Published online Mar 16, 2015. doi: 10.12998/wjcc.v3.i3.285
Peer-review started: July 31, 2014
First decision: November 18, 2014
Revised: December 11, 2014
Accepted: December 29, 2014
Article in press: December 31, 2014
Published online: March 16, 2015
Processing time: 224 Days and 19.8 Hours
Asthma and obesity are epidemiologically linked; however, similar relationships are also observed with other markers of the metabolic syndrome, such as insulin resistance and dyslipidemia, which cannot be accounted for by increased body mass alone. Obesity appears to be a predisposing factor for the asthma onset, both in adults and in children. In addition, obesity could make asthma more difficult to control and to treat. Although obesity may predispose to increased Th2 inflammation or tendency to atopy, other mechanisms need to be considered, such as those mediated by hyperglycaemia, hyperinsulinemia and dyslipidemia in the context of metabolic syndrome. The mechanisms underlying the association between asthma and metabolic syndrome are yet to be determined. In the past, these two conditions were believed to occur in the same individual without any pathogenetic link. However, the improvement in asthma symptoms following weight reduction indicates a causal relationship. The interplay between these two diseases is probably due to a bidirectional interaction. The purpose of this review is to describe the current knowledge about the possible link between metabolic syndrome and asthma, and explore potential application for future studies and strategic approaches.
Core tip: Asthma is a complex syndrome that encompasses multiple phenotypes. The relationship with obesity has been addressed in the past; however, the underlying mechanism of such a relationship seems to be more complex, and not explained by the body weight alone. The metabolic syndrome carries a condition of systemic inflammation that could potentially explain the influence on asthma onset and severity. This is a rather unexplored area that could potentially open new scenario in the diagnostic algorithm and in the strategic approach, with a more comprehensive assessment of the disease.
- Citation: Serafino-Agrusa L, Spatafora M, Scichilone N. Asthma and metabolic syndrome: Current knowledge and future perspectives. World J Clin Cases 2015; 3(3): 285-292
- URL: https://www.wjgnet.com/2307-8960/full/v3/i3/285.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v3.i3.285
Asthma is among the most common chronic diseases worldwide. The disease is poorly controlled despite available therapies in a large proportion of patients[1], with long-term impairment and disability[2-4]. Among factors impairing the control of symptoms and the lack of response to treatment, obesity is to be taken into account, as stated by recent guidelines[5].
It is well recognized that obesity and asthma are epidemiologically linked[6-9]. This relationship is also observed between asthma and other markers of the metabolic syndrome, such as insulin resistance and hypertension that cannot be accounted for by increased body mass alone[9-12]. The World Health Organization has reported that obesity has dramatically increased during the last few decades. In 2009-2010, more than one-third of United States adults (35.7%) were obese[13]. In this scenario, an estimated 300000 deaths per year are directly attributable to obesity, mainly due to heart diseases, diabetes, cancer, obstructive sleep apnea syndrome (OSAS), arthritis, and psychological disturbances, leading to the concept that obesity represents a risk factor for several pathologies in different clinical conditions[14]. In this regard, overweight and obesity have been demonstrated to be associated in a dose-dependent fashion with the risk of having asthma[15], and obesity appears to be a predisposing factor for the asthma onset, both in adults and in children, as assessed by several cross-sectional studies[16]. In addition, obesity could make asthma more difficult to control and to treat; interestingly, weight-loss interventions in overweight severe asthmatic patients have shown substantial improvements in the clinical status, lung function, symptoms, and overall asthma control[8,17,18]. However, the mechanism linking obesity and asthma is still a controversial issue.
The obese-asthma phenotype is characterized by a paucity of airway inflammation. Although obesity may predispose to increased Th2 inflammation or tendency to atopy, other mechanisms that are independent of inflammatory infliltrates need to be considered, such as hyperglycaemia, hyperinsulinemia and dyslipidemia in the context of metabolic syndrome. Metabolic syndrome is defined as a syndrome that involves three of the following characteristics: dyslipidemia (high levels of apoB lipoproteins and triglycerides, and/or low high density lipoprotein cholesterol), an impaired fasting glucose metabolism, hypertension or central obesity[19-21]. Metabolic syndrome is directly involved in the increased prevalence of coronary heart disease, atherosclerotic diseases, and diabetes mellitus type 2[20-22]. Other metabolic abnormalities have been reported in patients with metabolic syndrome (chronic proinflammatory and prothrombotic states, liver disease and sleep apnea)[20-22]. In the literature, some authors consider that the aforementioned criterion is a combination of risk factors rather than a specific syndrome[23]. On the other hand, epidemiological data reveals that there is a high prevalence of metabolic syndrome in both childhood and young adulthood, and pattern seems to be related to several inflammatory diseases including asthma[22].
