Published online Nov 6, 2025. doi: 10.12998/wjcc.v13.i31.110123
Revised: June 15, 2025
Accepted: September 1, 2025
Published online: November 6, 2025
Processing time: 154 Days and 3.6 Hours
Colchicine is an anti-inflammatory alkaloid that reduces cardiovascular events through its actions on the interleukin(IL)-1β/IL-6/C-reactive protein pathway, which promotes the degradation and rupture of atherosclerotic plaques. Low-dose colchicine (0.5 mg/day) has been shown to decrease major adverse car
Core Tip: Inflammation is now recognized as an essential component of atherosclerosis, and the anti-inflammatory agent colchicine, through a variety of actions, suppresses this response. Results from a meta-analysis of several randomized controlled trials that evaluated colchicine’s efficacy on cardiovascular outcomes led to its recent approval by the United States Food and Drug Administration for the management and prevention of atherosclerotic cardiovascular disease. It has been shown to reduce the risk of recurrent major adverse cardiovascular events, and clinical data now supports its use for secondary prevention in patients with established coronary artery disease, presumably due to its anti-inflammatory properties.
- Citation: English K, Uwibambe C. Role of anti-inflammatory agent colchicine in atherosclerotic cardiovascular disease. World J Clin Cases 2025; 13(31): 110123
- URL: https://www.wjgnet.com/2307-8960/full/v13/i31/110123.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v13.i31.110123
Within the past several decades, morbidity and mortality from coronary artery disease (CAD) have decreased through the advent of medications and lifestyle interventions[1-3]. Despite the management of known risk factors such as hyperlipidemia, hypertension, tobacco use, diabetes mellitus, and obesity, CAD remains the primary cause of mortality and disability-adjusted life years lost worldwide[4,5]. This burden of disease has a disproportionate impact on middle and low-income nations, resulting in over 7 million fatalities and 129 million disability-adjusted life years each year[4-7]. A significant proportion of residual risk leading to atherosclerosis is related to uncontrolled inflammation[8-10].
Inflammation plays a vital role in the development of CAD[11]. The progression of vulnerable plaques is associated with neutrophil-driven endothelial dysfunction, leading to heightened permeability to lipoproteins and their accumulation beneath the endothelium, along with the recruitment of leukocytes and activation of platelets, which increases the risk of plaque rupture[12-16]. Several studies have shown that elevated C-reactive protein (CRP), a marker of inflammation, is associated with the development of atherosclerosis even in patients on statin therapy with low-density lipoprotein (LDL)-cholesterol < 70 mg/dL[17-19]. Several clinical trials have demonstrated that colchicine, an anti-inflammatory agent frequently used to treat gout and pericarditis, among other inflammatory conditions, reduces major adverse cardiovascular events (MACE) even in patients with stable CAD and a recent acute myocardial infarction (MI)[20-22]. In this article, we provide a scoping review of colchicine and its mechanism of action, biochemical and molecular targets in various cardiovascular conditions, and safety and efficacy in the treatment of CAD.
Colchicine facilitates the prevention and treatment of CAD through various biochemical avenues[23]. Dysfunction of the endothelium plays a crucial role in the development and progression of atherosclerosis[23,24]. The drug prevents endothelial dysfunction through various mechanisms of action contingent on the dosage[25,26]. At reduced doses, it interferes with microtubule function and hinders cell migration, whereas at elevated doses, it obstructs mitosis[27,28]. Colchicine has also been shown to decrease the levels and expression of the tissue factor protein and gene, respectively, which play a vital role in the pathogenesis of coronary thrombosis induced by oxidized LDL[29,30]. By suppressing the upregulation of tissue factor, colchicine demonstrates protective benefits in atherosclerosis, providing an additional therapeutic option[29-31].
