Published online Nov 16, 2014. doi: 10.12998/wjcc.v2.i11.711
Revised: July 28, 2014
Accepted: September 4, 2014
Published online: November 16, 2014
Processing time: 198 Days and 5.5 Hours
We report the first case of a neonate with concurrent Chiari II malformation and achondroplasia. Although rare, both these conditions contribute to several deleterious anatomical changes at the cervicomedullary junction and thus predispose to acute hydrocephalus. Although our patient was initially asymptomatic, hydrocephalus ensued several weeks after birth and required cerebral spinal fluid diversion. We discuss the potential links between the two conditions, the pathophysiology, and the important clinical implications for the management of the increased risk of hydrocephalus.
Core tip: Achondroplasia and Chiari II malformations can induce similar anatomical changes at the cervicomedullary junction which increase the risk of acute hydrocephalus. Chiari decompression may not always be necessary, however, diligent and acute follow-up is important to monitor for signs of impeding hydrocephalus. If cerebral spinal fluid diversion is required, remember that shunt failure is common in the pediatric age group and also requires close follow-up.
- Citation: Awad AW, Aleck KA, Bhardwaj RD. Concomitant achondroplasia and Chiari II malformation: A double-hit at the cervicomedullary junction. World J Clin Cases 2014; 2(11): 711-716
- URL: https://www.wjgnet.com/2307-8960/full/v2/i11/711.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v2.i11.711
Achondroplasia is the most common cause of dwarfism, occurring at a frequency of 1 and 26000 live births[1]. Mutations of the FGFR3 gene, which encodes a tyrosine kinase receptor that is deferentially expressed during various stages of development, are responsible for the characteristic short-stature and macrocephaly[2,3]. The condition follows an autosomal dominant inheritance pattern with complete penetrance, however 80%-90% of cases are due to sporadic mutation[1,4]. The FGFR3 gene has been mapped to chromosome 4p16.3, and is part of a larger family of fibroblast growth factor receptor genes which play important roles in skeletal development[2,3].
The Chiari II malformation (CM II) is a well characterized congenital malformation of the central nervous system. Although the exact mechanism is still disputed, the condition is defined by a small posterior fossa and caudal displacement of the brainstem and cerebellum through the foramen magnum[5]. In addition, all cases of CM II are associated with a myelomeningocele[5] which can be further complicated by hydrocephalus, syringomyelia, heterotopias, and agenesis of the corpus callosum are only some of the commonly reported sequelae[5-7].
In our literature review we found two case reports of patients with both achondroplasia and Chiari I malformations[8,9], however to our knowledge there are no published case reports of concomitant achondroplasia and CM II. Here we report the first such case in a neonate, and discuss the potential links between the two conditions, the pathophysiology, and the management of the increased risk of hydrocephalous.
A 1 d old male born after 39-3/7 wk of gestation to a 39-year-old gravid 1, para 0 women presented after delivery for surgical correction of a sacral mass. During pregnancy, an elevated AFP was noted and ultrasound findings demonstrated enlarged ventricles, large for gestational age status, and a sacral myelomeningocele. The infant was delivered via cesarean section with no complications; at birth, 1 and 5 min APGARs were 8 and 9 respectively. The child’s cranium appeared relatively macrocephalic and measured in the 75th percentile with a short cranial base and mild frontal bossing. A 7 cm × 7 cm sacral myelomeningocele was noted and no cerebral spinal fluid (CSF) leak was evident. The extremities showed shortening of the femurs and humeri with relatively long fibulae and a trident confirmation of the hands. The patient had a short stature with an upper-lower segment ratio of 1.9 (normal 1.6 to 1.7), these exam findings initiated a genetic and skeletal survey for achondroplasia. The remainder of the physical and neurological exam were normal.
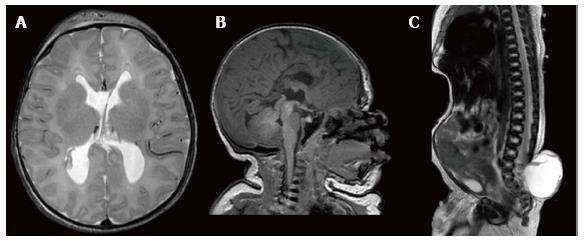
An axial T-2 magnetic resonance image (MRI) of the head showed no signs of hydrocephalus (Figure 1A). There was no evidence of a tonsillar herniation, however, a tight cervicomedullary junction was noted on the sagittal T-1 MRI of the head (Figure 1B), and the lateral and third ventricles were slightly enlarged. The corpus callosum and cerebral vermis were noted to be hypoplastic and a small posterior fossa with frontal bossing were evident. A sagittal T-2 MRI of the spine (Figure 1C) demonstrated a well circumscribed hyperintense sacral mass protruding dorsally, with a loss of posterior spinal elements distal to L3. There was some evidence of cauda equina involvement and tethering of the cord.
