Published online Nov 16, 2025. doi: 10.12998/wjcc.v13.i32.110553
Revised: August 8, 2025
Accepted: September 12, 2025
Published online: November 16, 2025
Processing time: 156 Days and 20 Hours
Patients with concurrent acute biliary pancreatitis (ABP) and acute cholangitis (AC) may experience exacerbated clinical consequences due to bile duct stones. However, studies exploring this topic remain limited.
To compare the clinical presentation and outcomes of patients experiencing AC with and without ABP.
This single-center retrospective cohort study included 358 patients with AC who underwent endoscopic retrograde cholangiopancreatography (ERCP) between January 2016 and December 2017. Patients were divided into two groups: AC with ABP (n = 90) and AC without ABP (n = 268). Clinical characteristics, laboratory data, ERCP results, primary study outcome [intensive care unit (ICU) admission], and secondary outcomes including 30-day mortality, length of hospital stay, and 30-day readmission rate were analyzed and compared.
All patients in the AC with ABP group had interstitial pancreatitis. The AC with ABP group had significantly higher white cell count (WBC) counts (13.1 × 10³/µL vs 10.4 × 10³/µL, P = 0.007) and more abnormal WBC results (61.1% vs 42.3%, P = 0.015). Liver biochemical tests, AC severity, ERCP success, adverse events, ICU admissions, 30-day mortality, hospital stay, and readmission rates did not differ significantly between the two groups. Univariate analysis showed no significant link between concurrent ABP and ICU admission, although significance was marginal in moderate/severe ABP cases (P = 0.051). In the multivariate analysis, age (P = 0.035) and cardiovascular dysfunction (P < 0.001) were independently associated with length of ICU stay.
Concurrent interstitial ABP and AC did not significantly affect outcomes. Age and cardiovascular dysfunction were stronger predictors of ICU admission and should guide clinical monitoring and management.
Core Tip: Concurrent acute cholangitis (AC) and acute biliary pancreatitis resulted in significantly higher white cell count (WBC) counts and abnormal WBC counts, suggesting a more intense inflammatory response. However, liver biochemical indices, AC severity, endoscopic retrograde cholangiopancreatography success or adverse events, and primary and secondary outcome measures did not differ significantly between the two groups. Multivariate analysis showed that age and cardiovascular dysfunction were independent factors for intensive care unit admission. Therefore, the presence of acute biliary pancreatitis in patients with AC does not appear to have a significant impact on intensive care unit admission rates or other major clinical outcomes.
- Citation: Liu KT, Lee MH, Lin CH, Tsou YK, Sung KF, Wang SF, Wu CH, Liu NJ. Acute cholangitis due to common bile duct stones: Clinical outcomes in patients with and without concurrent acute pancreatitis. World J Clin Cases 2025; 13(32): 110553
- URL: https://www.wjgnet.com/2307-8960/full/v13/i32/110553.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v13.i32.110553
Acute cholangitis (AC) occurs when bile duct obstruction leads to cholangio-venous reflux of pathogenic microorganisms or endotoxins with common bile duct stones (CBDS) frequently causing this issue[1]. The pathophysiology of acute biliary pancreatitis (ABP) involves obstruction of the pancreatic duct with gallstones that migrate from the bile duct into the common channel at its opening into the duodenum[2]. Persistent CBDS causes ongoing obstruction of the pancreatic duct and/or biliary tree, potentially resulting in pancreatic necrosis and/or AC[3]. Therefore, some patients with CBDS experience complications from both AC and ABP, which is called gallstone cholangio-pancreatitis[4]. ABP may represent a hybrid condition that encompasses a spectrum ranging from AC with mild acute pancreatitis (biliary-type acute pancreatitis) to necrotizing pancreatitis without biliary involvement (pancreatic-type acute pancreatitis)[1].
AC is uncommon in patients with ABP, accounting for only 2%-3% of patients diagnosed with AC in the early studies or using the Tokyo guidelines (TG18/TG13)[5-7]. There are few studies on the prevalence of ABP in patients with AC caused by CBDS. Currently, only one study has reported the presence of ABP in 23.0% (32/139) of gallstone-related AC cases[8]. The simultaneous presence of ABP may intensify the systemic inflammatory response associated with AC and worsen the clinical course by increasing both morbidity and mortality. Although theoretically interesting most studies have not addressed the clinical impact and outcome of concurrent ABP and AC, resulting in an unknown area in the field of these two diseases[9-11].
Therefore, we hypothesized that the presence of ABP in patients with CBDS-related AC may experience exacerbated disease severity and clinical outcomes such as intensive care unit (ICU) admission, hospital stay, and mortality. Clarifying this association will guide timely clinical triage, urgency of interventions, and arrangement of ICU admission. Therefore, this study was conducted to compare the clinical presentation, endoscopic retrograde cholangiopancreatography (ERCP) treatment, and clinical outcomes in patients with CBDS-related AC with or without ABP.
