Published online May 16, 2014. doi: 10.12998/wjcc.v2.i5.151
Revised: March 20, 2014
Accepted: April 3, 2014
Published online: May 16, 2014
Processing time: 142 Days and 17.7 Hours
A 24-year-old female patient with parathyroid carcinoma, the rarest endocrine malignancy, had two pregnancies. In the first pregnancy, she had severe nausea and fatigue. Hypercalcemia and hyperparathyroidism were diagnosed in the postpartum period. Hyperemesis gravidarum masked a diagnosis of hypercalcemia. Neck ultrasound and Tc-99m sestamibi found an enlarged lower right parathyroid gland. The gland was surgically removed, and an initial pathology report described atypical adenoma. Shortly afterward, she became pregnant again. During the second pregnancy, her calcium level was frequently controlled but was always in the normal range. Normocalcemia is explained by the specific physiology of pregnancy accompanied by hemodilution, hypoalbuminemia and maternal hypercalciuria (mediated by increased glomerular filtration). During lactation, calcium levels rose, and a new neck ultrasound showed a solitary mass in the area of prior surgery and an enlarged pretracheal lymph node. Fine needle aspiration of the solitary mass and node showed parathyroid carcinoma cells. The tumor mass was resected en bloc with the contiguous tissues and surrounding lymph nodes (pathology report; parathyroid carcinoma with metastases). Over the next five years, four consecutive surgeries were performed to remove malignant parathyroid tissue, lymph nodes and local metastases. Following the surgical procedures, no hypocalcemia was observed. More serious hypercalcemia recurred; the calcium level was difficult to control with a combination of pamidronate, cinacalcet and loop diuretic. No elements of multiple endocrine neoplasia were present.
Core tip: Parathyroid carcinoma is the rarest endocrine malignancy; it is extremely uncommon in pregnancy. Hyperemesis gravidarum can mask the symptoms of hypercalcemia. The calcium level can be lower during pregnancy due to its specific physiology. This is a case report of a 24-year-old female patient with parathyroid carcinoma and two consecutive pregnancies with good outcomes in the postnatal period despite poor prognosis due to malignant disease. In five years, four consecutive surgeries were performed to remove malignant parathyroid tissue, lymph nodes and local metastases. Hypercalcemia was difficult to control with a combination of pamidronate, cinacalcet and loop diuretic. There were no elements of multiple endocrine neoplasia.
- Citation: Baretić M, Tomić Brzac H, Dobrenić M, Jakovčević A. Parathyroid carcinoma in pregnancy. World J Clin Cases 2014; 2(5): 151-156
- URL: https://www.wjgnet.com/2307-8960/full/v2/i5/151.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v2.i5.151
Parathyroid carcinoma is a rare malignant disease in the general population. The United States National Cancer Database Report indicates that in the time period from 1985 to 1995, only 1% of patients with primary hyperparathyroidism had parathyroid cancers[1]. Some European countries, such as Italy, reported even higher prevalence (up to 5%)[2]. The etiology of parathyroid cancer remains unidentified; some authors mention neck irradiation as a risk factor, while others suggest long-standing secondary hyperparathyroidism[3,4]. Familial occurrences, such as hyperparathyroidism-jaw tumor syndrome, multiple endocrine neoplasia type 1 and 2A and familial isolated hyperparathyroidism[5], have been linked. It has been shown that mutations in the HRPT2 gene (a tumor suppressor gene called parafibromin) are associated with the development of parathyroid carcinoma, both in sporadic types and in cases with some familial clustering[6]. Parathyroid adenomas occur rarely in pregnancy, with the risk to both the mother and child directly connected to the level of serum calcium[7,8]. Parathyroid carcinoma is even more rare[9]. A case report of a woman with two subsequent pregnancies and parathyroid carcinoma is presented in this paper.