The patient’s radiographic and physical exam findings were consistent with a CM II. The patient was taken to the operating room on the same day of presentation to undergo repair of the myelomeningocele. A typical elliptical incision was made adjacent to the defect with midline extension caudal and rostral to the dome. The myelomeningocele was delicately dissected away from the dome skin and the surrounding fascial and dural layers. The termination of the placode was detethered from the dome skin, and the dorsal and ventral nerve roots were mobilized laterally. The lateral edges of the placode were approximated and sutured to form a closed cavity. The dura was then closed and no signs of CSF leak were evident after valsalva. Finally, the fascial layers and skin were re-approximated; some trimming of the excess skin was necessary. The operation was completed without complications and the patient’s recovery was monitored in the neonatal intensive care unit.
A genetic evaluation for achondroplasia was positive for a mutation of the FGFR3 gene on chromosome 4 showed a glycine to arginine substation mutation at the 380th amino acid residue confirming a diagnosis of achondroplasia. It was not immediately clear if the CM II was related to the patients’ concomitant achondroplasia. The patient was monitored post-operatively for 6 d. During that period the fontanelles remained soft and there were no signs of increased intracranial pressure (ICP). The patients’ family was counseled on the symptoms of hydrocephalous and the patient was discharged home with instructions to follow up in 2 wk.
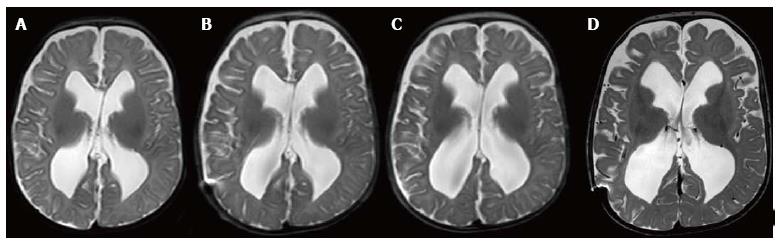
In the subsequent weeks, the patient had bi-weekly clinical and ultrasound exams which were normal. At 8 wk however, the patients head circumference was in the 97th percentile, the anterior fontanelle was full and pulsatile with signs of sagittal suture splaying, but the patient had no signs of respiratory distress. A T2 MRI (Figure 2A) showed evidence of ventriculomegaly with increased extra-axial spacing. Given the physical exam and radiographic findings we placed a ventriculoperitoneal (VP) shunt in the right lateral ventricle without incident.
After discharge the patient was monitored with serial MRIs (Figure 2B, C) which demonstrated subtle but continued ventriculomegaly. During this time the patient was asymptomatic and had normal exam findings.
Four months following shunt placement, the patient was found to have an enlarged head circumference that measured above the 95th percentile. The patient was reported to be less active than usual and the anterior fontanelle was full but soft. A T-2 MRI demonstrated the VP shunt catheter was no longer in the lateral ventricle; subsequently, the lateral and third ventricle had enlarged (Figure 2D) and there was evidence of encroachment of the cerebellar tonsils into the spinal canal. The patient required shut revision, which was completed without complications. At the time of the shunt revision, somatosensory evoked potentials were within normal limits and Chiari decompression was not carried out.
The patient is now 10 mo old and we continue to monitor his head circumference which has stabilized since shunt revision.
Embryogenesis is a complex physiologic phenomenon that is subject to a wide range of exogenous and endogenous forces that can have significant impact on developmental outcomes. At times, these changes are so gross they can be detected in-utero as in the case of a CM II, in which a myelomeningocele is evident in pre-partum ultrasound. Conversely, in conditions like achondroplasia, developmental changes become apparent shortly after birth. Although the exact mechanism by which these effects take place are not entirely delineated, understanding them is crucial to the development of preventive and therapeutic strategies.
As in our case, almost all cases of achondroplasia are due to a substitution mutation of the 380th amino acid Gly for an Arg residue, which resides in the transmembrane domain of the receptor protein[2,3]. Gene expression studies in mice have shown elevated levels of mRNA encoding the FGFR3 protein in the rudimentary cartilage of all premature bone during organogenesis[10]. The effects of achondroplasia on the developing calvarium can produce signs and symptoms similar to those seen in CM II. In addition to the characteristic macrocephalic changes commonly seen, changes in the shape and size of the foramen magnum, cervical stenosis, cervicomedullary compression and upward herniation of the brainstem are common[8,11].