In obese individuals, the risk for asthma in overweight and obese individuals is increased and does not differ with gender[24,25]. In a recent report, Dandona et al[26] showed that in obese asthma patients, with or without type 2 diabetes, there is an increased expression of pro-inflammatory mediators. Following gastric bypass surgery and weight loss, the expression of the aforementioned mediators and plasma metabolites fall significantly suggesting that the pro-inflammatory effect of obesity can be downregulated upon adipose tissue reduction. Assad et al[27] recently showed that BMI predicts asthma in women more than metabolic sybndrome[28], however, Agrawal et al[29] suggested that calculation of parameters was conducted on entirely different scales, thereby limiting comparison of strength. In another study, Brumpton et al[11] evaluated the associations of metabolic syndrome with the cumulative incidence of asthma in adults in 23245 individuals after an 11 years follow up (Nord-Trøndelag Health Study 1999-2008), showing that metabolic syndrome predisposes to. In a large mendelian randomization study, Granell et al[30] recently found that higher BMI increases the risk of asthma in non-atopic (1.90, 95%CI: 1.19-3.03) and atopic children (1.37, 95%CI: 0.89-2.11).
Obesity-associated asthma is characterized by the presence of neutrophilic airway inflammation, increased morbidity, and resistance to corticosteroids. The mechanisms underlying the relationship between metabolic syndrome and asthma are yet to be fully understood[31]. In the past, these two conditions were believed to occur in the same individual without any pathogenetic link. However, the improvement of in asthma symptoms following weight reduction implies a causal relationship between obesity and asthma[32,33]. The interplay between these two diseases could be based on a bidirectional interaction. For example, obese asthmatics are at higher risk of metabolic syndrome as opposed to obese individuals who do not suffer from asthma, suggesting that asthma per se can increase the risk of developing metabolic syndrome[34]. Similarly, metabolic syndrome has been demonstrated to increase the severity of asthma[35,36]. Recently, changes in the expression of pro-inflammatory mediators such as leptin, IL-6, TNF-α, C-reactive protein and adiponectin have been demonstrated in obese asthmatics[37], implying their potential role in the pathogenesis of obesity-associated asthma. However, due to the paucity of available literature in this area, it appears difficult to draw definite conclusions until additional experimental and epidemiological data are collected.
A cross-sectional study published by Bruno et al[38] recently analyzed the influence of BMI on asthma control in subjects with severe forms of the disease, demonstrating that the optimal state of asthma control is lower in obese than in normal weight and in overweight severe asthmatics and the number of asthma exacerbation episodes are significantly higher in obese than in normal or overweight severe asthmatics. These results may be explained with the inflammatory cascade that the adipose tissue generates. Indeed, the obese state is characterized by the so-called low-grade systemic inflammation[38]. Subcutaneous fat is the major source of fatty acids for the liver, and of free fatty acids in the circulating plasma[39,40]. Subcutaneous fat is related to insulin resistance and to visceral adipose tissue[39,40]. Abdominal subcutaneous fat from obese subjects has been reported to be an inflamed adipose state characterized by tissue macrophage accumulation. This pathologic tissue has been associated with impaired local vasodilatation, peripheral hyperinsulinemia, and insulin resistance[39-41]. Macrophage presence in the tissue is associated with an increase of plasma high-sensitivity C-reactive protein (hsCRP) levels and local amounts of TNF-α[39,40]. The precise mechanism of this event remains to be elucidated; however, adipokines have been proposed as important endocrine mediators since they are related to adipose tissue function and modulation. The following proteins are listed as adipokines, which are envisaged as markers of fat body mass and distribution, as well as tissue function: (1) leptin; (2) adiponectin; (3) ghrelin; (4) vaspin; (5) retinol binding protein 4; (6) apelin; (7) progranulin and MCP-1; (8) omentin; (9) resistin and chemerin; and (10) fetuin[42,43]. Adipose derived hormones may represent molecular links between asthma and inflammation. For example, adiponectin is known to exert anti-inflammatory effects, by inhibiting the eosinophil functions. Indeed, pre-treatment with adiponectin has been demonstrated to diminish the eotaxin-mediated chemotactic responses, by binding the adiponectin receptors AdipoR1 and AdipoR2 that are expressed in human eosinophils[44,45]. In addition, adiponectin has been shown to act as a protector to human bronchial epithelial cell that are involved in the pathogenesis of asthma[46].
High serum levels of resistin have been recently documented in asthmatic children[47]. More important, an in vitro study showed that the resistin production strongly increases in obese patients with severe persistent asthma[48], providing support to the notion that resistin can be depicted as a pro-inflammatory cytokine mainly in severely obese asthmatics. Conversely, high leptin levels are associated with a more severe disease and this even in non-obese asthmatics[49,50]. Leptin can upregulate systemic inflammation and may lead to an impairment in lung function[51]. Increased expression and secretion of pro-inflammatory cytokines such as TNF-α, IL-6, and IL-12 were detected when exposed to leptin[52]. Also, the systemic inflammation may contribute to drive insulin resistance, endothelial dysfunction and high blood pressure conditions. The results of a survey confirmed that leptin levels were highly associated with asthma especially in premenopausal women independent of BMI[51]. Guler et al[53] also suggested that serum leptin concentrations were a predictive factor for asthma in boys, even after adjusting for obesity. Previously, leptin-mediated increased bronchial hyperactivity in obese mice models had been documented[32].