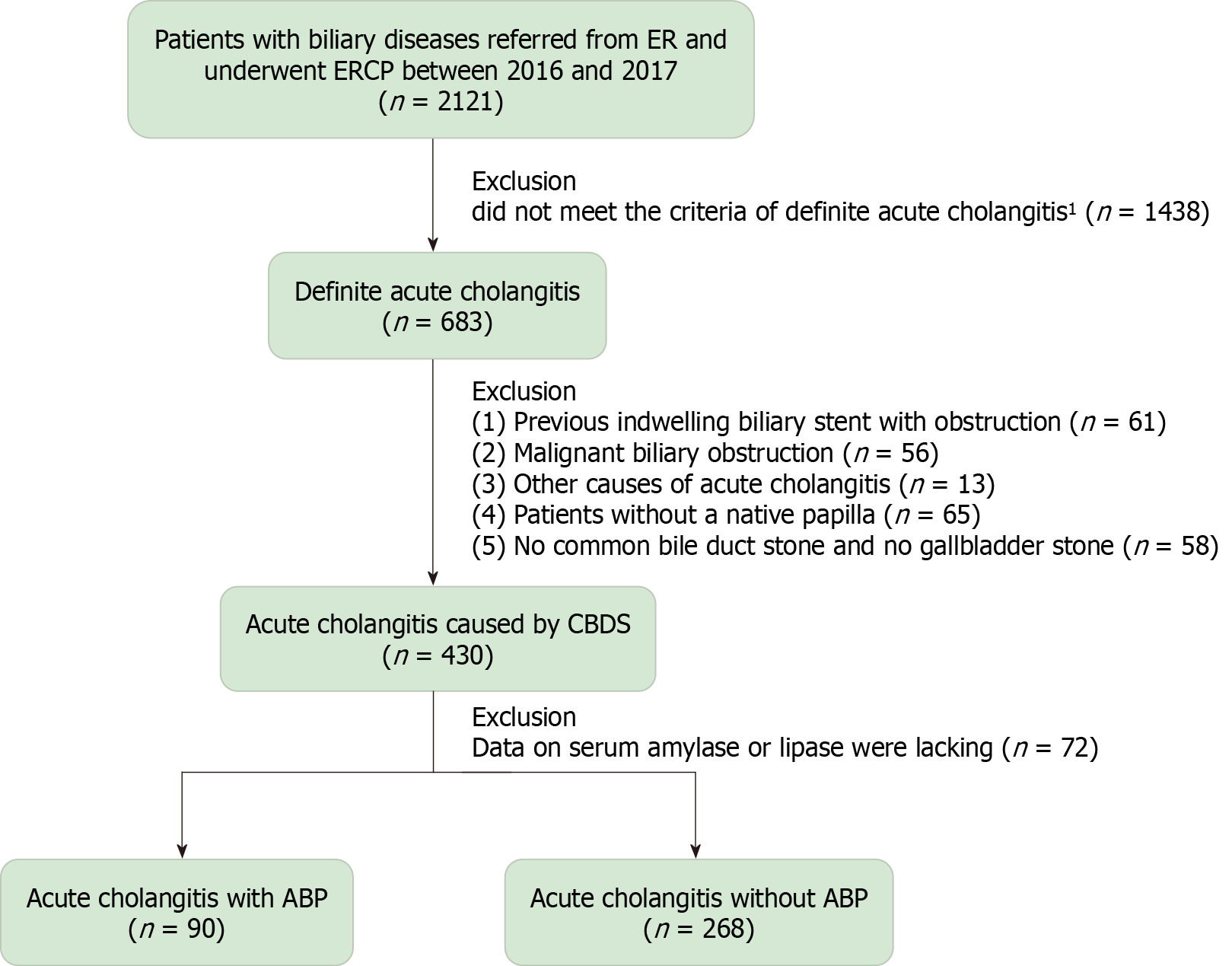
This retrospective cohort study was conducted at a tertiary medical center; a flowchart of the study is provided in Figure 1. ERCP remains the first-line approach to treating patients with AC at our institution, after more than two decades.
The diagnosis of AC was based on the TG18/TG13 criteria, which included systemic inflammation, cholestasis, and imaging findings. The diagnosis of AC was further categorized as definite AC or suspected AC (Supplementary Table 1)[6]. Systemic inflammation was defined by fever (> 38 °C) or an inflammatory response (WBC < 4000 or > 10000/µL or C-reactive protein ≥ 1 mg/dL). Cholestasis included jaundice (bilirubin ≥ 2 mg/dL) or abnormal liver function tests (alkaline phosphatase, γ-glutamyl transferase, aspartate aminotransferase, or alanine aminotransferase > 1.5 × the upper limit of normal). Imaging findings included bile duct dilatation or evidence of strictures, stones, or stents. According to TG18/13, AC severity was classified as mild, moderate, or severe, with severe AC involving organ dysfunction, including cardiovascular, respiratory, renal, hepatic, or hematological dysfunction (Supplementary Table 2)[6].
The diagnosis and severity of ABP were determined according to the revised Atlanta criteria[2,12]. Supplementary Table 3 Lists the revised diagnostic criteria for acute pancreatitis. Severe ABP was defined as the presence of persistent organ failure (lasting > 48 hours) and/or death. The detailed criteria for severity classification are listed in Supplementary Table 4.
The time to ERCP treatment was defined as the interval between the patient’s arrival at the emergency department and the completion of the ERCP procedure. ERCP-related adverse events, including perforation, pancreatitis, delayed bleeding, and cholangitis, were defined according to the lexicon for endoscopic adverse events published by the American Society for Gastrointestinal Endoscopy[13].