Although there are several proposed hypotheses describing the pathogenesis of CM II, the exact mechanism remains elusive. Animal models have demonstrated that open neural tube defects (NTD) are causative in the case of CM II. NTD that were surgically created in mouse, rat, and sheep models replicate the hindbrain herniation through the foramen magnum that is stereotypical of CM II[12-14]. This finding has two important implications; firstly it lends considerable evidence to the widely accepted “unified theory” of CM II[6]. This theory alleges the loss of CSF through the open caudal NTD causes a subsequent drop in ICP. This loss of pressure at a critical point during fetal development results in poor cranial vault expansion culminating into a small posterior fossa. The unexpectedly narrowed posterior fossa leads to the caudal displacement of the brainstem and cerebellum through the foramen magnum[6]. Secondly, the causal relationship of NTD and CM II has valuable corollaries for prevention. Folic acid supplementation has been found to reduce the incidence of NTD by 70%[15]. The incidence of CM II has not been well studied to determine whether the expected decrease exists following the increased use of folic acid supplementation.
Due to the association of folic acid and NTD, there has been considerable research evaluating the role of enzymes and transport proteins involved in its metabolism, namely MTRR, MTHFD1 and FOLR1-2[16]. However, these genes appear to have little relation to those of achondroplasia and make up only a small portion of candidate genes. The genetic causes of NTD appear to be far more complex and varied than originally anticipated; over 200 gene mutations in mice are known to cause NTD[17]. Similarly in humans, NTD defects arise in the setting of multiple syndromes (Pallister-Hall, Walker-Warburg and Fanconi anemia among others) and chromosomal abnormalities including Trisomies 13 and 18[18-20]. Of the wide range of potential gene candidates we focused on reports of mutations involving chromosome 4, where the FGFR3 gene is located. In a small series of 5 autopsy reports of fetuses with Wolf-Hirschhorn syndrome due to partial mutations (deletion/substitution) of the short arm of chromosome 4, three patients had sacral dimples, while 2 had partial or complete agenesis of the corpus callosum, all patients exhibited growth retardation, and had consistent craniofacial abnormalities including frontal bossing[21]. Furthermore, reports of achondroplasia and other spinal dysraphisms exist including tethered cords and lipomas indicating the range of phenotypes that ensue from genetic mutations in this region[22,23].
Familial studies of Chiari malformations are limited mainly to Chiari I malformations, as such, the genetics of CM II are not as well characterized. Animal models have suggested possible gene candidates. The Splotch mouse model for example can produce NTD and the hindbrain herniation characteristic of the CM II[6]. The genetic mutation responsible for this phenotype in mouse models was mapped to the human Pax-3 gene on chromosome 1, encodes transcription factors which play various roles in embryogenesis[24,25]. Further studies in humans however, demonstrated that mutations in Pax-3 generate distinct features not commonly seen in CM II, namely deafness and abnormal pigmentation which were later characterized into a distinct Waardenburg Syndrome[25,26].
Given our patients’ concurrent conditions, the concern for hydrocephalus was high. Brainstem herniation as a result of hydrocephalus in both CM II and achondroplasia can be acutely progressive, and greatly increases the risk of apneic spells and acute respiratory failure[1,11,27]. At the time of presentation we believed the degree of cervicomedullary constriction present did not warrant surgical Chiari decompression, as a result the patient underwent myelomeningocele repair with acute follow-up. Although our patient ultimately required CSF diversion, there were no overt signs of respiratory distress, spasticity, or dysphagia which would warrant a surgical Chiari decompression. In a large series of 148 patients with CM II, only 14% of patients required surgical decompression[7]. Treating the obstructive hydrocephalus by shutting is a more common method that is associated with fewer risks. In a series of 71 cases of CM II, 64 (90%) patients had hydrocephalus, 89% of which required VP shunting[28]. As in our case, shunt placement necessitates close follow-up and thorough patient education. Unfortunately, our patient experienced shunt failure due to tip migration, a risk associated with this treatment. A large series of 1015 patients who underwent VP shunting reported a failure rate of 46.3%, in the pediatric group however, failure rates were reported to be 79.2%, the majority of which occurred in the first 6 mo[29]. Importantly, in patients with spinal dysraphisms, as in our case, failure rates were 84.8%[29]. At last follow up (10 mo) the patient was symptom free with signs of improved CSF outflow. Due to the compounding effects of both underlying conditions on the potential hydrocephalus we continue to monitor the patient with routine monthly follow-up.
