INTRODUCTION
Gallbladder cancer (GBC) is the most prevalent malignancy in biliary tract and third in gastrointestinal (GI) tract[1,2]. It was first described in detail in the seventies[3]. It is a lethal disease for most patients in spite of growing awareness and improved diagnostic techniques[4]. Although rare, it has high incidence in certain world populations[5]. GBC has poor prognosis and the survival rate in 5 years is less than 10%[5,6]. It affects predominantly women, four times more as compared to men[6,7]. GBC is asymptomatic in nature which makes it difficult in diagnosis and treatment. The symptoms of GBC are alike to other GI tract problems viz. abdominal pain, abdominal lump, anorexia, nausea, jaundice and vomiting[2]. GBC is a neoplasm which is widely known for its distinct ethnic, gender and geographical variations[8]. Precise etiology of GBC is still not known but various factors have been incriminated cholelithiasis[8], external carcinogens[9], free radicals, lipid peroxidation products, inflammatory bowel disease and secondary bile acids[10]. Congenital malformations of biliary tract (more common in Japan, China) are also observed as the risk factor for GBC. Chronic inflammatory conditions, heavy metals exposure, a high carbohydrate diet, obesity, alcohol abuse and smoking are also known as possible risk factor for GBC[11,12]. Adenocarcinoma is most prevalent histo-pathological type of GBC[8].
BACKGROUND AND EPIDEMIOLOGY
The incidence of GBC worldwide is based on the gender, geography and ethnicity which suggest that both genetic and environmental factors can cause GBC. Various epidemiological studies suggested that geographical and racial difference also affects the frequency of GBC. A high geographical variability in occurrence of GBC also correlated with the ubiquity of cholelithiasis[12]. Incidence rate of GBC is higher in South American Countries like Chile, Bolivia and Ecuador and some Asian countries like some areas of India, Pakistan, Japan, and South Korea[12]. Intermediate rate of incidence have been observed in European countries. Its rate is lower in United States but Native Americans observed to have a high incidence. Further, urban areas have less risk compared to rural region. Incidence in Chile where mortality is 5.2% is highest in the world. There, it is the most prevalent cancer affecting women and the fourth important reason of cancer deaths[5]. Marked geographical differences seen in frequency of gallbladder carcinoma suggest a possible environmental cause besides race or ethnicity. The exact etiology of GBC has not been properly known till date. It is yet to be established. But several other factors like chronic cholecystitis, gallstones, choledochal cyst, female gender, age and exposure of carcinogens have been observed to be implicated in gallbladder carcinogenesis[13,14]. Randi et al[5] and other studies carried out where age adjusted incidence rates of GBC in various populations based on cancer registry data were considered concluded that maximum incidence rate was found in women in Delhi, India (21.5/100000), South Karachi, Pakistan (13.8/100000) and Quito, Ecuador (12.9/100000)[5,15,16]. Another study also described a very high incidence of cancer in Northern India (1.5/100000) and Native American Indian females (14.5/100000)[17]. Therefore, there is an urgent need of early detection of GBC[18]. Gastric cancer was the main cause of death in Japan since 1999. It was reported to be the sixth leading cause of cancer related death in Japan in 2007[19]. The incidence of GBC rises with age and reaches the peak during the seventh and eighth decades of life[6]. Age-standardized incidence rates (ASIR) has been found to be high above age of 45. Delhi has been listed as having the highest ASIR with 22.08 males and 35.67 females per 105 persons after the age of 65 years. Studies revealed a very distinct age-related pattern among both genders[20]. Female-to-male incidence ratio was generally around 3, but ranged from 1 in Far East Asia to over 5 in Spain and Columbia[5]. The incidence was observed to be higher from the Gangetic belt, but, due to lack of cancer registries in these areas, the precise incidence cannot be known accurately. The estimated occurrence of GBC in Varanasi is around 4.4% of all types cancers and around 16% of all GI cancers[2].
PATHOLOGY
Approximately 80% GBCs follow progression from dysplastic mucosa to carcinoma in situ and invasive carcinoma[21]. Morphological and molecular changes also suggest the same[22]. Approximately 60%, 30% and 10% tumors originate in the fundus, body and neck of the GB respectively[23]. Among all the GBC, adenocarcinoma constitute about 85% in comparison to others like epidermoid carcinomas (6.5%) and adenocanthomas (4.5%). Besides these, other histologic types of GBC are small (oat) cell carcinomas[24], carcinoid tumors and anaplastic carcinomas[8]. Glandular, medullary, scirrhous, papillary, and colloid type are the parts of adenocarcinoma with incidence rate of 35.3%, 23.2%, 15.7% 14.5% and 11.3% respectively[25]. Infiltrative, papillary and combined papillary-infiltrative forms are the different forms of GC[26]. Thickening and induration of GB wall occurred in infiltrating tumors which invades the subserosal plane and gallbladder wall into liver and neighboring structures. Papillary carcinoma shows a polypoid cauliflower like appearance which fills the GB lumen with very low wall invasion as it has the best prognosis. Recent researches also divide GC into metaplastic and non-metaplastic types[27]. Pseudopyloric and intestinal are two types of metaplastic variety. They are associated with chronic inflammation and cholelithiasis. Intestinal variety is associated with increasing age[28].