The changes in the adipose tissue in metabolic syndrome favour the production of mediators that modulate the transcription factors. When they are activated by their ligands, they are able to control genes that are involved in intermediate metabolism[54]. In this regard, peroxisome proliferator-activated receptors (PPAR)-gamma agonists may attenuate the upper airway allergic inflammation by induction of Treg cells and inhibiting the proliferation of effector T cells[55].
Diet-induced dyslipidemia may affect the trafficking of immune cells to the lung in diseases such as asthma[56]. In pulmonary physiology, circulating low density and high density lipoproteins (LDL and HDL) are both taken up by specific receptors, and consequently block local cholesterol biosynthesis[56]. Alveolar cholesterol homeostasis has been demonstrated to affect surfactant synthesis in normal lung physiology[56]. Conversely, HDL promotes surfactant production, and lung fibroblast growth. Adipose tissue reduction by diet or surgery, modulation of cholesterol, or glucose metabolism, has an important effect in asthmatic patients. The apolipoprotein E (ApoE)-low density lipoproteic receptor pathway appears to be involved in the pathogenesis of a murine model of allergic asthma[1]. However, the mechanism by which this protein modulates asthma pathogenesis has never been fully elucidated. ApoE has been hypothesized to as negatively modulate the degree of airway hyperresponsiveness[57]. Perhaps, this mechanism can also apply to humans. Low levels of serum HDL were found to be associated with an increased risk for asthma in adolescence[58], and a recent analysis on 85555 adults demonstrated that high triglycerides and low HDL were associated with wheezing, supporting their role as markers of inflammation[59]. Recently, the association between LDL and asthma was investigated by Scichilone et al[60], who found that in mild asthmatics, the least pro-inflammatory LDL (LDL-1 and LDL-2) are lower than in healthy subjects, whereas the most pro-inflammatory (LDL-3 and LDL-4) are higher. In addition, the serum concentrations of LDL-3 (most pro-inflammatory) were negatively associated with lung function, suggesting their contribution to the occurrence of the inflammatory changes of the airways[60]. Insulin excess can also directly alter lung cellular physiology and this would represent a fundamental common molecular link between asthma and metabolic syndrome[61]. There is substantial data that mechanistically links insulin and insulin like growth factor-1 to lung development and function. It is conceivable, although not proven, that hyperinsulinemia may lead to development of lung disease, particularly asthma[62]. Experimental studies that directly address this possibility are strongly advocated.
Recent observations seem to focus on the mitochondrial dysfunction as main mediator of the pathogenetic link between metabolic syndrome and asthma. Defective mitochondrial biogenesis in the adipose tissue is well documented in metabolic syndrome[63-66]. However, the involvement of mitochondria alterations among the risk factors of metabolic syndrome and asthma is unknown[67-72].
Oxidative stress on both pulmonary and extra-pulmonary inflammation in obesity may play a major role[73,74]. Oxidative stress is characterized by increased reactive oxygen species (ROS), which induce functional changes of the airways. On this basis, increased oxidative stress may be recognized as a potential mechanism by which obesity results in increased asthma severity. In this regard, the renin angiotensin aldosterone system, a potent inducer of oxidative stress, is often activated in patients with metabolic syndrome, and results in increased levels of angiotensin II. Angiotensin II seems to be able to determine bronchial hyperresponsiveness[75] and airway remodelling[76]; however, the mechanisms by which this occurs are not yet fully understood.
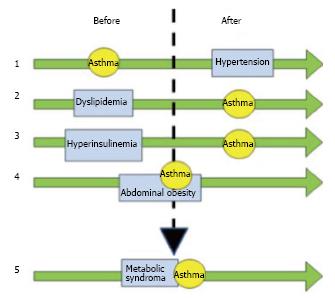
Figure 1 describes the temporal and causal relationships between asthma and features of metabolic syndrome. The role of lipoproteins in the pathogenesis of asthma pathogenesis supported the use of statins in asthmatic patients[77-83]; however, there are still some controversies[84-86]. Even though the aim of statin use in asthmatic patients is related to cholesterol metabolism, most of the reports have highlighted the anti inflammatory properties[77-79,86,87]. An in vitro study showed that lovastatin attenuates the differentiation and proliferation of asthmatic bronchial fibroblasts[85], airway smooth muscle cells[86]. Both simvastatin and atorvastatin treatment reduce inflammatory cells in sputum[86]. The mevalonate-dependent and-independent pathways have been identified as potential opportunities for novel treatments with statins in asthma develop new treatments for asthma[78]. Even though statin therapy could be beneficial for a subgroup of asthmatic patients that are either overweight or obese, similar important advantages can be obtained by diet and exercise[20,26]. Biphosphonates could have a beneficial effect in asthmatic patients; alendronate has been shown to have a protective effect by decreasing eosinophil airway inflammation by chemokine secretion, eotaxin, and down-regulating cytokine secretion induced by Th2 and Th17 cells[88]. Retinoic acid[89], retinoids[90] and fenretidine[91] appear to have a beneficial effect on the inflammatory asthmatic response by decreasing the inflammatory milieu. As a consequence, signal transduction pathways inhibition by these compounds could decrease the occurrence of bronchial constriction. Further studies are required to ascertain the possible beneficial effect of new therapeutic elements to control hypertension and endocrine disorders in asthmatic patients with metabolic syndrome. In patients with severe uncontrolled or non responding asthma[92,93], biological therapies seem to be relevant. Interestingly, chemokines and chemokine receptors, CCR3 and CCR4, have been involved in adipose tissue mass increase and insulin resistance[94,95]. Thus, therapy involving chemokines or chemokine receptor inhibition could potentially provide beneficial effects on asthmatic patients with metabolic syndrome[96]. Finally, a therapeutic option in asthmatic patients with diabetes could be represented by thiazolidinediones, oral diabetes medications that selectively activate PPAR receptor gamma, which have potent anti-inflammatory properties thus reducing the number of exacerbations[97,98].