A retrospective review was conducted of 683 patients who underwent ERCP at our center between January 2016 and December 2017 and met the TG18/TG13 criteria for definitive AC[6]. Data were retrieved from the database at the Therapeutic Endoscopy Center[11]. Exclusion criteria included the following: (1) AC caused by factors other than CBDS, such as a previously indwelling biliary stent (n = 61), malignant biliary obstruction (n = 56), or other causes (n = 13); (2) The absence of a native papilla (n = 65); (3) Neither CBDS nor gallstones found on any imaging studies before the ERCP, including abdominal ultrasound, computed tomography (CT) scan, or magnetic resonance cholangiopancreatography (n = 58); and (4) Data lacking on serum amylase or lipase (n = 72).
Medical records were reviewed to collect demographic information, including sex and age, clinical manifestations, such as body temperature, systolic blood pressure, heart rate, blood oxygen saturation, respiratory rate, and urine output, and laboratory values, including WBC count, platelet count, prothrombin time-international normalized ratio, and serum levels of creatinine, alanine aminotransferase, aspartate aminotransferase, total bilirubin, alkaline phosphatase, lipase, amylase, and albumin. Gallbladder status (in situ with or without stones or post-cholecystectomy), time to ERCP, and ERCP outcomes, including biliary cannulation success rate, papillary procedures, bile duct clearance (with or without stones), need for stent placement, and adverse events, were also documented. Patient symptoms, vital signs, and laboratory test results used to assess AC severity were collected while patients were in the emergency room. Serum C-reactive protein levels were not collected because C-reactive protein is not a routine test item in the emergency room. Serum albumin level data were available for only 55 patients (12.8%) and were excluded from the analysis.
Patients were divided into two groups: AC with ABP, and AC without ABP. The primary outcome comparison was ICU admission. The decision for ICU admission was made by the doctor on duty using the following common criteria: Respiratory failure requiring intubation and an unstable condition despite the use of norepinephrine. Secondary outcomes were 30-day mortality, length of hospital stay (LOHS), and 30-day readmission rate.
Continuous variables were presented as medians with interquartile ranges while categorical variables were expressed as numbers and percentages. The Mann-Whitney U test was used for comparisons of continuous variables, and Pearson’s χ2 test or Fisher’s exact test was applied to appropriate categorical variables. Logistic regression analysis was conducted to identify factors associated with ICU admissions, with variables having a P value < 0.05 in the univariate analysis included in the multivariate analysis. Results from the univariate and multivariate analyses were reported as odds ratios (ORs) with 95% confidence intervals (CIs). Statistical significance was defined as two-tailed P values < 0.05. All statistical analyses were performed using SPSS software (version 29.0; IBM Corp., Armonk, NY, United States).
This study included a total of 358 patients who met the inclusion criteria. Of these, 90 patients (25.1%) were diagnosed with concurrent ABP and AC. All patients had interstitial edematous pancreatitis on their initial CT scan in the emergency room. This group included 7 patients (7.8%) with severe ABP and 9 patients (10.0%) with moderate ABP. The remaining 268 patients (74.9%) were diagnosed with AC.
Table 1 Lists the patient demographic, laboratory, and clinical characteristics. The median age, sex, and body temperature at onset were comparable between the AC with ABP group and the AC without ABP group. The AC with ABP group had a significantly higher WBC count (13.1 × 103/µL vs 10.4 × 103/µL, P = 0.007) and a higher percentage of patients with an abnormal WBC count (61.1% vs 42.3%, P = 0.015). There were no significant differences in any of the liver biochemical tests. Blood cultures were obtained for 293 patients, yielding a positive rate of 19.2% (14/73) in the AC with ABP group and 25.9% (57/220) in the AC without ABP group (P = 0.245).