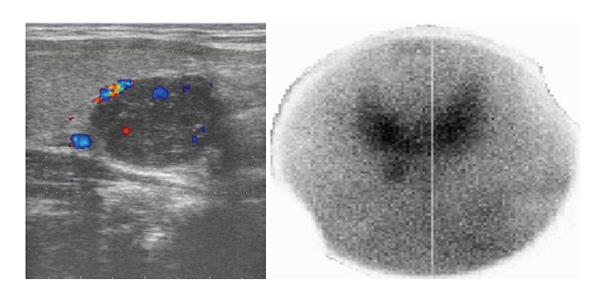
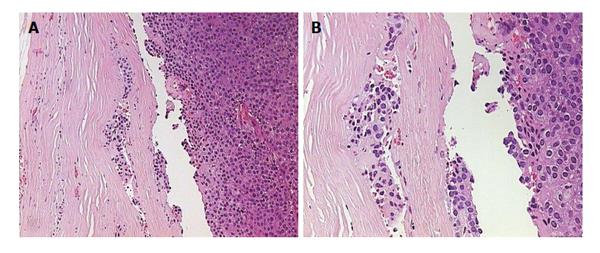
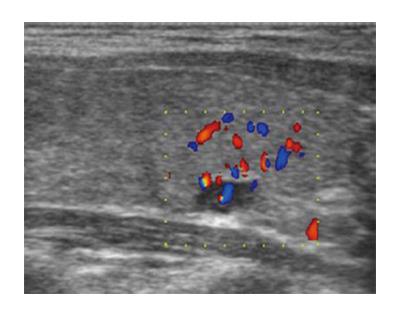
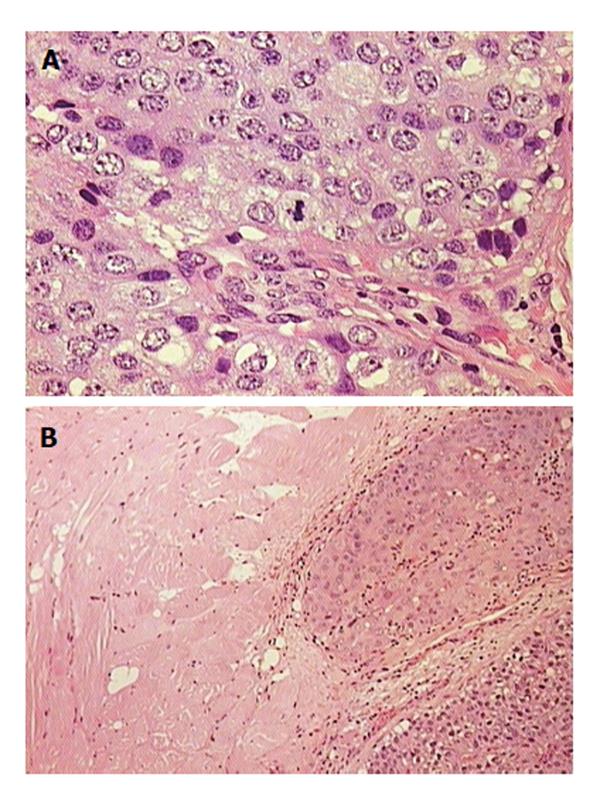
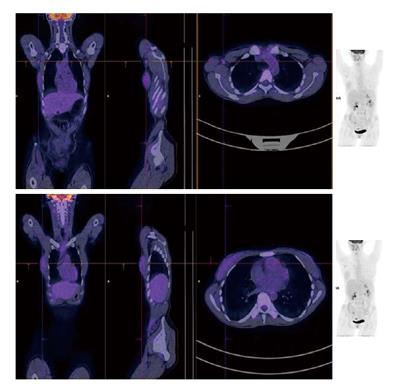
A 24-year-old woman had severe hyperemesis gravidarum during pregnancy, with constant nausea and vomiting 3-4 times daily. Symptoms started in the 10th week and persisted until the end of pregnancy, sometimes accompanied by fatigue and weakness. Vaginal delivery was at 37 wk of pregnancy. Shortly after the birth, her infant had neonatal convulsions; laboratory tests showed severe prolonged hypocalcemia of the child, and the mother’s calcium was checked. The mother’s level of calcium was high, and she was referred to an endocrinologist. High parathyroid hormone (PTH) and low phosphorus pointed to hyperparathyroidism. Initial laboratory results confirmed that suspicion: calcium was 3.62 mmol/L (normal range, 2.14-2.53 nmol/L); ionized calcium, 1.87 mmol/L (normal range, 1.18-1.32); phosphorus, 0.53 mmol/L (normal range, 0.79-1.42 nmol/L); alkaline phosphatase, 187 U/L (normal range, 64-153 U/L); bone alkaline phosphatase, 133 U/L (normal range for premenopausal women, 14.2-42.7 U/L); and intact PTH, 36 pmol/L (normal range, 1-6.0 pmol/L). The patient had no previous history of familial hyperparathyroidism or other endocrine conditions, neck irradiation, chronic renal disease or kidney stones. There were no systemic manifestation of hypercalcemia, and later bone densitometry indicated osteoporosis (T score of the lumbar spine was -3.8) but without bone fractures. Neck Doppler ultrasound recognized an enlarged lower-right parathyroid gland, described as a well-defined mass hypoechoic to thyroid tissue with a homogenous echo pattern measuring 21 mm × 15 mm × 15 mm with few vascular structures (PTH in aspirate was higher than 150 pmol/L, Figure 1). Tc-99m sestamibi planar scan showed an area of increased uptake on the right side of the neck, additional evidence of hyperfunctioning parathyroid tissue. A pinhole collimator was used to increase the resolution of scintigraphy Fine needle aspiration showed epithelial cells with pronounced anisonucleosis (variation in the size of the cell nuclei). The cytoplasmic borders were not sharply defined; the described cells had many naked nuclei. Sliver reaction (Grimelius) stained weekly positive. Hypercalcemia was treated by hydration with isotonic sodium chloride solution, loop diuretics and pamidronate. Two months after delivery, the patient underwent surgical removal of the described parathyroid gland. The removed gland measured 2 cm × 3 cm, and the pathological report noted tissue made up of main and oxyphil cell types. There was no significant remodeling of the stroma cells or polymorphism; tumor cells were monotonous, small cells with focal cytological atypia. Mitotic figures were not observed (Figure 2A). The tumor was surrounded by a thick capsule, with cells infiltrating the capsule without breaking through it (Figure 2B). There was no invasion of tumor cells in blood vessels and no invasion of the surrounding fat tissue. However, malignant cells surrounded blood vessels in the tumor tissue, and the final pathological diagnosis was atypical adenoma of the parathyroid gland. The patient was repeatedly followed after surgery: she was normocalcemic and became pregnant again. The time interval between the two pregnancies was 15 mo. During the second pregnancy, calcium