergic pathway (bronchodilatation) is stimulated by pulmonary stretch receptors. Contraction is mediated by acetylcholine and relaxation is mediated by transmitters of the non-adrenergic, non-cholinergic pathway. Cholinergic tone is extremely sensitive to the ventilitory mechanism and is reduced when the respiratory rate is reduced[37]. Thus, CPAP may achieve its positive effects by reducing the respiratory rate and stimulating the non-adrenergic, non-cholinergic pathway through pulmonary stretch receptors. The acute effects of CPAP on autonomic modulation have been demonstrated in other pathological conditions, but there is a lack of scientific evidence regarding the benefits of this method in patients (especially children) with asthma[50].
In a study conducted by our research group[54] involving the evaluation of clinical variables and the ANS in children in the throes of an asthma attack, the activation of vagal tone was found with the administration of CPAP. Parasympathetic activation stimulates both contraction and relaxation of the smooth muscles of the airways. Cholinergic tone is reduced with positive end-expiratory pressure and the reduction in the respiratory rate[37,55].
The findings suggest that the non-cholinergic parasympathetic pathway is activated by the administration of CPAP due to the significant increase in peak flow, which continues after the removal of NIV, suggesting a bronchodilatation response as well as the mechanical stimulation of the opening of the airways. Pulmonary stretch and the activation of the non-cholinergic pathway is believed to assist in the inhibition of the cholinergic bronchoconstriction pathway[37].