The scope of the current review is to explore the possible link between metabolic syndrome and asthma. Early endocrine disturbances seem to predispose to severe or difficult to treat asthma. Hypertensive and diabetic patients should be screened for respiratory function in the effort to identify cases of airway hyperreactivity or subclinical asthma. Specifically designed studies are needed to address the influence of metabolic syndrome on asthma occurrence and severity, and to unveil the potential underlying common mechanisms. Future studies will hopefully provide convincing evidence on useful therapeutic schemes that today are still unrevealed.
P- Reviewer: Pereira-Vega A, Popescu FD, Zhao D S- Editor: Ji FF L- Editor: A E- Editor: Lu YJ
| 1. | Ulrik CS, Claudius BK, Tamm M, Harving H, Siersted HC, Backer V, Hellquist B, Dahl R, Høgholm A, Jøhnk IK. Effect of asthma compliance enhancement training on asthma control in patients on combination therapy with salmeterol/fluticasone propionate: a randomised controlled trial. Clin Respir J. 2009;3:161-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 2. | Global Strategy for Asthma Management and Prevention. Global Initiative for Asthma (GINA) 2012. Available from: http: //www.ginasthma.org/. |
| 3. | Program NAEaP. Expert Panel Report 3 (EPR-3): Guidelines for the Diagnosis and Management of Asthma-Summary Report 2007. J Allergy Clin Immunol. 2007;120:S94-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 984] [Article Influence: 51.8] [Reference Citation Analysis (0)] |
| 4. | BTS/SIGN Asthma Guideline; British Guideline for the Management of Asthma. Available from: http: //www.brit-thoracic.org.uk. |
| 5. | Global Strategy for Asthma Management and Prevention. Global Initiative for Asthma (GINA) 2014. Available from: http: //www.ginasthma.org/. |
| 6. | Demoly P, Gueron B, Annunziata K, Adamek L, Walters RD. Update on asthma control in five European countries: results of a 2008 survey. Eur Respir Rev. 2010;19:150-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 94] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 7. | Gershon AS, Wang C, Guan J, To T. Burden of comorbidity in individuals with asthma. Thorax. 2010;65:612-618. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 94] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 8. | Beuther DA, Sutherland ER. Overweight, obesity, and incident asthma: a meta-analysis of prospective epidemiologic studies. Am J Respir Crit Care Med. 2007;175:661-666. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 738] [Cited by in RCA: 802] [Article Influence: 42.2] [Reference Citation Analysis (0)] |
| 9. | Thuesen BH, Husemoen LL, Hersoug LG, Pisinger C, Linneberg A. Insulin resistance as a predictor of incident asthma-like symptoms in adults. Clin Exp Allergy. 2009;39:700-707. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 109] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 10. | Adeyeye OO, Ogbera AO, Ogunleye OO, Brodie-Mens AT, Abolarinwa FF, Bamisile RT, Onadeko BO. Understanding asthma and the metabolic syndrome - a Nigerian report. Int Arch Med. 2012;5:20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 11. | Brumpton BM, Camargo CA, Romundstad PR, Langhammer A, Chen Y, Mai XM. Metabolic syndrome and incidence of asthma in adults: the HUNT study. Eur Respir J. 2013;42:1495-1502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 123] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 12. | Park J, Kim TB, Joo H, Lee JS, Lee SD, Oh YM. Diseases concomitant with asthma in middle-aged and elderly subjects in Korea: a population-based study. Allergy Asthma Immunol Res. 2013;5:16-25. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 26] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 13. | Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of obesity among adults: United States, 2011-2012. NCHS Data Brief. 2013;1-8. [PubMed] |
| 14. | Cazzola M, Segreti A, Calzetta L, Rogliani P. Comorbidities of asthma: current knowledge and future research needs. Curr Opin Pulm Med. 2013;19:36-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 43] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 15. | Weiss ST, Shore S. Obesity and asthma: directions for research. Am J Respir Crit Care Med. 2004;169:963-968. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 124] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 16. | Ford ES. The epidemiology of obesity and asthma. J Allergy Clin Immunol. 2005;115:897-909; quiz 910. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 410] [Cited by in RCA: 408] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 17. | Boulet LP. Asthma and obesity. Clin Exp Allergy. 2013;43:8-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 137] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 18. | McGinley B, Punjabi NM. Obesity, metabolic abnormalities, and asthma: establishing causal links. Am J Respir Crit Care Med. 2011;183:424-425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 19. | Expert Panel on Detection Ea, and Treatment of High Blood Cholesterol in Adults. Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III). JAMA. 2001;285:2486-2497. [PubMed] |
| 20. | Dandona P, Aljada A, Chaudhuri A, Mohanty P, Garg R. Metabolic syndrome: a comprehensive perspective based on interactions between obesity, diabetes, and inflammation. Circulation. 2005;111:1448-1454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 917] [Cited by in RCA: 906] [Article Influence: 43.1] [Reference Citation Analysis (0)] |