levels were normal, and the patient had no nausea or sickness, but in the third trimester her TSH level dropped significantly (TSH < 0.05 mIJ/L, normal range 0.4-4.5 mIJ/L). Free T3 and T4 remained in the normal range; thus, she was not treated with thyrostatic drugs. After the second delivery, while lactating, her calcium level rose again (3.48 mmol/L). Other lab results suggested a recurrence of hyperparathyroidism (high alkaline phosphatase 158 IU and PTH 12 pmol/L). The second baby had no convulsions or hypocalcemia after birth, unlike the first. A new neck ultrasound described a solitary mass measuring 5 mm × 3 mm × 5 mm in the area of the previous surgery (Figure 3). An enlarged lymph node was present in the pretracheal region (lower third of neck) (Figure 4A). Fine needle aspiration of the solitary mass described naked nuclei and malignant cells with pronounced macronucleosis and intranuclear inclusions, leading to a diagnosis of carcinoma of the parathyroid gland. The same cytological description was found in an aspirate of a pretracheal node, confirming metastasis of the parathyroid carcinoma. Single photon emission computed tomography (SPECT) of the neck and mediastinum showed no significant focal uptake of Tc-99m sestamibi in the region of the neck and mediastinum. Shortly after delivery, thyrostatic drugs were introduced in therapy. The patient underwent a second surgery: the tumor mass was resected en bloc, together with contiguous tissues to which the tumor adhered. Other parathyroid glands were not removed. Tracheoesophageal, paratracheal, and upper mediastinal lymph nodes were excised; due to hyperthyroidism total thyroidectomy was performed as well. The pathology report described parathyroid carcinoma with mitotic figures, trabecular pattern and invasion of skeletal muscle (Figure 4). There were areas of tumor necrosis, and immunohistochemical results showed that the tissue was positive for PTH. Metastases of the parathyroid tumor were also found in the pretracheal lymph nodes. Postoperative hypocalcemia was prolonged but not severe. Patient received supplemental calcium and vitamin D, together with levothyroxine. In further evaluation, the levothyroxine dosage was modified according to the TSH level. Chromogranin A levels were normal (44 μg/L, normal levels < 100 μg/L), and there were no elements of multiple endocrine neoplasia. Gradually, the calcium level increased, and vitamin D and calcium supplementation were excluded from therapy. A year later, magnetic resonance imaging of the neck and mediastinum showed a lymph node enlarged to 12 mm, located dorsally from the right subclavia. The consulted oncologist advised that surgical treatment was an option (disease was not disseminated at that point), and a third surgery was performed, removing all tissue surrounding the malignant parathyroid gland, lymph nodes and local metastases. Following the surgery, hypercalcemia persisted. Early SPECT images performed 15 min after Tc-99m sestamibi injection showed two foci of radiopharmaceutical uptake anterior to the lower third of the neck region. Delayed tomographic images obtained 2 h after Tc-99m sestamibi injection showed no significant focal uptake of radiopharmaceutical in the neck and mediastinum region. Focal accumulation of fluorodeoxyglucose (F-18 FDG) was observed in several enlarged lymph nodes of the right axillary region, and diffuse F-18 FDG uptake was observed in the right breast (Figure 5). Additional ultrasonography and fine needle aspiration cytology of one of the enlarged axillary lymph nodes were obtained (inflammatory changes). Breast ultrasonography found no suspicious-looking lesions. During the last neck exploration (fourth surgery), the surgeon described tumor formation on the left side of the neck, with subcutaneous location and muscle infiltration. A right paratracheal tumorous mass was located behind the right carotid bifurcation. The pathology report of the tissue was identical to that described in prior surgeries. Hypercalcemia persisted and was difficult to control, even with a combination of pamidronate, cinacalcet and loop diuretic. Calcium is a tumor marker that indicates a persistence of small, disseminated, hormonally active metastases. Further F-18 FDG positron emission tomography-computed tomography (PET/CT) evaluation is planned, in addition to a test for the HRPT2 mutation.
Parathyroid cancer is a rare condition, and only few cases have been reported during pregnancy in the literature[9-13]. This condition is associated with significant neonatal morbidity and mortality, as well as maternal morbidity, and it is very difficult to treat[12,13]. In this case report, the described patient had two consecutive pregnancies with good outcomes both for mother and children, despite later poor prognosis for the mother due to malignant disease. Hyperemesis gravidarum, e.g., nausea, fatigue and weakness, are non-specific symptoms in pregnancy, but they are also symptoms of an elevated calcium level. The first pregnancy obscured the diagnosis of hypercalcemia. During the second pregnancy, the patient was normocalcemic. This result could be explained by the specific physiology of pregnancy, including hemodilution, hypoalbuminemia and maternal hypercalciuria, caused by an increase of the glomerular filtration rate. Sickness and vomiting in pregnancy usually start before the 9th wk and, in most cases, resolve by 16 wk of pregnancy. Otherwise, hypercalcemia is one possible cause. Nausea and vomiting are not the only clinical manifestation described in similar cases. Some of those patients have had hypertension, abdominal pain, pancreatitis and pre-eclampsia. For all of these patients, parathyroid carcinoma was diagnosed during pregnancy, and most of them underwent surgery in the third trimester[9-13].
Tc-99m sestamibi scintigraphy has been used for the detection of hyperfunctioning parathyroid tissue, including parathyroid carcinoma[14]. Tc-99m sestamibi uptake correlates with parathyroid oxyphil cell content and the size of hyperfunctioning parathyroid tissue. False-negative sestamibi scans can occur with parathyroid glands that contain predominantly clear cells, small size of the hyperfunctioning parathyroid tissue and rapid wash-out of the radiopharmaceutical from parathyroid cells[15]. A finding of focal accumulation of Tc-99m sestamibi in the lower third of the neck on early images and no significant uptake of radiopharmaceutical on delayed images is most likely due to rapid wash-out of Tc-99m sestamibi from the tumor cells.
Following the first surgery, the next scan was negative. F-18 FDG PET/CT is recognized as a powerful tool to detect tumors, especially malignant ones, due to the increased glucose metabolism of tumor cells[16]. FDG PET/CT is used in parathyroid carcinoma to detect recurrent disease. A negative finding on FDG PET/CT is most likely due to small tumor size.