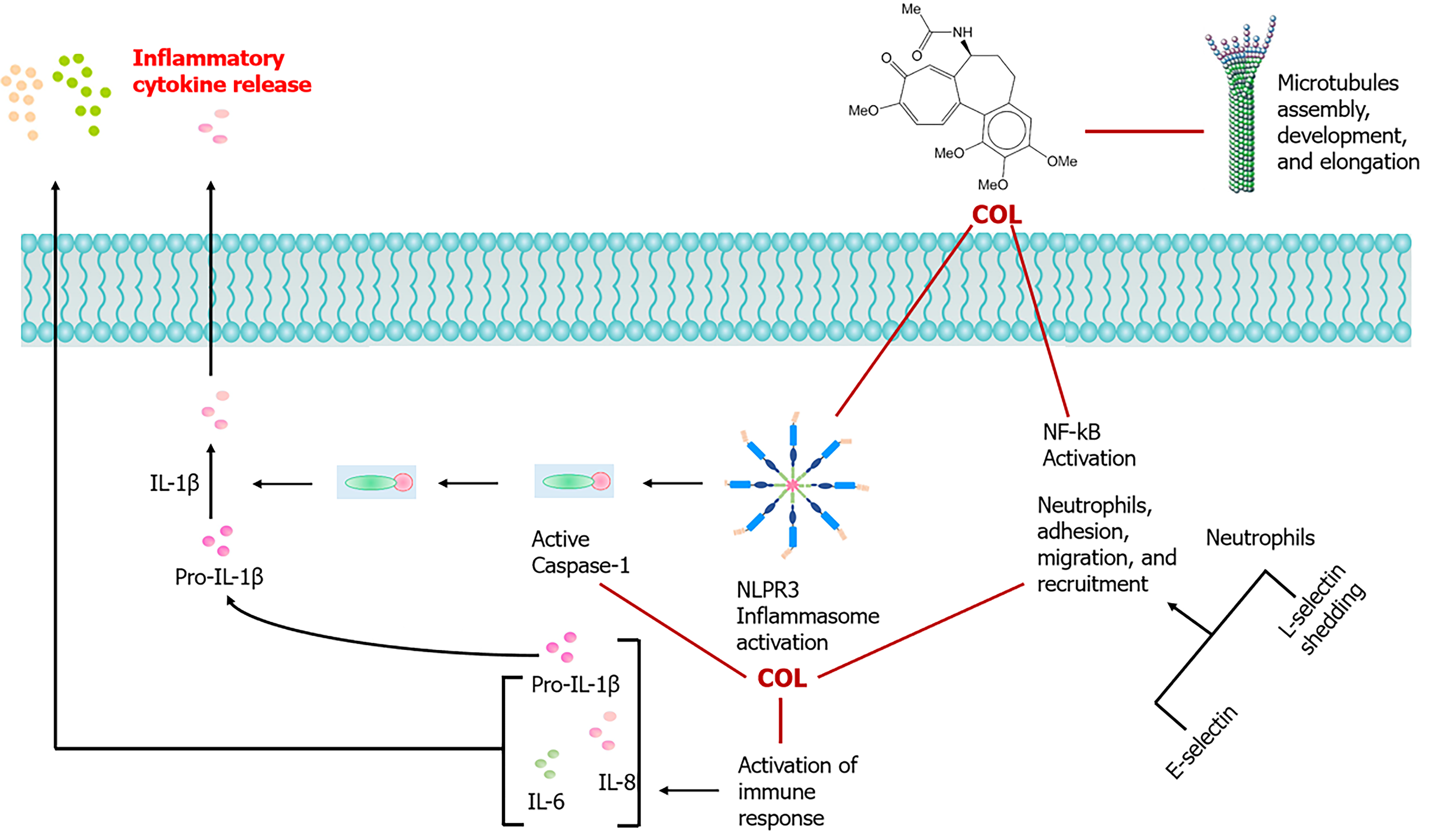
Colchicine also plays a role in atherosclerosis by inhibiting the chemotaxis of macrophages and neutrophils through the suppression of interleukin (IL)-18 and C-type lectin-like receptor production[32,33]. Macrophages accumulate in damaged vessel walls and produce foam cells through the uptake of modified lipids[34]. The drug hinders the expression of adhesion molecules L and E-selectin by promoting microtubule dysfunction and obstructing the advancement of plaque development (Figure 1)[35,36]. Research has shown that colchicine administered through nanoparticles coated with macrophage membranes can prevent the formation of foam cells[37]. Colchicine elevates cyclic adenosine monophosphate levels in leukocytes, which in turn reduces IL-1 production[38,39]. Furthermore, it reduces the generation of neutrophil extracellular traps, thereby inhibiting the release of inflammatory cytokines from injured macrophages and endothelial cells[38-40]. This mechanism is especially advantageous for patients experiencing acute coronary syndrome who have received percutaneous coronary intervention[36-38,40,41].