| Variables | AC with ABP, n = 90 | AC without ABP, n = 268 | P value |
| Age in years | 73 (64-82) | 63 (52-75) | 0.305 |
| Male sex | 52 (57.8) | 154 (57.5) | 0.958 |
| Body temperature in °C | 37.4 (36.9-38.0) | 37.5 (36.9-38.4) | 0.797 |
| WBC count as × 103/µL | 13.1 (9.2-17.3) | 10.4 (8.5-15.3) | 0.007 |
| Abnormal WBC count | 55 (61.1) | 124 (42.3) | 0.015 |
| AST in U/L | 178.5 (83-261) | 193 (111-468) | 0.722 |
| ALT in U/L | 175 (89.5-290.5) | 222 (136-447) | 0.790 |
| ALK-P in U/L | 215 (135.5-300.5) | 181 (123-267) | 0.316 |
| Total bilirubin in mg/dL | 3.5 (2.1-5.3) | 3.6 (2.5-6.2) | 0.795 |
| Creatinine in mg/dL | 1.08 (0.90-1.27) | 0.88 (0.73-1.15) | 0.201 |
| Amylase | 1165 (611-2368) | 47.6 (40.0-69.4) | < 0.01 |
| Lipase | 2054 (1128-4289) | 28 (24-40) | < 0.01 |
| Blood culture performed, n = 293 | 73 (81.1) | 220 (82.1) | 0.835 |
| Positive blood culture | 14/73 (19.2) | 57/220 (25.9) | 0.245 |
| Gallbladder status | |||
| In situ with stone | 55 (61.1) | 156 (58.2) | 0.069 |
| In situ without stone | 28 (31.1) | 63 (23.5) | 0.152 |
| Post cholecystectomy | 7 (7.8) | 49 (18.3) | 0.018 |
| CBDS on images before ERCP | 61 (67.8) | 171 (63.8) | 0.495 |
| Abdominal ultrasound, n = 89 | 1/19 (5.3) | 9/70 (12.9) | 0.353 |
| CT scan, n = 356 | 58/90 (64.4) | 154/266 (57.9) | 0.274 |
| MRCP, n = 19 | 3/4 (75) | 15/15 (100) | 0.047 |
| Median CBDS diameter in mm | 5.6 (4.0-8.7) | 6.8 (4.7-10.0) | 0.775 |
| Severity of AC | |||
| Mild | 39 (43.3) | 141 (52.6) | 0.088 |
| Moderate | 28 (31.1) | 71 (26.5) | 0.397 |
| Severe | 23 (25.6) | 56 (20.9) | 0.356 |
| Organ dysfunction | |||
| Cardiovascular | 4 (4.4) | 12 (4.5) | 0.989 |
| Neurological | 6 (6.7) | 14 (5.2) | 0.606 |
| Respiratory | 6 (6.7) | 7 (2.6) | 0.075 |
| Renal | 11 (12.2) | 20 (7.5) | 0.165 |
| Hepatic | 4 (4.4) | 3 (1.1) | 0.044 |
| Hematological | 8 (8.9) | 16 (6.0) | 0.352 |
Patients who underwent cholecystectomy before the AC episode were significantly less prevalent in the AC with ABP group than in the AC without ABP group (7.8% vs 18.3%, P = 0.018). Before ERCP, 89 patients (24.9%) underwent transabdominal ultrasonography, 356 patients (99.4%) underwent CT scan, and 19 patients (5.3%) underwent magnetic resonance cholangiopancreatography. CBDS was evident on at least one of these images for 61 patients (67.8%) in the AC with ABP group and 171 patients (63.8%) in the AC without ABP group (P = 0.495). The median stone diameter was 5.6 mm and 6.8 mm in the two groups, respectively (P = 0.775).
There were no significant differences in AC severity and the proportion of organ dysfunction between the AC with ABP and AC without ABP groups.
The results of ERCP are listed in Table 2. The time to ERCP treatment in the AC with ABP group was significantly shorter than in the AC without ABP group (median: 45.4 hours vs 62.6 hours, P = 0.030). When stratified by AC severity, the time to ERCP treatment in patients with moderate AC was also significantly shorter in the AC with ABP group (median: 35.9 hours vs 46.6 hours, P = 0.029). However, this difference was not observed in patients with mild or severe AC.
| Variables | AC with ABP, n = 90 | AC without ABP, n = 268 | P value |
| Time to ERCP in hours | |||
| Overall population | 45.4 (30.4-69.3) | 62.6 (26.0-83.1) | 0.030 |
| Mild AC | 42.1 (18.4-71.8) | 46.3 (28.6-84.0) | 0.243 |
| Moderate AC | 35.9 (19.5-57.6) | 46.6 (28.9-76.4) | 0.029 |
| Severe AC | 63.4 (28.6-91.8) | 67.5 (28.8-94.6) | 0.538 |
| ERCP procedure | |||
| Successful biliary cannulation | 90 (100) | 264 (98.5) | 0.244 |
| Papillary procedures, n = 354 | |||
| EST | 89 (98.9) | 253 (94.4) | 0.167 |
| EPBD | 0 | 4 (1.5) | 0.241 |
| No EST or EPBD | 1 (1.1) | 10 (3.7) | 0.206 |
| Bile duct sweep, n = 341 | 86 (95.6) | 255 (95.1) | 0.844 |
| With stones | 72/86 (83.7) | 230/255 (90.2) | 0.103 |
| Without stones | 14/86 (16.3) | 25/255 (9.8) | 0.103 |
| Stent insertion | 5 (5.6) | 23 (8.6) | 0.338 |
| Adverse event due to ERCP | 5 (5.6) | 14 (5.2) | 0.921 |
| Delayed bleeding | 2 (2.2) | 6 (2.2) | 0.982 |
| Pancreatitis | 0 | 6 (1.9) | 0.344 |
| AC | 2 (2.2) | 1 (0.4) | 0.159 |
| Perforation | 2 (2.2) | 1 (0.4) | 0.159 |
There was no statistically significant difference in the success rate of deep bile duct cannulation between the two groups (100% vs 98.5%, P = 0.244). Similarly, the frequency of papillary procedures, such as endoscopic sphincterotomy or endoscopic papillary balloon dilatation, did not significantly differ between the groups. Bile duct sweeping was performed in 341 patients: 86 patients (95.6%) in the AC with ABP group and 255 patients (95.1%) in the AC without ABP group (P = 0.844). Among those who underwent bile duct sweeping, stones were removed from 72 patients (83.7%) in the AC with ABP group and 230 patients (90.2%) in the AC without ABP group (P = 0.103).