There are no official guidelines for the treatment of hyperparathyroidism in pregnancy; even less is known about how to treat parathyroid carcinoma. The options are a conservative approach or surgery. Both approaches carry the risk of drug and procedure side effects, though the risk imposed by the elevated calcium level is sometimes greater. If surgery is an option, it should be performed in the third trimester of pregnancy. A literature analysis described 16 cases of primary hyperparathyroidism treated surgically after 27 wk of gestation, and carcinoma was found in a high percentage: 12.5% of cases. These patients had a low postoperative incidence of clinically significant complications for both the fetus and the mother[17]. When overt hyperparathyroidism is diagnosed in the postpartum period, surgery is the first choice of treatment. If parathyroid cancer is suspected (on the basis of the clinical picture and cytology, even before confirmation by pathology report), complete surgical resection with negative margins and removal of surrounding lymph nodes is the recommended course of action. In this case, the first pathological report was atypical adenoma. Atypical parathyroid adenomas have some histological findings that are suggestive of cancer but not enough to make the diagnosis. Atypical adenomas have a thick capsule, fibrous trabeculae, and a trabecular growth pattern with nuclear pleomorphism; they have no local or vascular invasion and no lymph node or distant metastasis[18].
This case suggests that any unusual parathyroid histology should be reviewed, and re-resection should be performed if there is suspicion of carcinoma. In such cases, pregnancy should not be a reason for withholding aggressive surgery, especially in very young patients.
A 24-year-old female patient with parathyroid carcinoma and two consecutive pregnancies.
Hypercalcemia, hyperparathyroidism.
Adenoma of the parathyroid gland.
Calcium, 3.62 mmol/L (normal range, 2.14-2.53 nmol/L); ionized calcium, 1.87 mmol/L (normal range, 1.18-1.32); phosphorus, 0.53 mmol/L (normal range, 0.79-1.42 nmol/L); alkaline phosphatase, 187 U/L (normal range, 64-153 U/L); bone alkaline phosphatase, 133 U/L (normal range for premenopausal women, 14.2-42.7 U/L); intact parathyroid hormone (PTH), 36 pmol/L (normal range, 1-6.0 pmol/L). PTH in aspirate of right lower parathyroid gland higher than 150 pmol/L.
Neck ultrasound; enlarged right lower parathyroid gland (well-defined mass hypoechoic to thyroid tissue with a homogenous echo pattern measuring 21 mm × 15 mm × 15 mm. Tc-99m sestamibi scan; an area of increased uptake on the right side of neck.
Initial pathological diagnosis atypical adenoma of parathyroid gland; tissue made up of main and oxyphil cell-type, no significant remodeling of stroma cells or polymorphism, tumor cells monotonous, small cells with focal cytological atypia, mitotic figures not observed. Tumor is surrounded by thick capsule; cells infiltrate capsule, not breaking through. There is no invasion of tumor cells in blood vessels and no invasion of surrounding fat tissue. Next pathology report described a parathyroid carcinoma with mitotic figures, trabecular pattern and invasion of skeletal muscle.
In five years, four consecutive surgeries were performed to remove tissue surrounding the malignant parathyroid gland, lymph nodes and local metastases. Hypercalcemia was difficult to control with a combination of pamidronate, cinacalcet and loop diuretic.
HRPT2 gene or parafibromin is a tumor suppressor gene associated with the development of parathyroid carcinoma in both sporadic types and some familial clustering cases.
This case report represents a patient with parathyroid carcinoma and two pregnancies. Pregnancy itself can obscure proper diagnosis of the disease, as the unique physiology of pregnancy can alter clinical findings. It is necessary to be aggressive during the surgical treatment of parathyroid carcinoma.
This is an interesting case report clarifing the clinical findings of a 24-year-old female patient with parathyroid carcinoma and two consequent pregnancies with good outcome, despite poor prognosis due to malignant disease. Generally, the manuscript was straight-forward and well-written on this topic. This article is of potential interest to the readers.