| 21. | Kassi E, Pervanidou P, Kaltsas G, Chrousos G. Metabolic syndrome: definitions and controversies. BMC Med. 2011;9:48. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 993] [Cited by in RCA: 1005] [Article Influence: 67.0] [Reference Citation Analysis (0)] |
| 22. | Sovio U, Skow A, Falconer C, Park MH, Viner RM, Kinra S. Improving prediction algorithms for cardiometabolic risk in children and adolescents. J Obes. 2013;2013:684782. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 18] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 23. | Yarnell JW, Patterson CC, Bainton D, Sweetnam PM. Is metabolic syndrome a discrete entity in the general population? Evidence from the Caerphilly and Speedwell population studies. Heart. 1998;79:248-252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 36] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 24. | Jensen ME, Gibson PG, Collins CE, Wood LG. Airway and systemic inflammation in obese children with asthma. Eur Respir J. 2013;42:1012-1019. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 70] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 25. | Garmendia JV, Moreno D, Garcia AH, De Sanctis JB. Metabolic syndrome and asthma. Recent Pat Endocr Metab Immune Drug Discov. 2014;8:60-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 24] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 26. | Dandona P, Ghanim H, Monte SV, Caruana JA, Green K, Abuaysheh S, Lohano T, Schentag J, Dhindsa S, Chaudhuri A. Increase in the mediators of asthma in obesity and obesity with type 2 diabetes: reduction with weight loss. Obesity (Silver Spring). 2014;22:356-362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 44] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 27. | Assad N, Qualls C, Smith LJ, Arynchyn A, Thyagarajan B, Schuyler M, Jacobs DR, Sood A. Body mass index is a stronger predictor than the metabolic syndrome for future asthma in women. The longitudinal CARDIA study. Am J Respir Crit Care Med. 2013;188:319-326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 52] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 28. | Bel EH. Another piece to the puzzle of the “obese female asthma” phenotype. Am J Respir Crit Care Med. 2013;188:263-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 29. | Agrawal A, Prakash YS, Linneberg A. Body mass index is not a stronger predictor than the metabolic syndrome for future asthma in women. Am J Respir Crit Care Med. 2014;189:231-232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 30. | Granell R, Henderson AJ, Evans DM, Smith GD, Ness AR, Lewis S, Palmer TM, Sterne JA. Effects of BMI, fat mass, and lean mass on asthma in childhood: a Mendelian randomization study. PLoS Med. 2014;11:e1001669. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 78] [Cited by in RCA: 89] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 31. | Agrawal A, Prakash YS. Obesity, metabolic syndrome, and airway disease: a bioenergetic problem? Immunol Allergy Clin North Am. 2014;34:785-796. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 32. | Shore SA. Obesity and asthma: lessons from animal models. J Appl Physiol (1985). 2007;102:516-528. [PubMed] |
| 33. | Shore SA. Obesity, airway hyperresponsiveness, and inflammation. J Appl Physiol (1985). 2010;108:735-743. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 124] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 34. | Del-Rio-Navarro BE, Castro-Rodriguez JA, Garibay Nieto N, Berber A, Toussaint G, Sienra-Monge JJ, Romieu I. Higher metabolic syndrome in obese asthmatic compared to obese nonasthmatic adolescent males. J Asthma. 2010;47:501-506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 28] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 35. | Rasmussen F, Hancox RJ, Nair P, Hansen HS, Siersted HC, Nybo M. Associations between airway hyperresponsiveness, obesity and lipoproteins in a longitudinal cohort. Clin Respir J. 2013;7:268-275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 36. | Farah CS, Salome CM. Asthma and obesity: a known association but unknown mechanism. Respirology. 2012;17:412-421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 91] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 37. | Lugogo NL, Bappanad D, Kraft M. Obesity, metabolic dysregulation and oxidative stress in asthma. Biochim Biophys Acta. 2011;1810:1120-1126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 48] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 38. | Bruno A, Pace E, Cibella F, Chanez P. Body mass index and comorbidities in adult severe asthmatics. Biomed Res Int. 2014;2014:607192. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 39. | Sepe A, Tchkonia T, Thomou T, Zamboni M, Kirkland JL. Aging and regional differences in fat cell progenitors - a mini-review. Gerontology. 2011;57:66-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 176] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 40. | Bremer AA, Jialal I. Adipose tissue dysfunction in nascent metabolic syndrome. J Obes. 2013;2013:393192. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 96] [Cited by in RCA: 116] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 41. | Staiano AE, Katzmarzyk PT. Ethnic and sex differences in body fat and visceral and subcutaneous adiposity in children and adolescents. Int J Obes (Lond). 