MOLECULAR AND GENETICS RELATED TO GBC
Different epidemiological studies and diversity in incidence of GBC studies also suggest an association of gene in its etiopathogenesis. Few facts are available about genetic changes in GBC. K-ras gene mutations have been found in 39%-59% of GBC, whereas p53 mutations have been reported in 35%-92% of patients having GBC[6,29]. Shukla et al[30] reported that telomerase activity was significantly raised in gall bladder cancer tissue. Telomerase activity was mainly concentrated in poorly-differentiated adenocarcinomas (83.33%) and increased expression was present in advanced stages. The presence of telomerase may serve as a molecular marker for the diagnosis of gall bladder carcinoma and may have prognostic and therapeutic implications in the treatment of patients[30]. It has been reported in various studies that genetic alterations in k-ras, p53 and p16 take a significant part in gallbladder carcinogenesis[31-36]. Inactivation of tumor suppressor genes involves the different genetic mutational events which lead to the one allele and allelic loss of the other allele. This is the main and complex mechanism associated with it. This allelic loss is known as loss of heterozygosity (LOH) which can be detected by using microsatellite markers. Recurrent LOH has also been detected in GC at different chromosomal locations like 1p, 3p, 5q, 8p, 9q, 13q and 17p[37]. Other reports also suggested in their study that many chromosomal regions have important tumor suppressor genes which are also detected in this neoplasm. 3p (20% to 52%); 5q21 (APCMCC gene, 6% to 66 %); 8p22-24 (22% to 44%); 13q14 (RB gene, 20% to 30%); and 18q (DCC gene, 18% to 31%) are the some gene locations other that the TP53 and CDKN gene[37]. In all the cholecystitis and adenoma patients Retinoblastoma (Rb) gene have been detected; however, Rb gene is deleted in 18%-67% of carcinoma of the gallbladder patients[38,39]. The precise molecular abnormality which causes neoplastic transformation in the gallbladder epithelium still unclear. An accurate pathway related to molecular changes which further result in neoplastic transformation in gallbladder epithelium has not fully understood. This more understanding of molecular changes events will give better tools to detect GBC in early stage.
ROUTES OF SPREAD: LOCO-REGIONAL AND DISTANT
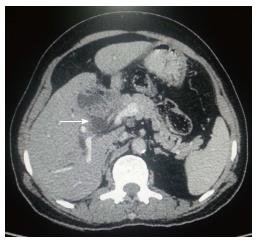
Vascular, lymphatic, intraperitoneal, neural and intraductal routes are the leading routes of spread. Intraductal spread in GBC has a better prognosis[40]. Invasion of liver and lymph nodes has been reported in 69% and 45% of patients respectively in 984 patients from nine series[8]. Loco regional spread in GC is more common than distant metastasis. Metastases usually occur in liver (Figures 1A-B), lymph nodes, adjacent organs and peritoneum. Lymph nodes are usually found in about 60% of cases while metastasis in liver is about 76%-86% cases. Intraperitoneal spread is common with ascites, omental nodules and peritoneal implants[41-43]. 76.8%, 71% and 24.6% cases had liver, lymph node involvement and peritoneal deposits respectively in a study consists of 69 patients which undergo exploratory laparotomy[1]. Lymphatic drainage from the gallbladder occurs in a predictive fashion and correlates with the pattern of lymph node metastasis seen in GBC. Initially, cystic duct and pericholedochal nodes are involved, followed by more distant metastasis to posterior nodes to the head of the pancreas and then to interaortocaval lymph nodes. This primary route is called cholecysto-retropancreatic pathway. Secondary route of lymphatic drainage includes the retroportal and right celiac lymph nodes through the gastrohepatic ligament, called cholecysto-celiac pathway. The third one is called cholecysto-mesenteric route consisting of a pathway from the posterior of gallbladder to the aortocaval lymph nodes via pancreas[44]. It is common for GBC spread directly into the liver and porta hepatis which lead to narrowing or obstruction of the common hepatic or right hepatic duct. Surgical specimens from 48 patients who had undergone radical/extended cholecystectomies were examined in 5-mm stepwise tissue sections in one large surgical series[45]. Zero to three score was given to nodal spread (0: no metastases; 1: cystic, paradochal, hilar; 2: peripancreatic head, portal, hepatic artery; 3: celiac, periduodenal, perimesenteric). Out of 48 