| 1. | Hundahl SA, Fleming ID, Fremgen AM, Menck HR. Two hundred eighty-six cases of parathyroid carcinoma treated in the U.S. between 1985-1995: a National Cancer Data Base Report. The American College of Surgeons Commission on Cancer and the American Cancer Society. Cancer. 1999;86:538-544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 2. | Favia G, Lumachi F, Polistina F, D’Amico DF. Parathyroid carcinoma: sixteen new cases and suggestions for correct management. World J Surg. 1998;22:1225-1230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 82] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 3. | Christmas TJ, Chapple CR, Noble JG, Milroy EJ, Cowie AG. Hyperparathyroidism after neck irradiation. Br J Surg. 1988;75:873-874. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 56] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 4. | Boyle NH, Ogg CS, Hartley RB, Owen WJ. Parathyroid carcinoma secondary to prolonged hyperplasia in chronic renal failure and in coeliac disease. Eur J Surg Oncol. 1999;25:100-103. [PubMed] |
| 5. | Yoshimoto K, Endo H, Tsuyuguchi M, Tanaka C, Kimura T, Iwahana H, Kato G, Sano T, Itakura M. Familial isolated primary hyperparathyroidism with parathyroid carcinomas: clinical and molecular features. Clin Endocrinol (Oxf). 1998;48:67-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 27] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 6. | Howell VM, Haven CJ, Kahnoski K, Khoo SK, Petillo D, Chen J, Fleuren GJ, Robinson BG, Delbridge LW, Philips J. HRPT2 mutations are associated with malignancy in sporadic parathyroid tumours. J Med Genet. 2003;40:657-663. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 275] [Cited by in RCA: 229] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 7. | Amaya García M, Acosta Feria M, Soto Moreno A, Dios Fuentes E, Navarro González E, Quijada Thong D, Del Valle A, Acosta Delgado D, Astorga Jiménez R. Primary hyperparathyroidism in pregnancy. Gynecol Endocrinol. 2004;19:111-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 27] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 8. | Diaz-Soto G, Linglart A, Sénat MV, Kamenicky P, Chanson P. Primary hyperparathyroidism in pregnancy. Endocrine. 2013;44:591-597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 61] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 9. | Paul RG, Elston MS, Gill AJ, Marsh D, Beer I, Wolmarans L, Conaglen JV, Meyer-Rochow GY. Hypercalcaemia due to parathyroid carcinoma presenting in the third trimester of pregnancy. Aust N Z J Obstet Gynaecol. 2012;52:204-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 10. | Montoro MN, Paler RJ, Goodwin TM, Mestman JH. Parathyroid carcinoma during pregnancy. Obstet Gynecol. 2000;96:841. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 11. | Parham GP, Orr JW. Hyperparathyroidism secondary to parathyroid carcinoma in pregnancy. A case report. J Reprod Med. 1987;32:123-125. [PubMed] |
| 12. | Hess HM, Dickson J, Fox HE. Hyperfunctioning parathyroid carcinoma presenting as acute pancreatitis in pregnancy. J Reprod Med. 1980;25:83-87. [PubMed] |
| 13. | Panchani R, Varma T, Goyal A, Tripathi S. Parathyroid carcinoma masquerading as morning sickness in pregnancy. Indian J Endocrinol Metab. 2013;17:S198-S200. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 14. | Abdelgadir Adam M, Untch BR, Olson JA. Parathyroid carcinoma: current understanding and new insights into gene expression and intraoperative parathyroid hormone kinetics. Oncologist. 2010;15:61-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 38] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 15. | Westreich RW, Brandwein M, Mechanick JI, Bergman DA, Urken ML. Preoperative parathyroid localization: correlating false-negative technetium 99m sestamibi scans with parathyroid disease. Laryngoscope. 2003;113:567-572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 37] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 16. | Evangelista L, Sorgato N, Torresan F, Boschin IM, Pennelli G, Saladini G, Piotto A, Rubello D, Pelizzo MR. FDG-PET/CT and parathyroid carcinoma: Review of literature and illustrative case series. World J Clin Oncol. 2011;2:348-354. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 61] [Cited by in RCA: 65] [Article Influence: 4.3] [Reference Citation Analysis (12)] |
| 17. | Schnatz PF, Thaxton S. Parathyroidectomy in the third trimester of pregnancy. Obstet Gynecol Surv. 2005;60:672-682. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 62] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 18. | Fernandez-Ranvier GG, Khanafshar E, Jensen K, Zarnegar R, Lee J, Kebebew E, Duh QY, Clark OH. Parathyroid carcinoma, atypical parathyroid adenoma, or parathyromatosis? Cancer. 2007;110:255-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 100] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
P- Reviewers: Kara PO, Olsha O, Wang CC, Xiao DL, Zampieri N S- Editor: Song XX L- Editor: A E- Editor: Liu SQ