2012;36:1261-1269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 127] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 42. | Blüher M. Clinical relevance of adipokines. Diabetes Metab J. 2012;36:317-327. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 140] [Cited by in RCA: 142] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 43. | Ali Assad N, Sood A. Leptin, adiponectin and pulmonary diseases. Biochimie. 2012;94:2180-2189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 78] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 44. | Yamamoto R, Ueki S, Moritoki Y, Kobayashi Y, Oyamada H, Konno Y, Tamaki M, Itoga M, Takeda M, Ito W. Adiponectin attenuates human eosinophil adhesion and chemotaxis: implications in allergic inflammation. J Asthma. 2013;50:828-835. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 30] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 45. | Yamauchi T, Iwabu M, Okada-Iwabu M, Kadowaki T. Adiponectin receptors: a review of their structure, function and how they work. Best Pract Res Clin Endocrinol Metab. 2014;28:15-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 227] [Cited by in RCA: 259] [Article Influence: 21.6] [Reference Citation Analysis (8)] |
| 46. | Zhu XL, Qin XQ, Xiang Y, Tan YR, Qu XP, Liu HJ. Adipokine adiponectin is a potential protector to human bronchial epithelial cell for regulating proliferation, wound repair and apoptosis: comparison with leptin and resistin. Peptides. 2013;40:34-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 47. | Ziora D, Machura E, Ziora KT, Swietochowska E, Oswiecimska JM, Kasperska-Zajac A. Serum resistin levels are elevated in schoolchildren with atopic asthma. Neuro Endocrinol Lett. 2013;34:212-216. [PubMed] |
| 48. | Rojas-Dotor S, Segura-Méndez NH, Miyagui-Namikawa K, Mondragón-González R. Expression of resistin, CXCR3, IP-10, CCR5 and MIP-1α in obese patients with different severity of asthma. Biol Res. 2013;46:13-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 49. | Leivo-Korpela S, Lehtimäki L, Vuolteenaho K, Nieminen R, Kankaanranta H, Saarelainen S, Moilanen E. Adipokine resistin predicts anti-inflammatory effect of glucocorticoids in asthma. J Inflamm (Lond). 2011;8:12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 35] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 50. | Quek YW, Sun HL, Ng YY, Lee HS, Yang SF, Ku MS, Lu KH, Sheu JN, Lue KH. Associations of serum leptin with atopic asthma and allergic rhinitis in children. Am J Rhinol Allergy. 2010;24:354-358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 22] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 51. | Sood A, Ford ES, Camargo CA. Association between leptin and asthma in adults. Thorax. 2006;61:300-305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 167] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 52. | Sarraf P, Frederich RC, Turner EM, Ma G, Jaskowiak NT, Rivet DJ, Flier JS, Lowell BB, Fraker DL, Alexander HR. Multiple cytokines and acute inflammation raise mouse leptin levels: potential role in inflammatory anorexia. J Exp Med. 1997;185:171-175. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 568] [Cited by in RCA: 559] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 53. | Guler N, Kirerleri E, Ones U, Tamay Z, Salmayenli N, Darendeliler F. Leptin: does it have any role in childhood asthma? J Allergy Clin Immunol. 2004;114:254-259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 163] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 54. | Fuentes E, Guzmán-Jofre L, Moore-Carrasco R, Palomo I. Role of PPARs in inflammatory processes associated with metabolic syndrome (Review). Mol Med Rep. 2013;8:1611-1616. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 72] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 55. | Wang W, Zhu Z, Zhu B, Ma Z. Pioglitazone attenuates allergic inflammation and induces production of regulatory T lymphocytes. Am J Rhinol Allergy. 2010;24:454-458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 56. | Gowdy KM, Fessler MB. Emerging roles for cholesterol and lipoproteins in lung disease. Pulm Pharmacol Ther. 2013;26:430-437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 109] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 57. | Yao X, Remaley AT, Levine SJ. New kids on the block: the emerging role of apolipoproteins in the pathogenesis and treatment of asthma. Chest. 2011;140:1048-1054. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 23] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 58. | Yiallouros PK, Savva SC, Kolokotroni O, Behbod B, Zeniou M, Economou M, Chadjigeorgiou C, Kourides YA, Tornaritis MJ, Lamnisos D. Low serum high-density lipoprotein cholesterol in childhood is associated with adolescent asthma. Clin Exp Allergy. 2012;42:423-432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 36] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 59. | Fenger RV, Gonzalez-Quintela A, Linneberg A, Husemoen LL, Thuesen BH, Aadahl M, Vidal C, Skaaby T, Sainz JC, Calvo E. The relationship of serum triglycerides, serum HDL, and obesity to the risk of wheezing in 85,555 adults. Respir Med. 2013;107:816-824. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 46] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 60. | Scichilone N, Rizzo M, Benfante A, Catania R, Giglio RV, Nikolic D, Montalto G, Bellia V. Serum low density lipoprotein subclasses in asthma. Respir Med. 2013;107:1866-1872. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 31] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 61. | Hoefner DM, Hodel SD, O’Brien JF, Branum EL, Sun D, Meissner I, McConnell JP. Development of a rapid, quantitative method for LDL subfractionation with use of the Quantimetrix Lipoprint LDL System. Clin Chem. 2001;47:266-274. [PubMed] |