patients, 8 (17%), 7 (14.5%) and 9 (18.8%) were found to have group 1 and 3 nodes respectively. Out of 16 patients with group 2 or 3 nodes, only 3 were considered for curative resection. Such studies recommend a possible niche for regional radiation therapy where more distant nodal groups are commonly comprised for pancreatic cancer and cholangiocarcinoma (CC). Some studies also described that GBC have a greater risk for synchronous distant metastatic spread than in CC as GBC usually have a regional pattern of spread. Ninety-seven patients of GBC and 76 patients of hilar CC were included in a study who were subjected to potentially curative resection and analyzed for patterns of initial disease recurrence[46]. GBC patients had a significantly lesser time to recurrence (11.5 mo for GBC, vs 20.3 mo; P = 007) than hilar CC patients. In addition, GBC patients were far more likely to have a distant site involved at the initial period of recurrence (85% for GBC, vs 41%; P = 001). They summarized in their study that patients of GBC were having less chances to benefit from locoregional therapy given the high rate of synchronous distant disease. Such conflicting results have made difficult to recommend chemo radiation. Spontaneous biliary-enteric fistulas are developed by gallstones (90%), peptic ulcer disease (6%) and malignancy or trauma (4%). Cholecysto-duodenal type (61% to 77%) is the most common communication, followed by cholecysto-colonic (14% to 17%) and cholecysto-gastric (6%)[47] (Figures 2-5). Contrast cholangiographic studies were used in the diagnosis of gallbladder and biliary tract diseases before the emergence of USG and computed tomography (CT). Involvement of hepatic flexure and mesocolon has been observed in 33.3% of cases which was demonstrated by eccentric or circumferential wall thickening. A gallbladder mass lesion closely abutting hepatic flexure with no obvious eccentric wall thickening was observed in 2.3% cases[48]. According to Arminski[49] metastases occur to every organ including liver, lymph nodes, adrenal, kidney, spleen, brain, breast, thyroid, heart and uterus, those to the skeletal system are least frequent. Adrenal metastases (Figures 6 and 7) and venous occlusion due to tumor thrombus are unusual in newly diagnosed gallbladder carcinoma patients. Incidence of vascular metastasis is rare, but can occur. Portal vein invasion or tumor thrombus can be seen in aggressive or late cases. Vascular invasion leads to the localized involvement of the liver in the neighboring primary lesion preferably than disseminated multiple nodules[50]. Disseminated metastases appear in the late stage of the disease and caused due to retroperitoneal veins invasion. Median survival rate in patients of carcinoma gallbladder with distant metastases is only 3 to 4 mo and these patients may not be offered any intervention[42] Indian studies suggest that cases from this geographic belt are more aggressive[51]. Accuracy rate of preoperative staging by using multislice CT has an overall range from 83% to 86%[52,53]. GBC can have myriad of manifestations and spread[54]. The rarity of bony metastases from primary carcinoma has been documented by other authors[49,55-57]. In cases of skeletal metastases of the carcinoma of the gall bladder, 90% are purely osteolytic, 10% are mixed lytic and blastic type with purely osteoblastic lesions being unknown[58] (Figures 8). GC have non-specific laboratory finding. Most common laboratory finding are liver function abnormality. Serum alkaline phosphatase (ALP), direct bilirubin (conjugated bilirubin), and aspartate aminotransferase (AST) concentrations are usually deranged in more than 50% of cases. The patient is mildly hypoalbuminemic and hemoglobin level is usually less than 11 g/dL in 10% of patients[59].
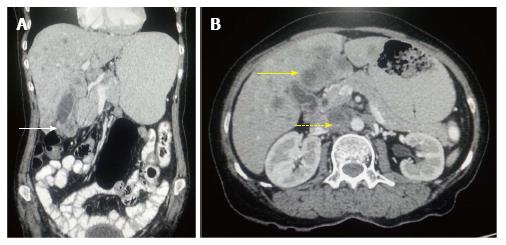
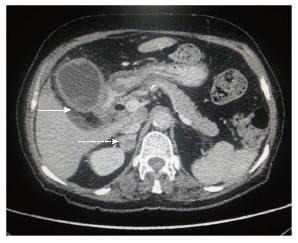
Figure 1 Carcinoma gallbladder: Nodal and hepatic metastasis.
A: Coronal contrast-enhanced computed tomography (CECT) abdominal section shows relatively defined heterogenous mass involving fundus of gall bladder (arrow) with loss of fat plane with adjacent hepatic segment; B: Axial CECT abdominal section shows multiple hepatic metastasis (arrow) along with interaortocaval lymph nodal metastasis (dashed arrow).
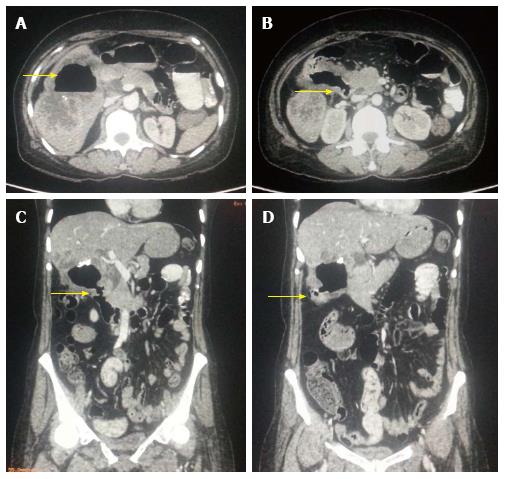
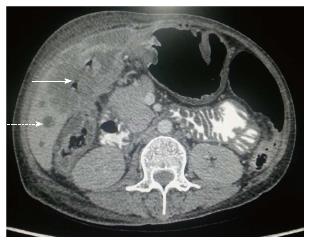
Figure 2 Aggressive gallbladder cancer and entero-biliary fistula.