Plastic stents were placed in 28 patients (7.8%) because of unstable clinical conditions, bile duct stricture (including Mirizzi syndrome), coagulopathy or use of anticoagulants, incomplete stone removal, or significant bleeding after endoscopic sphincterotomy. The incidence of stenting was similar in the two groups. The overall ERCP-related adverse events, including perforation, delayed post-endoscopic sphincterotomy bleeding, biliary tract infection, and pancreatitis, were not significantly different between the two groups (5.6% vs 5.2%, P = 0.921).
Table 3 shows the primary and secondary outcomes for the overall population and for the population when stratified by AC severity. ICU admission rates were similar in the AC with ABP and AC without ABP groups (6.7% vs 4.5%, P = 0.411). Secondary outcomes included 30-day mortality (1.1% vs 0.4%, P = 0.416), LOHS (median: 7 days vs 7 days, P = 0.554), and 30-day readmission rate (8.9% vs 6.7%, P = 0.492), which did not differ significantly between the two groups.
| Variables | AC with ABP, n = 90 | AC without ABP, n = 268 | P value |
| Overall population | (n = 90) | (n = 268) | |
| ICU admission | 6 (6.7) | 12 (4.5) | 0.411 |
| 30-day mortality | 1 (1.1) | 1 (0.4%) | 0.416 |
| LOHS in days | 7.0 (5.5-9.5) | 7.0 (5.0-9.0) | 0.554 |
| 30-day readmission | 8 (8.9) | 18 (6.7) | 0.492 |
| Mild AC | (n = 39) | (n = 141) | |
| ICU admission | 0 | 0 | |
| 30-day mortality | 0 | 0 | |
| LOHS in days | 6.0 (5.0-8.0) | 6.0 (5.0-8.0) | 0.823 |
| 30-day readmission | 4 (10.2) | 5 (3.5) | 0.097 |
| Moderate AC | (n = 28) | (n = 71) | |
| ICU admission | 0 | 0 | |
| 30-day mortality | 0 | 0 | |
| LOHS in days | 6.0 (5.0-8.5) | 6.0 (5.0-9.0) | 0.666 |
| 30-day readmission | 1 (3.6) | 7 (9.9) | 0.301 |
| Severe AC | (n = 23) | (n = 56) | |
| ICU admission | 6 (26.0) | 12 (21.4) | 0.201 |
| 30-day mortality | 3 (13.0) | 6 (10.7) | 0.510 |
| LOHS in days | 9.0 (7.0-10.5) | 7.5 (6.0-11.0) | 0.409 |
| 30-day readmission | 3 (13.0) | 6 (10.7) | 0.767 |
The reasons for readmission within 30 days in the two groups are shown in Table 4. A total of 26 patients (7.3%) were readmitted within 30 days, including 3 cases of acute cholecystitis (0.8%), 3 cases of acute pancreatitis (0.8%), 4 cases of recurrent AC (1.1%), 2 cases of delayed bleeding after endoscopic sphincterotomy (0.6%), 7 cases of planned laparoscopic cholecystectomy (2.0%), and 7 cases of unrelated biliary or pancreatic diseases (2.0%).
| Reason | AC with ABP, n = 90 | AC without ABP, n = 268 | Overall, n = 358 |
| Acute cholecystitis | 1 (1.1) | 2 (0.8) | 3 (0.8) |
| Acute pancreatitis | 2 (2.2) | 1 (0.4) | 3 (0.8) |
| AC | 0 | 4 (1.5) | 4 (1.1) |
| Post-EST bleeding | 0 | 2 (0.8) | 2 (0.6) |
| Scheduled laparoscopic cholecystectomy | 3 (3.3) | 4 (1.5) | 7 (2.0) |
| Other unrelated disorders | 2 (2.2) | 5 (1.9) | 7 (2.0) |
When stratified by AC severity, ICU admission, 30-day mortality, LOHS, and 30-day readmission rates were comparable between the two groups for mild, moderate, and severe disease.
We performed a subgroup analysis restricting ABP to severe or moderate severity, and the results showed that the AC combined with severe/moderate ABP group had a significantly higher ICU admission rate (18.8% vs 4.5%, P = 0.013), a significantly higher 30-day mortality rate (6.3% vs 0.4%), and a significantly longer hospital stay (median, 8.5 days vs 7 days).
Table 5 presents the factors associated with ICU admission. In the univariate analysis, age, sex, WBC count, time to ERCP within 12 hours, any ERCP-related adverse event, and organ failure (including cardiovascular, neurological, or hepatic dysfunction) were associated with ICU admission. Concurrent pancreatitis (OR: 1.524, 95%CI: 0.670-4.186, P = 0.414) was not significantly associated with ICU admission. However, when only severe and moderate ABP cases were considered, this factor showed marginal significance (OR: 5.462, 95%CI: 0.992-30.077, P = 0.051).