In conclusion, both achondroplasia and the CM II are relatively common as independent conditions; however, they very rarely occur concomitantly. Importantly, the CM II is a consequence of NTDs which appear to have multifocal genetic and environmental etiologies. Although the genes involved in achondroplasia appear to be distinct from those of the CM II, due to the complexity of embryologic development, there may be key interactions between the downstream pathways which may account for some of the similar anatomic changes seen at the cervicomedullary junction. As such the management of the potential hydrocephalus that may arise within patients with this unique predisposition requires acute and diligent follow up and patient education.
A newborn boy presented with macrocephaly, short limbs, and a sacral mass.
Concurrent achondroplasia and Chiari II malformation.
Myelomeningocele has a unique gross presentation however a sacrococcygeal teratoma should be considered and the two conditions can be easily differentiated using magnetic resonance imaging. The differential of a newborn with macrocephaly is quite large and includes congenital infections, obstructive hydrocephalus, metabolic conditions (Canavan’s disease, Alexander’s disease, etc.), and osteogenensis imperfecta are only a few to consider.
A genetic test indicated a missense mutation of the FGFR3 gene on chromosome 4 confirming a diagnosis of Achondroplasia.
MRI of the head and spinal column confirmed a diagnosis of Chiari II malformation.
Surgical myelomeningocele repair and ventriculoperitoneal shunting due to hydrocephalous is a standard treatment.
This is the first reported case of both a Chiari II malformation and achondroplasia in the same patient. Cases of concurrent Chiari I malformation and achondroplasia have been reported in references 7 and 8.
Spina bifida is an incomplete closure of the spine during embryogenesis. A myelomeningocele is a form of spina bidifia that includes a herniation of the spinal meninges through the spinal defect.
Shunt failure is more common in pediatric patients with spinal dysraphisms, generally occurring within the first 6 mo.
The manuscript of Awad et al describes a neonate case with concomitant achondroplasia and Chiari II malformation. According to a literature review performed by the authors this is the first case report of a patient with this combination. This is definitely an interesting case that is thoroughly documented. Everything seems to be very well described.
| 1. | Oberklaid F, Danks DM, Jensen F, Stace L, Rosshandler S. Achondroplasia and hypochondroplasia. Comments on frequency, mutation rate, and radiological features in skull and spine. J Med Genet. 1979;16:140-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 125] [Article Influence: 2.7] [Reference Citation Analysis (1)] |
| 2. | Shiang R, Thompson LM, Zhu YZ, Church DM, Fielder TJ, Bocian M, Winokur ST, Wasmuth JJ. Mutations in the transmembrane domain of FGFR3 cause the most common genetic form of dwarfism, achondroplasia. Cell. 1994;78:335-342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 967] [Cited by in RCA: 894] [Article Influence: 27.9] [Reference Citation Analysis (0)] |
| 3. | Vajo Z, Francomano CA, Wilkin DJ. The molecular and genetic basis of fibroblast growth factor receptor 3 disorders: the achondroplasia family of skeletal dysplasias, Muenke craniosynostosis, and Crouzon syndrome with acanthosis nigricans. Endocr Rev. 2000;21:23-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 87] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 4. | Murdoch JL, Walker BA, Hall JG, Abbey H, Smith KK, McKusick VA. Achondroplasia--a genetic and statistical survey. Ann Hum Genet. 1970;33:227-244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 87] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 5. | Stevenson KL. Chiari Type II malformation: past, present, and future. Neurosurg Focus. 2004;16:E5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 105] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 6. | McLone DG, Knepper PA. The cause of Chiari II malformation: a unified theory. Pediatr Neurosci. 1989;15:1-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 353] [Cited by in RCA: 303] [Article Influence: 8.2] [Reference Citation Analysis (1)] |
| 7. | Messing-Jünger M, Röhrig A. Primary and secondary management of the Chiari II malformation in children with myelomeningocele. Childs Nerv Syst. 2013;29:1553-1562. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 33] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 8. | Thomas IT, Frias JL, Williams JL, Friedman WA. Magnetic resonance imaging in the assessment of medullary compression in achondroplasia. Am J Dis Child. 1988;142:989-992. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 9. | Nakai T, Asato R, Miki Y, Tanaka F, Matsumoto S, Konishi J. A case of achondroplasia with downward displacement of the brain stem. Neuroradiology. 