A: Axial contrast-enhanced computed tomography (CECT) abdominal section shows predominantly hypodense mass lesion replacing gall bladder fossa with presence of air (arrow) raising suspicion of fistula formation. Adjacent hepatic segments are also infiltrated by the mass; B and C: Axial and Coronal CECT abdominal sections clearly reveal the fistulous communication with D2 segment of duodenum (arrow); D: Coronal CECT abdominal section also show coexisting cholecysto-colonic fistula with hepatic flexure of colon (arrow).
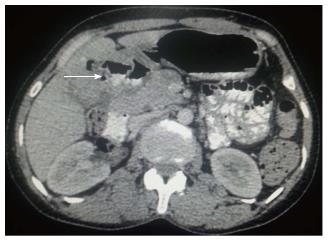
Figure 3 Axial contrast-enhanced computed tomography abdominal section shows ill-defined heterogenous mass replacing gall bladder fossa with loss of fat plane with adjacent hepatic segments.
Cholecystoduodenal and cholecysto-gastric fistulas are seen with D1 segment of duodenum and antropyloric region of stomach (arrow).
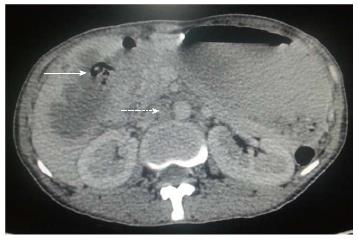
Figure 4 Axial contrast-enhanced computed tomography abdominal section shows ill-defined mass replacing gall bladder fossa with direct infiltration of hepatic segment V.
Cholecystoduodenal fistula (arrow) is noted along with retroperitoneal lymph nodal metastasis (dashed arrow).
Figure 5 Axial contrast-enhanced computed tomography abdominal section shows ill-defined heterogenous mass replacing gall bladder fossa with direct infiltration of hepatic segments.
Cholecystoduodenal fistula (arrow) is noted along with multiple hepatic metastasis (dashed arrow).
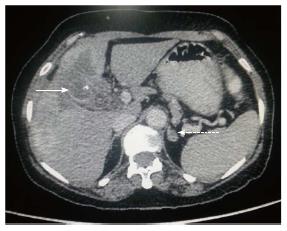
Figure 6 Axial contrast-enhanced computed tomography abdominal section shows diffuse irregular nodular enhancing wall thickening of gall bladder (arrow) along with circumferential wall thickening of distal stomach and proximal duodenum.
Associated right adrenal metastasis is also seen (dashed arrow).
Figure 7 Axial contrast-enhanced computed tomography abdominal section shows ill-defined heterogenous mass replacing gall bladder fossa with direct infiltration of adjacent hepatic segments.
Associated left adrenal lesion is also seen (dashed arrow) raising suspicion of adrenal metastasis.
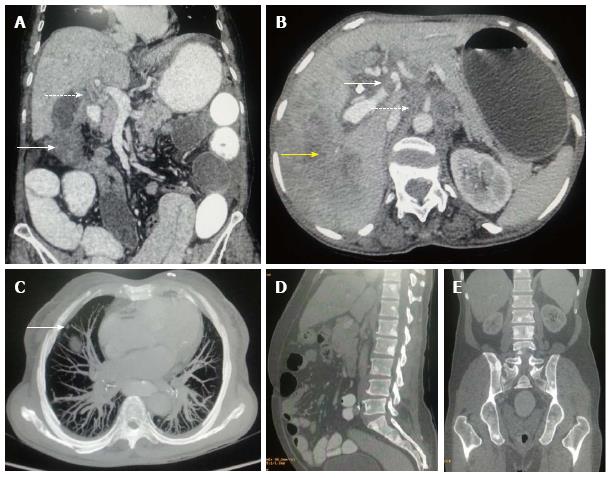
Figure 8 Gallbladder carcinoma: regional and distant metastasis.
A: Axial contrast-enhanced computed tomography abdominal section shows heterogenous mass lesion involving fundus of gall bladder fossa (arrow) with associated circumferential enhancing wall thickening of CBD (dashed arrow) indicating cholangitis or intraductal spread of malignancy; B: Axial CECT abdominal section shows hypodense filling defect in left branch of portal vein indicating thrombus formation (arrow). Hepatic infiltration is seen in segment VII (yellow arrow) with retroperitoneal lymph nodal metastasis (dashed arrow); C: MIP axial CT imaging shows soft tissue nodule in anterior segment of right lung indicating pulmonary metastasis (arrow); D and E: Sagittal and coronal CECT abdominal sections (bone window) show multiple osteoblastic skeletal metastatic lesions. CBD: Corticobasal degeneration; CECT: Contrast-enhanced computed tomography.