| 62. | Singh S, Prakash YS, Linneberg A, Agrawal A. Insulin and the lung: connecting asthma and metabolic syndrome. J Allergy (Cairo). 2013;2013:627384. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 59] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 63. | Kim JA, Wei Y, Sowers JR. Role of mitochondrial dysfunction in insulin resistance. Circ Res. 2008;102:401-414. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 815] [Cited by in RCA: 783] [Article Influence: 43.5] [Reference Citation Analysis (0)] |
| 64. | Nisoli E, Clementi E, Carruba MO, Moncada S. Defective mitochondrial biogenesis: a hallmark of the high cardiovascular risk in the metabolic syndrome? Circ Res. 2007;100:795-806. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 173] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 65. | Bugger H, Abel ED. Molecular mechanisms for myocardial mitochondrial dysfunction in the metabolic syndrome. Clin Sci (Lond). 2008;114:195-210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 153] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 66. | Aroor AR, Mandavia C, Ren J, Sowers JR, Pulakat L. Mitochondria and Oxidative Stress in the Cardiorenal Metabolic Syndrome. Cardiorenal Med. 2012;2:87-109. [PubMed] |
| 67. | Mabalirajan U, Dinda AK, Kumar S, Roshan R, Gupta P, Sharma SK, Ghosh B. Mitochondrial structural changes and dysfunction are associated with experimental allergic asthma. J Immunol. 2008;181:3540-3548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 176] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 68. | Mabalirajan U, Rehman R, Ahmad T, Kumar S, Singh S, Leishangthem GD, Aich J, Kumar M, Khanna K, Singh VP. Linoleic acid metabolite drives severe asthma by causing airway epithelial injury. Sci Rep. 2013;3:1349. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 73] [Cited by in RCA: 92] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 69. | Mabalirajan U, Dinda AK, Sharma SK, Ghosh B. Esculetin restores mitochondrial dysfunction and reduces allergic asthma features in experimental murine model. J Immunol. 2009;183:2059-2067. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 56] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 70. | Aich J, Mabalirajan U, Ahmad T, Khanna K, Rehman R, Agrawal A, Ghosh B. Resveratrol attenuates experimental allergic asthma in mice by restoring inositol polyphosphate 4 phosphatase (INPP4A). Int Immunopharmacol. 2012;14:438-443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 42] [Article Influence: 3.0] [Reference Citation Analysis (1)] |
| 71. | Ahmad T, Mabalirajan U, Sharma A, Aich J, Makhija L, Ghosh B, Agrawal A. Simvastatin improves epithelial dysfunction and airway hyperresponsiveness: from asymmetric dimethyl-arginine to asthma. Am J Respir Cell Mol Biol. 2011;44:531-539. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 64] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 72. | Aguilera-Aguirre L, Bacsi A, Saavedra-Molina A, Kurosky A, Sur S, Boldogh I. Mitochondrial dysfunction increases allergic airway inflammation. J Immunol. 2009;183:5379-5387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 207] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 73. | Holguin F, Fitzpatrick A. Obesity, asthma, and oxidative stress. J Appl Physiol (1985). 2010;108:754-759. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 81] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 74. | Keaney JF, Larson MG, Vasan RS, Wilson PW, Lipinska I, Corey D, Massaro JM, Sutherland P, Vita JA, Benjamin EJ. Obesity and systemic oxidative stress: clinical correlates of oxidative stress in the Framingham Study. Arterioscler Thromb Vasc Biol. 2003;23:434-439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1001] [Cited by in RCA: 1031] [Article Influence: 44.8] [Reference Citation Analysis (0)] |
| 75. | Sakai H, Nishizawa Y, Nishimura A, Chiba Y, Goto K, Hanazaki M, Misawa M. Angiotensin II induces hyperresponsiveness of bronchial smooth muscle via an activation of p42/44 ERK in rats. Pflugers Arch. 2010;460:645-655. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 18] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 76. | Ramsay SG, Kenyon CJ, Whyte N, McKay IC, Thomson NC, Lindop GB. Effects of angiotensin II on remodelling of the airway and the vasculature in the rat. Clin Sci (Lond). 2000;98:1-7. [PubMed] |
| 77. | Maneechotesuwan K, Ekjiratrakul W, Kasetsinsombat K, Wongkajornsilp A, Barnes PJ. Statins enhance the anti-inflammatory effects of inhaled corticosteroids in asthmatic patients through increased induction of indoleamine 2, 3-dioxygenase. J Allergy Clin Immunol. 2010;126:754-762.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 105] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 78. | Zeki AA, Kenyon NJ, Goldkorn T. Statin drugs, metabolic pathways, and asthma: a therapeutic opportunity needing further research. Drug Metab Lett. 2011;5:40-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 27] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 79. | Huang CC, Chan WL, Chen YC, Chen TJ, Chou KT, Lin SJ, Chen JW, Leu HB. Statin use in patients with asthma: a nationwide population-based study. Eur J Clin Invest. 