| Factor | Univariate analysis | Multivariate analysis | |||
| OR (95%CI) | P value | OR (95%CI) | P value | ||
| Age | Every 1-year increase | 1.103 (1.050-1.159) | < 0.001 | 1.179 (1.012-1.373) | 0.035 |
| Sex | Male | 2.218 (0.839-5.862) | 0.108 | ||
| WBC count | 1.148 (0.555-1.230) | < 0.001 | 1.014 (0.875-1.174) | 0.857 | |
| Acute pancreatitis | Yes | 1.524 (0.670-4.186) | 0.414 | ||
| Severe plus moderate acute pancreatitis | Yes | 5.462 (0.992-30.077) | 0.051 | ||
| Time to ERCP within 12 hours | Yes | 6.880 (2.211-21.414) | < 0.001 | 11.984 (0.346-415.629) | 0.170 |
| Any ERCP-related AE | Yes | 6.133 (1.800-20.897) | 0.004 | 3.123 (0.125-77.908) | 0.488 |
| Cardiovascular dysfunction1 | Yes | 292.067 (62.939-1355.333) | < 0.001 | 1201.997 (28.078-51457.080) | < 0.001 |
| Neurological dysfunction (conscious disturbance) | Yes | 29.909 (9.935-90.038) | < 0.001 | 28.347 (1.829-439.339) | 28.347 |
| Respiratory dysfunction (PaO2/FiO2 ratio < 300) | Yes | Infinite | 0.998 | ||
| Renal dysfunction (Cr > 2.0 mg/dL) | Yes | 2.229 (0.608-8.165) | 0.226 | ||
| Hepatic dysfunction (PT-INR > 1.5) | Yes | 15.375 (3.135-75.408) | < 0.001 | 2.443 (0.077-77.235) | 0.612 |
| Hematological dysfunction (PLT < 100 × 103/µL) | Yes | 3.010 (0.807-11.220) | 0.101 | ||
In the multivariate analysis independent factors associated with ICU admission were age (OR: 1.179, 95%CI: 1.012-1.373, P = 0.035) and cardiovascular dysfunction (OR: 1201.997, 95%CI: 28.078-51457.080, P < 0.001).
The diagnosis of AC according to the TG18/TG13 can be divided into definite AC or suspected AC[6]. Using only definite AC diagnostic criteria, we found that 18.7% of patients with AC also had ABP. The inflammation of surrounding tissue due to ABP may theoretically increase the difficulty of ERCP, but there are few studies conducted on this topic[14]. Pecsi et al[14] compared ERCP in patients with ABP and those with AC without ABP. They found no difference in overall biliary cannulation success (95.8% vs 97.2%) or adverse event rates between the two groups. Likewise, in our study the overall success rate of biliary cannulation was comparable in the AC with ABP group and the AC without ABP group (100% vs 98.5%). The overall adverse event rate was also similar (6.7% vs 4.9%).
Pecsi et al[14] reported a higher incidence of normal cholangiograms in patients with ABP compared with patients with AC (20.0% vs 12.3%, P = 0.026). This observation may be due to more frequent spontaneous passage of CBDS into the duodenum before ERCP in patients with ABP[15,16]. However, the direct comparison of the rates of spontaneous CBDS passage in patients with AC alone and patients with both AC and ABP has not been conducted. In our study, the detection rates of CBDS on imaging before ERCP were similar between the two groups and stone retrieval rates via bile duct sweeping during ERCP were also comparable. These findings suggest that the spontaneous CBDS passage rate did not significantly differ between the groups.
Patients with both AC and ABP are expected to exhibit a more pronounced inflammatory response than patients with AC alone. This potentially results in worse clinical outcomes. However, studies specifically investigating this association are lacking. Our patients with AC and ABP demonstrated significantly higher WBC counts and a higher incidence of abnormal WBC values, indicating an enhanced inflammatory response in those with both conditions. Unexpectedly, clinical outcomes, including ICU admission rates, 30-day mortality, LOHS, and 30-day readmission rates, were similar between the two groups.
This discrepancy may lie in the criteria used to classify disease severity. Both the TG18/TG13 and the revised Atlanta criteria rely on the presence of organ failure to define severe illness. Based on the revised Atlanta criteria, severe ABP is defined by the presence of persistent organ failure (lasting > 48 hours) and/or death. Organ failure is defined as shock, pulmonary insufficiency, renal failure, and/or gastrointestinal bleeding or modified Marshall score of 2 or more in the three accepted organ systems. Moderately severe ABP is defined as transient organ failure (resolves within 48 hours) and/or the development of local complications. As a result, we may have categorized patients with severe or moderately severe ABP, even when the ABP was mild.
Another potential explanation is the spontaneous passage of CBDS prior to ERCP, particularly in patients with ABP. Spontaneous passage likely resolves the obstruction. In these cases, the inflammatory response may overestimate the actual severity of ABP, and clinical outcomes remain stable. Furthermore, CT imaging in our cohort did not reveal peripancreatic fluid collections or pancreatic necrosis, suggesting that most cases were mild[1]. Due to the overlap in the severity classification between the TG18/TG13 and Atlanta criteria, the severity of ABP in the patients with both AC and ABP should be assessed dynamically and continuously instead of relying on the Atlanta classification.
The clinical outcomes of concurrent AC and ABP were similar to those of AC alone, suggesting that concurrent AC and ABP can be treated according to the AC management guidelines for ERCP[17,18]. However, the mean time to ERCP treatment was shorter in the AC with ABP group compared with the AC group, particularly among patients with moderate AC. ERCP timing is primarily based on the physician’s discretion based on the patient’s hemodynamic status and clinical condition. Although patients with severe AC experience longer delays before ERCP treatment than patients with moderate or mild AC due to their unstable condition, we found similar times to ERCP between the two groups. Therefore, we believe this factor did not significantly influence the overall study outcomes.