STAGING
Staging is the main part for the management and reporting of GBC. Gallbladder carcinoma is staged primarily at the time of surgery. Pathologic staging is decided at the time of surgery when the resection has been performed. Resection area (R) should be reported as it acts as the most important prognostic factor for GBC[60]. American Joint Committee on Cancer has given a TNM Staging System which usually determined by the depth of invasion, expansion of GC into adjacent structures, lymph node involvement and metastatic spread[61,62] The primary stage of GC which decides the treatment is the “T” stage. Surgery is usually performed for T1/T2 (tumor confined to GB wall) if metastasis is absent. Tumors extending beyond the GB wall are considered T3 and T4. T3 tumors can be resected but with en bloc resection of adjacent organs while T4 tumors are unresectable[17]. But a simple cholecystectomy cannot be used to completely remove a T2 stage tumor as there is no serosa on the GB on the side from where it is attached to liver[60]. A minimum presence of three regional lymph nodes is required for accurate “N” staging. Hilar, celiac, periduodenal, peripancreatic and superior mesenteric nodes as well as nodes along the pancreatic head are included in the regional lymph nodes. Lymph nodes outside the hepato-duodenal ligament are regarded as metastatic disease[17]. Metastasis to liver and peritoneum is common and occasionally to the lungs and pleura. Direct tumor invasion to the liver should not be regarded as distant metastasis[60].
IMAGING MODALITIES
Abdominal ultrasound
The most prominent imaging modality to assess symptoms of biliary tract disease along with suspected GC is Ultrasound (US). It can differentiate between carcinoma and chronic cholecystitis with a sensitivity of 44% in early stages of disease[63]. Sensitivity differs for different types as reported by a retrospective study using US in early GC[64]. Ultrasound can easily detect invasion of liver parenchyma and loss of normal tissue interface. The sensitivity and accuracy of US in advanced GC are 85% and 80% respectively[65]. Bach et al[66] in their study reported the sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) in non-resectable carcinoma. The gallbladder wall is not visualized or poorly visualized as a thin echogenic line on ultrasound. An echogenic double-rim effect is produced when the gallbladder wall becomes thickened from wall edema in inflammatory diseases[67]. Increase in gallbladder wall thickness can be seen in association with many conditions including chronic cholecystitis and neoplasia[68]. Sonography is often the first requested imaging technique in suspicion of gallbladder diseases due to cost effectiveness and easy availability[69-71]. CT and MRI are preferred over US for detection of early lesions and accurate staging and characterization[69,71,72]. US should be used along with other modalities as a complementary tool[73]. Hederström et al[63] in their study concluded that US had poor sensitivity to differentiate GBC from chronic cholecystitis. There is significant chance of missing a malignancy. A cholecystectomy in such cases is useful. Another study reported that 6 were correctly diagnosed by surgery or autopsy out of 11 ultrasonographically proved GC. It concluded that sonography can suggest the diagnosis of gallbladder carcinoma, but inflammatory changes in the gallbladder may simulate or mask the signs of malignancy[74]. A study by Wibbenmeyer et al[75] suggested that several sonographic findings were more common in patients with GBC in comparison to patients with benign gallbladder pathologies. Evaluation of these signs may be helpful in such conditions[75]. Doppler US has been found to be useful in detecting invasion of GC into the liver, the portal vein, and the bile ducts, but it has limitation in detection of lymph node and peritoneal metastases[66]. Contrast enhanced US may be helpful in improving the diagnosis of GC. The usefulness of contrast enhanced US for differential diagnosis of polypoid GB lesions has been described by Hattori et al[76]. Classification of contrast enhanced patterns were described as linear, scattered, and diffuse or branched. It had a sensitivity of 100%, specificity of 76.9% and accuracy of 84.5% in case of diffuse or branched type pattern[76]. US guided fine needle aspiration cytology (FNAC) has been routinely for tissue diagnosis. It has an accuracy of 95% without major complications[77]. FNAC has been reported to have a sensitivity of 88%, specificity of 100%, PPV of 100% and NPV of 52%[78].
Endoscopic ultrasonography
Endoscopic ultrasonography (EUS) has been widely used for peri-operative staging of GC. It looks as a hypoechoic mass with or without GB wall calcifications[79]. The overall accuracy rate of 91.9% has been reported in differentiating neoplastic from non-neoplastic masses[80]. Mitake et al[81] also demonstrated the effectiveness of EUS in determination of the extent of tumor invasion and GC diagnosis. They reported an overall accuracy rate for tumor invasion depth to be 76.5% and differentiation between early and advanced stage tumors was possible in 79.5%. Furthermore, the usefulness of EUS has increased by the development of contrast agents. The depth of GC invasion was accurately assessed in 11 of 14 cases (78.6%) by conventional EUS, and in 13 of 14 cases (92.9%) by contrast enhanced EUS in a comparative study. If we compare the conventional ultrasonography to endoscopic ultrasonography, the endoscopic ultrasonography has been observed to be more precise for staging[82,83] A combination of diagnostic methods and biopsies may bring down the incidence of explorative laparotomies performed for GC[71]. Other aids like percutaneous liver biopsies, cholecystocentesis and culture of bile have been suggested in diagnosis of equivocal cases[84]. Use of EUS alone or in combination with EUS-guided FNA of gallbladder can improve the diagnosis of GC[85,86]. EUS-guided FNA is a safe and reliable modality for carcinoma gallbladder[79] .