1995;37:293-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 14] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 10. | Peters K, Ornitz D, Werner S, Williams L. Unique expression pattern of the FGF receptor 3 gene during mouse organogenesis. Dev Biol. 1993;155:423-430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 359] [Cited by in RCA: 362] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 11. | Ruiz-Garcia M, Tovar-Baudin A, Del Castillo-Ruiz V, Rodriguez HP, Collado MA, Mora TM, Rueda-Franco F, Gonzalez-Astiazaran A. Early detection of neurological manifestations in achondroplasia. Childs Nerv Syst. 1997;13:208-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 36] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 12. | Inagaki T, Schoenwolf GC, Walker ML. Experimental model: change in the posterior fossa with surgically induced spina bifida aperta in mouse. Pediatr Neurosurg. 1997;26:185-189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 12] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 13. | Paek BW, Farmer DL, Wilkinson CC, Albanese CT, Peacock W, Harrison MR, Jennings RW. Hindbrain herniation develops in surgically created myelomeningocele but is absent after repair in fetal lambs. Am J Obstet Gynecol. 2000;183:1119–1123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 115] [Article Influence: 4.4] [Reference Citation Analysis (1)] |
| 14. | Weber Guimarães Barreto M, Ferro MM, Guimarães Bittencourt D, Violin Pereira LA, Barini R, Sbragia L. Arnold-Chiari in a fetal rat model of dysraphism. Fetal Diagn Ther. 2005;20:437-441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 15. | Prevention of neural tube defects: results of the Medical Research Council Vitamin Study. MRC Vitamin Study Research Group. Lancet. 1991;338:131-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2872] [Cited by in RCA: 2567] [Article Influence: 73.3] [Reference Citation Analysis (0)] |
| 16. | Greene ND, Stanier P, Copp AJ. Genetics of human neural tube defects. Hum Mol Genet. 2009;18:R113-R129. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 216] [Cited by in RCA: 227] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 17. | Harris MJ, Juriloff DM. Mouse mutants with neural tube closure defects and their role in understanding human neural tube defects. Birth Defects Res A Clin Mol Teratol. 2007;79:187-210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 239] [Cited by in RCA: 248] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 18. | Goetzinger KR, Stamilio DM, Dicke JM, Macones GA, Odibo AO. Evaluating the incidence and likelihood ratios for chromosomal abnormalities in fetuses with common central nervous system malformations. Am J Obstet Gynecol. 2008;199:285.e1-285.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 38] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 19. | Detrait ER, George TM, Etchevers HC, Gilbert JR, Vekemans M, Speer MC. Human neural tube defects: developmental biology, epidemiology, and genetics. Neurotoxicol Teratol. 2005;27:515-524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 259] [Cited by in RCA: 248] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 20. | Chen CP. Syndromes, disorders and maternal risk factors associated with neural tube defects (V). Taiwan J Obstet Gynecol. 2008;47:259-266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 16] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 21. | Tachdjian G, Fondacci C, Tapia S, Huten Y, Blot P, Nessmann C. The Wolf-Hirschhorn syndrome in fetuses. Clin Genet. 1992;42:281-287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 45] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 22. | Phadke RV, Phadke SR, Chakravarti AL. Spinal dysraphism in achondroplasia. Pediatr Neurosurg. 1990;16:32-4; discussion 34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 4] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 23. | Archibeck MJ, Smith JT, Carroll KL, Davitt JS, Stevens PM. Surgical release of tethered spinal cord: survivorship analysis and orthopedic outcome. J Pediatr Orthop. 1997;17:773-776. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 24. | Barber TD, Barber MC, Cloutier TE, Friedman TB. PAX3 gene structure, alternative splicing and evolution. Gene. 1999;237:311-319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 61] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 25. | Chalepakis G, Goulding M, Read A, Strachan T, Gruss P. Molecular basis of splotch and Waardenburg Pax-3 mutations. Proc Natl Acad Sci USA. 1994;91:3685-3689. [PubMed] |
| 26. | Tassabehji M, Newton VE, Leverton K, Turnbull K, Seemanova E, Kunze J, Sperling K, Strachan T, Read AP. PAX3 gene structure and mutations: close analogies between Waardenburg syndrome and the Splotch mouse. Hum Mol Genet. 1994;3:1069-1074. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 71] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 27. | Aryanpur J, Hurko O, Francomano C, Wang H, Carson B. Craniocervical decompression for cervicomedullary compression in pediatric patients with achondroplasia. J Neurosurg. 1990;73:375-382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 51] [Article Influence: 1.4] [Reference Citation Analysis (1)] |
| 28. | Elgamal EA. Natural history of hydrocephalus in children with spinal open neural tube defect. Surg Neurol Int. 2012;3:112. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 33] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 29. | Reddy GK, Bollam P, Caldito G. Long-term outcomes of ventriculoperitoneal shunt surgery in patients with hydrocephalus. World Neurosurg. 2014;81:404-410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 267] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
P- Reviewer: Zaucke F S- Editor: Song XX L- Editor: A E- Editor: Lu YJ