A multicenter study involving 6063 patients from Japan and Taiwan (including our center) divided patients into two groups according to whether they underwent early/urgent ERCP[19]. There was no difference in 30-day mortality between the two groups for patients with mild or severe AC. However, the 30-day mortality was significantly lower in patients with moderate AC who underwent early/urgent ERCP. This suggests that the TG18/TG13 AC severity grading criteria may be more favorable for patients with moderate AC who benefit the most from timely biliary drainage. The report also observed significantly higher 30-day mortality in patients with grade II AC and abnormal WBC counts, suggesting that early ERCP may be particularly beneficial for our patients with AC and ABP with elevated WBC counts. Because the AC with ABP group had a significantly higher rate of abnormal WBC counts (a key criterion for moderate AC), clinicians should prioritize early ERCP treatment in this patient population.
Our study had several limitations. First, the inherent biases of observational and retrospective study designs (e.g., selection bias) should be acknowledged. However, due to the limited number of studies on this topic, our study still provides valuable insights; Second, CT scans were performed while the patient was treated in the emergency room, potentially leading to the underdiagnosis of some patients with moderately severe ABP because signs of impaired pancreatic perfusion and peripancreatic necrosis are not apparent until several days later[12]; Third, albumin data (one criterion for moderate AC) were available in only 12.8% of patients. Therefore, patients with moderate AC may be misclassified as mild AC and vice versa. Fourth, the time to ERCP treatment was longer for patients with severe AC than for patients with moderate or mild AC regardless of the presence of ABP because of the unstable conditions of patients with severe AC. However, we believe this factor did not influence the overall study outcomes because the times were comparable in both groups. In the multivariate analysis, we identified age and cardiovascular dysfunction as independent predictors of ICU admission. Notably, pancreatitis itself was not an independent risk factor for ICU admission, contrasting with our initial hypothesis that concurrent ABP would worsen clinical outcomes. Therefore, risk stratification in AC should incorporate age and cardiovascular dysfunction as well as inflammatory markers and concomitant ABP.
This study demonstrated that the presence of interstitial ABP in patients with AC did not significantly impact ICU admission rates or other major outcomes. However, age and cardiovascular dysfunction were critical determinants of ICU admission. The findings highlighted the importance of managing organ dysfunction and monitoring older patients closely.
| 1. | Isogai M. Pathophysiology of severe gallstone pancreatitis: A new paradigm. World J Gastroenterol. 2024;30:614-623. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (6)] |
| 2. | Tenner S, Vege SS, Sheth SG, Sauer B, Yang A, Conwell DL, Yadlapati RH, Gardner TB. American College of Gastroenterology Guidelines: Management of Acute Pancreatitis. Am J Gastroenterol. 2024;119:419-437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 199] [Article Influence: 99.5] [Reference Citation Analysis (0)] |
| 3. | Williams E, Beckingham I, El Sayed G, Gurusamy K, Sturgess R, Webster G, Young T. Updated guideline on the management of common bile duct stones (CBDS). Gut. 2017;66:765-782. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 212] [Cited by in RCA: 278] [Article Influence: 30.9] [Reference Citation Analysis (1)] |
| 4. | Isogai M. Proposal of the term "gallstone cholangiopancreatitis" to specify gallstone pancreatitis that needs urgent endoscopic retrograde cholangiopancreatography. World J Gastrointest Endosc. 2021;13:451-459. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 1] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (3)] |
| 5. | Chang L, Lo SK, Stabile BE, Lewis RJ, de Virgilio C. Gallstone pancreatitis: a prospective study on the incidence of cholangitis and clinical predictors of retained common bile duct stones. Am J Gastroenterol. 1998;93:527-531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 66] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 6. | Kiriyama S, Kozaka K, Takada T, Strasberg SM, Pitt HA, Gabata T, Hata J, Liau KH, Miura F, Horiguchi A, Liu KH, Su CH, Wada K, Jagannath P, Itoi T, Gouma DJ, Mori Y, Mukai S, Giménez ME, Huang WS, Kim MH, Okamoto K, Belli G, Dervenis C, Chan ACW, Lau WY, Endo I, Gomi H, Yoshida M, Mayumi T, Baron TH, de Santibañes E, Teoh AYB, Hwang TL, Ker CG, Chen MF, Han HS, Yoon YS, Choi IS, Yoon DS, Higuchi R, Kitano S, Inomata M, Deziel DJ, Jonas E, Hirata K, Sumiyama Y, Inui K, Yamamoto M. Tokyo Guidelines 2018: diagnostic criteria and severity grading of acute cholangitis (with videos). J Hepatobiliary Pancreat Sci. 2018;25:17-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 468] [Cited by in RCA: 483] [Article Influence: 60.4] [Reference Citation Analysis (0)] |