CT
CT is a common imaging modality for the identification of primary tumor and tumor staging. Hepatic parenchyma is the most commonly invaded site, followed by the bile duct and neighboring organs. CT scan can also accurately determine peritoneal and lymphatic metastasis[87]. Pericholedochal nodes and cystic nodes are commonly involved[88]. Kim et al[89] using preoperative CT reported a precision of 71%. Helical CT was suggested to evaluate the spread and depth invasion of GC to assess resectability[90]. There are many advantages of helical CT over conventional CT[52]. In a retrospective study diagnostic precision for T staging was reported to be 83%-86% in comparison to conventional CT[52]. In another study overall accuracy of CT for staging GC was 71% and 79% for T1 and T2 tumors, 46% for T3 tumors, and 73% for T4 tumors. There was statistically significant difference between thickened wall and intraluminal mass type of tumors (P < 0.05). The accuracy was (89%) for the intraluminal mass type, (83%) for massive type, whiles it was 54% in the thickened wall type[89]. Another prospective Japanese study using spiral CT reported affectability, specificity, PPV, NPP and the general exactness to be 88%, 87%, 88%, 87%, and 87%, respectively[91]. Although CT is not routinely used to explore patients with gallbladder disease symptoms, it is an important examination for suspected cases of gallbladder carcinoma. The most widely recognized CT finding in gallbladder carcinoma is a mass that fills the greater part of an irregular and distorted gallbladder[92] (Figure 9). Gallbladder carcinoma appears as a symmetric or asymmetric gallbladder wall thickening that may be hard to distinguish from the scarred gallbladder seen in chronic cholecystitis. Non-specific gallbladder wall thickening can be seen in acute and chronic cholecystitis, xanthogranulomatous cholecystitis, adenomyomatosis, diffuse hepatic or systemic disorders like hepatitis, portal hypertension, and congestive heart failure[46,93]. Yun et al[87] used dual phase CT to assess thickness as well as enhancement pattern of gallbladder wall seen in gallbladder melanoma as well as chronic cholecystitis in arterial and venous phase. They reported a difference in enhancement patterns of malignancy as compared to chronic cholecystitis using dual phase CT. Kim et al[89] assessed the enhancement pattern of abnormal GB wall thickening using MDCT to differentiate between carcinoma and inflammatory diseases. They concluded that there is a distinct pattern of enhancement of inner wall compared to non-enhancing surface covering. Different signs of gallbladder carcinoma can be found due to biliary obstruction and liver involvement[53]. CT was found to be 85% precise in evaluation loco-regional spread of gallbladder malignancy[94]. It can be useful in guiding aspiration/biopsy from gallbladder in few cases[95]. It is prudent to correlate CT scan findings with clinical and laboratory findings in elderly individuals, particularly women presenting with acute cholecystitis and abnormal liver function. Imaging findings suspicious of carcinoma are diffuse irregular gallbladder wall thickening, intraluminal mass along with enlarged local lymph nodes[96]. Lee et al[97] compared the efficacy of ultrasound (US) and CT in cases of intraluminal and infiltrating gallbladder carcinoma with and without gallstones. They found CT to be superior than US. Some authors suggest that combination of CT with ultrasound increases the diagnostic accuracy of gallbladder carcinoma associated with cholecystitis[98].
Figure 9 Axial contrast-enhanced computed tomography abdominal section shows heterogenous mass involving gall bladder fossa and leading to obstruction of biliary system due to intraductal spread (arrow).
Magnetic resonance imaging
Initially due to disadvantage of poor spatial and contrast resolution, magnetic resonance imaging (MRI) was not widely used to evaluate GB disease[18]. Nonetheless, with recent advances and introduction of dynamic techniques after the administration of paramagnetic contrast, MR cholangiopancreatography (MRCP) and MR angiography (MRA), have been utilized for tumor staging[70]. Primary GC appears hypodense on T1 weighted images and hyperdense on T2 images with sensitivity of 67%-100% and specificity of 89%-100%[95]. The detection rate of lymph node metastasis was only (57%). The sensitivity and specificity for vascular invasion can be increased by combining MR cholangiography with three-dimensional MR angiography[99,100].