2011;41:507-512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 76] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 80. | Yuan C, Zhou L, Cheng J, Zhang J, Teng Y, Huang M, Adcock IM, Barnes PJ, Yao X. Statins as potential therapeutic drug for asthma? Respir Res. 2012;13:108. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 42] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 81. | Silva D, Couto M, Delgado L, Moreira A. A systematic review of statin efficacy in asthma. J Asthma. 2012;49:885-894. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 82. | Moini A, Azimi G, Farivar A. Evaluation of atorvastatin for the treatment of patients with asthma: a double-blind randomized clinical trial. Allergy Asthma Immunol Res. 2012;4:290-294. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 29] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 83. | Maneechotesuwan K, Kasetsinsombat K, Wamanuttajinda V, Wongkajornsilp A, Barnes PJ. Statins enhance the effects of corticosteroids on the balance between regulatory T cells and Th17 cells. Clin Exp Allergy. 2013;43:212-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 56] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 84. | Menzies D, Nair A, Meldrum KT, Fleming D, Barnes M, Lipworth BJ. Simvastatin does not exhibit therapeutic anti-inflammatory effects in asthma. J Allergy Clin Immunol. 2007;119:328-335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 93] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 85. | Si XB, Zhang S, Huo LY, Dai WL, Wang HL. Statin therapy does not improve lung function in asthma: a meta-analysis of randomized controlled trials. J Int Med Res. 2013;41:276-283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 17] [Article Influence: 1.3] [Reference Citation Analysis (1)] |
| 86. | Agrawal A, Mabalirajan U, Ahmad T, Ghosh B. Emerging interface between metabolic syndrome and asthma. Am J Respir Cell Mol Biol. 2011;44:270-275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 84] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 87. | Capra V, Rovati GE. Rosuvastatin inhibits human airway smooth muscle cells mitogenic response to eicosanoid contractile agents. Pulm Pharmacol Ther. 2014;27:10-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 27] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 88. | Sasaki O, Imamura M, Yamazumi Y, Harada H, Matsumoto T, Okunishi K, Nakagome K, Tanaka R, Akiyama T, Yamamoto K. Alendronate attenuates eosinophilic airway inflammation associated with suppression of Th2 cytokines, Th17 cytokines, and eotaxin-2. J Immunol. 2013;191:2879-2889. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 89. | Wu J, Zhang Y, Liu Q, Zhong W, Xia Z. All-trans retinoic acid attenuates airway inflammation by inhibiting Th2 and Th17 response in experimental allergic asthma. BMC Immunol. 2013;14:28. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 46] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 90. | Tsuchiya H, Ikeda Y, Ebata Y, Kojima C, Katsuma R, Tsuruyama T, Sakabe T, Shomori K, Komeda N, Oshiro S. Retinoids ameliorate insulin resistance in a leptin-dependent manner in mice. Hepatology. 2012;56:1319-1330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 55] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 91. | Mcilroy GD, Delibegovic M, Owen C, Stoney PN, Shearer KD, McCaffery PJ, Mody N. Fenretinide treatment prevents diet-induced obesity in association with major alterations in retinoid homeostatic gene expression in adipose, liver, and hypothalamus. Diabetes. 2013;62:825-836. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 59] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 92. | Arron JR, Scheerens H, Matthews JG. Redefining approaches to asthma: developing targeted biologic therapies. Adv Pharmacol. 2013;66:1-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 93. | Garcia G, Taillé C, Laveneziana P, Bourdin A, Chanez P, Humbert M. Anti-interleukin-5 therapy in severe asthma. Eur Respir Rev. 2013;22:251-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 98] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 94. | Ota T. Chemokine systems link obesity to insulin resistance. Diabetes Metab J. 2013;37:165-172. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 80] [Cited by in RCA: 86] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 95. | Malagón MM, Díaz-Ruiz A, Guzmán-Ruiz R, Jiménez-Gómez Y, Moreno NR, García-Navarro S, Vázquez-Martínez R, Peinado JR. Adipobiology for novel therapeutic approaches in metabolic syndrome. Curr Vasc Pharmacol. 2013;11:954-967. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 96. | Linderholm AL, Bratt JM, Schuster GU, Zeki AA, Kenyon NJ. Novel therapeutic strategies for adult obese asthmatics. Immunol Allergy Clin North Am. 2014;34:809-823. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 97. | Rinne ST, Feemster LC, Collins BF, Au DH, Perkins M, Bryson CL, O’Riordan TG, Liu CF. Thiazolidinediones and the risk of asthma exacerbation among patients with diabetes: a cohort study. Allergy Asthma Clin Immunol. 2014;10:34. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 30] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 98. | Perez MK, Piedimonte G. Metabolic asthma: is there a link between obesity, diabetes, and asthma? Immunol Allergy Clin North Am. 2014;34:777-784. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 47] [Article Influence: 3.9] [Reference Citation Analysis (0)] |