| 7. | Rajragavan S, Gao Y, Sim S, Tan C, Pang N, Bonney G, Kow W, Madhavan K, Iyer S. Antibiotic Use in Patients with Acute Pancreatitis and Concomitant Suspected Cholangitis – Are We Overtreating Due to Diagnostic Ambiguity? HPB. 2022;24:S244. [DOI] [Full Text] |
| 8. | Neoptolemos JP, Carr-Locke DL, Leese T, James D. Acute cholangitis in association with acute pancreatitis: incidence, clinical features and outcome in relation to ERCP and endoscopic sphincterotomy. Br J Surg. 1987;74:1103-1106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 42] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 9. | Schwed AC, Boggs MM, Pham XD, Watanabe DM, Bermudez MC, Kaji AH, Kim DY, Plurad DS, Saltzman DJ, de Virgilio C. Association of Admission Laboratory Values and the Timing of Endoscopic Retrograde Cholangiopancreatography With Clinical Outcomes in Acute Cholangitis. JAMA Surg. 2016;151:1039-1045. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 27] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 10. | Tan M, Schaffalitzky de Muckadell OB, Laursen SB. Association between early ERCP and mortality in patients with acute cholangitis. Gastrointest Endosc. 2018;87:185-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 61] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 11. | Huang YC, Wu CH, Lee MH, Wang SF, Tsou YK, Lin CH, Sung KF, Liu NJ. Timing of endoscopic retrograde cholangiopancreatography in the treatment of acute cholangitis of different severity. World J Gastroenterol. 2022;28:5602-5613. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 6] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (2)] |
| 12. | Banks PA, Bollen TL, Dervenis C, Gooszen HG, Johnson CD, Sarr MG, Tsiotos GG, Vege SS; Acute Pancreatitis Classification Working Group. Classification of acute pancreatitis--2012: revision of the Atlanta classification and definitions by international consensus. Gut. 2013;62:102-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4932] [Cited by in RCA: 4689] [Article Influence: 360.7] [Reference Citation Analysis (48)] |
| 13. | Cotton PB, Eisen GM, Aabakken L, Baron TH, Hutter MM, Jacobson BC, Mergener K, Nemcek A Jr, Petersen BT, Petrini JL, Pike IM, Rabeneck L, Romagnuolo J, Vargo JJ. A lexicon for endoscopic adverse events: report of an ASGE workshop. Gastrointest Endosc. 2010;71:446-454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1238] [Cited by in RCA: 2012] [Article Influence: 125.8] [Reference Citation Analysis (1)] |
| 14. | Pécsi D, Gódi S, Hegyi P, Hanák L, Szentesi A, Altorjay I, Bakucz T, Czakó L, Kovács G, Orbán-Szilágyi Á, Pakodi F, Patai Á, Szepes Z, Gyökeres T, Fejes R, Dubravcsik Z, Vincze Á; Hungarian Endoscopy Study Group. ERCP is more challenging in cases of acute biliary pancreatitis than in acute cholangitis - Analysis of the Hungarian ERCP registry data. Pancreatology. 2021;21:59-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 15. | Isogai M, Yamaguchi A, Harada T, Kaneoka Y, Washizu J, Aikawa K. Gallstone pancreatitis: positive correlation between severe pancreatitis and passed stone. J Hepatobiliary Pancreat Surg. 2005;12:116-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 16. | Tranter SE, Thompson MH. Spontaneous passage of bile duct stones: frequency of occurrence and relation to clinical presentation. Ann R Coll Surg Engl. 2003;85:174-177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 71] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 17. | Buxbaum JL, Buitrago C, Lee A, Elmunzer BJ, Riaz A, Ceppa EP, Al-Haddad M, Amateau SK, Calderwood AH, Fishman DS, Fujii-Lau LL, Jamil LH, Jue TL, Kwon RS, Law JK, Lee JK, Naveed M, Pawa S, Sawhney MS, Schilperoort H, Storm AC, Thosani NC, Qumseya BJ, Wani S. ASGE guideline on the management of cholangitis. Gastrointest Endosc. 2021;94:207-221.e14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 55] [Article Influence: 11.0] [Reference Citation Analysis (1)] |
| 18. | Miura F, Okamoto K, Takada T, Strasberg SM, Asbun HJ, Pitt HA, Gomi H, Solomkin JS, Schlossberg D, Han HS, Kim MH, Hwang TL, Chen MF, Huang WS, Kiriyama S, Itoi T, Garden OJ, Liau KH, Horiguchi A, Liu KH, Su CH, Gouma DJ, Belli G, Dervenis C, Jagannath P, Chan ACW, Lau WY, Endo I, Suzuki K, Yoon YS, de Santibañes E, Giménez ME, Jonas E, Singh H, Honda G, Asai K, Mori Y, Wada K, Higuchi R, Watanabe M, Rikiyama T, Sata N, Kano N, Umezawa A, Mukai S, Tokumura H, Hata J, Kozaka K, Iwashita Y, Hibi T, Yokoe M, Kimura T, Kitano S, Inomata M, Hirata K, Sumiyama Y, Inui K, Yamamoto M. Tokyo Guidelines 2018: initial management of acute biliary infection and flowchart for acute cholangitis. J Hepatobiliary Pancreat Sci. 2018;25:31-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 318] [Cited by in RCA: 291] [Article Influence: 36.4] [Reference Citation Analysis (0)] |
| 19. | Kiriyama S, Takada T, Hwang TL, Akazawa K, Miura F, Gomi H, Mori R, Endo I, Itoi T, Yokoe M, Chen MF, Jan YY, Ker CG, Wang HP, Wada K, Yamaue H, Miyazaki M, Yamamoto M. Clinical application and verification of the TG13 diagnostic and severity grading criteria for acute cholangitis: an international multicenter observational study. J Hepatobiliary Pancreat Sci. 2017;24:329-337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 88] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/