Positron emission tomography
18F-2fluoro-2-deoxy- D-glucose (FDG) uptake by tumor cells gives positron emission tomography (PET) imaging the combined benefit of utilizing metabolic activity and imaging features together. The main drawback of FDG-PET is that it is not yet generally accessible for routine clinical use and the low pervasiveness of GC. Till date, there is little data on the feasible contribution of these techniques in the useful imaging diagnosis of GC. In a recent prospective cohort study in patients presenting with radiologically suspicious gallbladder lesions[101] a staging diagnostic pre-surgical FDG-PET study was performed. Total diagnostic precision was 83.33% for the finding of the primary lesion, 88.89% for the assessment of involvement of lymph node and 85.1% for the assessment of metastatic spread. Karim et al[102] examined the efficiency of different imaging techniques employed in GBC diagnosis.
Preoperative evaluation and staging
MDCT is widely available nowadays and has a reported precision of up to 84% in determining the T stage of primary gallbladder carcinoma[103] and 85% in foreseeing resectability due to its capacity to depict hepatic and vascular invasion, lymph nodal and distant metastases[104]. MDCT is commonly performed as unenhanced and contrast-enhanced dual phase, from which multiplanar and 3D volume-rendered reconstruction images are generated to provide complete anatomic information. Additional coronal oblique images may be acquired for surgical management[104]. Kim et al[89] claimed that the all-in-one standard protocol of using MR cholangio-pancreatography and contrast enhanced MR angiography may yield a sensitivity of up to 100% with regard to bile duct and vascular invasion. However it is only 67% with regard to hepatic involvement and 56% with regard to lymph node metastases. PET/CT may have a promising role in the diagnosis associated with unsuspected metastases, which might change staging and treatment[105,106]. To date, potential research which specifically assess CT, MRI, and PET/CT in their capabilities to detect and stage gallbladder carcinoma are yet to be executed. The particular spread of gallbladder carcinoma to the liver parenchyma in addition to surrounding internal organs is possibly due to lack of muscularis mucosa in addition to submucosa inside the gallbladder wall structure and primary venous drainage with the liver parenchyma on the hepatic abnormal veins. As per the sixth release of American Joint Committee on Cancer staging manual for gallbladder carcinoma[107] primary gallbladder carcinoma can be delegated T1, kept to the lamina propria or the muscle layer of the gallbladder (T1a and T1b, separately); T2, stretching out to the serosa; T3, puncturing the serosa or directly invading the liver or one other contiguous structure (stomach, duodenum, colon, pancreas, omentum, extrahepatic bile channels); or T4, invading the primary portal vein, the hepatic artery, or numerous extrahepatic organs. Lymphatic spread is present in more than half of patients at initial finding and first reaches cystic, pericholedochal, hilar, periduodenal, peripancreatic, and predominant mesenteric nodes, which are viewed as regional or N1 nodes. Portacaval, inter-aortocaval, and more distant nodes are viewed as distant or M1 disease. Gallbladder carcinoma can spread through intraductal route along the cystic duct, hematogenous route and neural pathways, and intraperitoneal “drop” metastases[70]. T1 or T2 involvement without nodal metastasis are termed as stage IA or IB respectively. T3 disease without nodal spread are stage IIA. T1, T2, or T3 disease with N1 lymph node involvement is characterized as stage IIB. A T4 disease without distant metastasis is considered as stage III. Any patient with distant disease comes in to the category of stage IV.
Cytology
A carcinoma at an early stage can be ignored, and the diagnosis can only be made after microscopic examination of paraffin-embedded tissue. Imprint cytology of the gallbladder mucosa is a simple, quick, and excellent method for the detection of GBC[108]. Ultrasound-guided fine-needle aspiration cytology is likewise a safe diagnostic method for GBC[109]. Endoscopic retrograde cholangio-pancreaticography of biliary tree and GBC can also be considered for assessment of clinically suspicious carcinoma[110].
Tumor markers
Nowadays, tumor markers have a significant role in the detection and assessment of GBC. Investigation of CA242, CA15-3, CA19-9, and CA125 are genuinely proficient markers for segregating patients of carcinoma of the gallbladder from cholelithiasis. CA242 and CA125 when utilized together accomplished best sensitivity and specificity. Serum markers appear to be less effective when utilized independently, however it can be a useful complementary tool in combination[111]. No biochemical markers are useful in early detection of GC. Cholestasis and hyperbilirubinemia indicate late stage disease. Carcinoembryonic antigen (CEA) more than 4 ng/mL has a sensitivity of 50% and specificity of 93%[112]. Presence of CA19-9 suggest poor prognosis. A value more than 20 IU/mL has a specificity of 79%. CA19-9 is frequently increased in the presence of biliary obstruction therefore it is less specific in patients of jaundice[112]. Incresed levels of serum alpha fetoprotein (AFP) have been seen in few patients but have little significance[113].
Electrophoretic pattern of proteins
Electrophoretic examination of serum protein has demonstrated protein bands in patients of carcinoma of the gallbladder in comparison to electrophoretic pattern in cholelithiasis[114].
Gallbladder membrane lipids
Fourier enhance infrared (FTIR) spectroscopy is usually very sensitive for the molecular composition of tissues, and has the potential to distinguish premalignant tissues. Lipids have been elevated inside the plasma tissue layer of GBC. This proportion associated with high intensity might be a marker to help in identification of cancer through FTIR[115].
TREATMENT OPTIONS
The only likely curative treatments intended for gallbladder carcinoma is usually surgical resection. Unfortunately, most patients with GBC have unresectable disease. Only 10%-30% of patients can be considered for surgery on presentation[29]. The surgical alternatives for the treatment of GBC have evolved over the years. The methods range from a simple cholecystectomy to a radical or extended cholecystectomy, which incorporates the gallbladder in addition to 2 cm of liver tissue from the gallbladder bed. The radical cholecystectomy has been further modified to incorporate more significant liver resections, like segmentectomies (4b/5), right hepatectomies and trisectionectomy. Extended procedures additionally incorporate regional lymphadenectomy of the porta hepatis and periduodenal and pancreatic stations. Few surgeons incorporate a resection of the bile duct to clear the lymphatics in the porta hepatis. A few specialists now incorporate periaortic lymph node dissection for staging purposes and if the tumor is distal or includes the head of the pancreas, a pancreatico-duodenectomy is added to accomplish R0 resection status[116,117]. Incidental GBC is found during cholecystectomy in 1%-2% of the cases. GBC should be suspected in the event of a tough gallbladder dissection or if there is presence of regional lymphadenopathy. A simple cholecystectomy is definitely an adequate treatment for Tis and T1 stage. Five-year survival for patients with T1 tumors is more than 85% with simple cholecystectomy[117,118]. For the T1 stage patients, the estimation of radical resection depends upon whether it is a T1a tumor or T1b tumor. In a study, the authors compared treatment of simple cholecystectomy and radical resection of T1a patients; however they didn’t find any variation in survival or recurrence in the two groups[119]. Additionally, no positive lymph nodes were found in the sampled 147 lymph nodes in 12 patients who underwent radical resection. Simple cholecystectomy is needed for T1a GBC. For T1b (muscle invasion), there is proof that a more aggressive surgical methodology is needed. T1b tumors had lymph node metastases in 15% of cases, though only 2.5% of T1a tumors are accounted to have lymph node association[117]. Extrahepatic biliary resection is advocated for the management of T3 and T4 tumors[120]. The authors propose that the limit for extrahepatic biliary resection ought to be decreased in patients with penetration of GBC through the subserosa[120]. Adjuvant combination chemotherapy and molecular targeted therapy are emerging as powerful therapeutics choices in those with advanced GBC. These days, adjuvant combination chemotherapy and molecular targeted therapy are regularly utilized as viable treatment for advanced GBC[121,122]. Adjuvant radiation treatment is utilized in locally advanced GBC or gallbladder disease with regional disease and has better survival rate[123,124]. It has been found that chemotherapy did not give effective treatment to unresectable GBC. Different regimens are already studied which include mixtures of 5-FU, leucovorin, mitomycin, adriamycin, in addition to nitrosoureas. A study observed a 64% reaction rate to gemcitabine with or without cisplatin to patients with stage 4 GBCs during phase II trial. Average time to development was 28 wk and the average general survival was 42 wk[125]. In other phase II trial utilizing a combination of gemcitabine and carboplatin in 20 patients with unresectable GBC, the reaction rate was 37%, average time to development was 34 wk, and the 1-year survival rate was 43%. The study concluded that chemotherapy with mixture of gemcitabine and carboplatin is viable in the treatment of advanced gallbladder carcinoma[126]. Palliative treatment may be done if GBC is found to be unresectable at the time of surgical investigation. The particular rate of biliary obstruction within affected individuals by GBC is higher than 60%[117]. A study concluded that combination of gemcitabin and oxaliplatin works well in inoperable GBC with overall response rate of more than 20%. It depicted that it may even incite complete pathological reaction. One year survival was found in more than 20% patients[127]. Other phase III study predicts that survival rate has been found higher in those patients who receive combination of gemcitabine and cisplatin in comparison to gemcitabine alone. Different studies are in process of clinical trials for molecular targeted agents which restrain angiogenesis and EGFR pathways[128]. New effective treatment and drugs are an urgent need for GBC. Recently a new study has been done in which the authors look at the effects of triptolide on GBC cells to identify its anticancer effects. They concluded in their study that triptolide induce apoptosis in gallbladder cells and thus can be used as a potential drug for treatment of GBC[129].
CONCLUSION
The clinical and radiologic diagnosis of gallbladder carcinoma at an early stage is challenging. It is crucial for radiologists to examine the gallbladder in its entirety, especially in patients who are at a greater risk of developing GBC, for important morphologic abnormalities that may suggest carcinoma. Identification of characteristic imaging appearances of primary GBC and comprehension of its pathways of spread and staging criteria will help in formulating appropriate treatment regimens.
P- Reviewer: Caldarella C S- Editor: Gong XM L- Editor: A E- Editor: Lu YJ
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/