Lastly, colchicine suppresses plaque formation through the inhibition of smooth muscle cells (SMCs) and platelet activation[38-42]. SMCs play a crucial role in vascular contraction and the synthesis of extracellular matrix proteins such as proteoglycans and collagen (COLL)[43,44]. Dysregulation of vascular SMCs is a hallmark feature of atherosclerosis[45]. Lipid molecules accumulate in the sub-endothelium, which triggers the proliferation and migration of SMCs, initiating plaque formation[43-46]. Extracellular matrix proteins are then secreted, leading to the formation of fibrous caps within the atheroma[44-47]. SMCs also interact with oxidized LDL to form foam cells within plaques that then undergo apoptosis, releasing proinflammatory cytokines and further facilitating atherosclerotic plaque formation[43-48]. Colchicine disrupts this process by inhibiting microtubule polymerization[49]. Platelet activation is known to promote the pathogenesis of atherosclerosis, as seen in unstable angina and MI[45-50]. Upon activation, platelets cluster together and release proinflammatory cytokines, resulting in thrombus formation[46-51]. The processes of microtubule depolymerization and polymerization are essential to platelet activation[45-52]. Colchicine disrupts this mechanism, consequently inhibiting the release of inflammatory cytokines and the aggregation of platelets[53]. A study conducted by Cimmino et al[54] investigated whether colchicine interfered with platelet aggregation by action on cytoskeleton rearrangement. In the experiment, platelets obtained from healthy volunteers were activated using adenosine diphosphate (ADP), COLL, and thrombin-activating receptor peptide (TRAP), both with and without a 10 μM colchicine pretreatment. Following the stimulation, aggregation was assessed through light aggregometry over time. Results revealed that colchicine pretreatment significantly reduced ADP/COLL/TRAP-induced platelet aggregation. The effects appeared to be mediated by microtubule depolymerization, and cytoskeleton disarrangement associated with the inactivation of myosin phosphatase targeting subunit and LIM domain kinase 1 that interfered with coilin activity. A notable limitation of the experiment was that it was a mechanistic study conducted in vitro. As such, more targeted clinical trials are needed to accurately determine the in vivo colchicine dosage and duration of treatment required to achieve cardiac benefit in both the short and long term.
There are several molecular mechanisms through which colchicine exerts its anti-inflammatory effects. The primary mechanism of action that has been extensively researched regarding colchicine is its capacity to bind to tubulins, which consequently inhibits the formation and polymerization of microtubules[55,56]. Colchicine particularly blocks mitotic cells in metaphase[55-57]. It attaches to soluble tubulin, creating tubulin-colchicine complexes that adhere to the ends of microtubules, thereby inhibiting polymerization[55,57]. At low concentrations, the drug limits microtubule growth, while at higher concentrations, it promotes microtubule depolymerization[55-58]. It selectively accumulates within neutrophils and erythrocytes, which lack P-glycoproteins that typically facilitate clearance[53-59]. The buildup within neutrophils hinders chemotaxis and triggers the release of adhesion molecules, including L-selectin, consequently obstructing additional recruitment of neutrophils[35,36,56,58,59]. Colchicine has also been demonstrated to inhibit neutrophil adhesion and mobility during crystal-induced neutrophil activation through the selective inhibition of tyrosine phosphorylation[35,36,60]. A study conducted by Popa-Nita et al[61] demonstrated that colchicine diminishes the crystal-induced tyrosine phosphorylation of Tec, which is likely the primary kinase involved in the activation of neutrophils by crystals. Lastly, colchicine suppresses monosodium urate crystal-induced NACHT-LRRPYD-containing protein 3 inflammasomes, responsible for caspase-1 activation and subsequent IL-1β and IL-18 processing and release[62,63]. The mechanism through which colchicine achieves this inhibition still remains unknown. These variations in the mechanism of action of the drug are thought to play a role in its beneficial effects on atherosclerosis[59-64]. The long-term use of low-dose colchicine (0.5 mg daily) is safe and well tolerated[57-59]. More serious adverse effects, such as bone marrow suppression and myotoxicity, are likely to occur at higher doses in patients with liver or renal impairment[57-59,61,63,64].
Atherosclerosis progresses through the accumulation of inflammatory cells and cholesterol within the arterial wall, leading to the formation of vessel-occluding plaques that can result in chronic ischemia and risk of severe cardiovascular complications, including MI and stroke, as a consequence of acute plaque rupture[65,66]. This process also involves an inflammatory response to cholesterol, which leads to the formation of crystals within arterial walls[65-67]. Thus, despite the use of potent cholesterol-reducing agents, patients face an elevated lifetime risk of recurring MACE due to inadequate control of the inflammatory process[68-70].
Recent clinical trials such as COLCOT and CANTOS have provided substantial evidence that reducing the inflammatory response in patients with atherosclerotic cardiovascular disease leads to better clinical outcomes (Table 1)[21,71-75]. Despite the efficacy of IL-1β inhibitor canakinumab in reducing the rate of recurrent cardiovascular events compared to placebo in the CANTOS trial, low-dose colchicine is the sole anti-inflammatory agent approved by the FDA for clinical application in patients diagnosed with established CAD[71,72,76]. A meta-analysis conducted by Zhou et al[77] of five large randomized controlled trials that included more than 14000 patients found that early long-term use of low-dose colchicine in patients who suffer an acute MI decreases the risk of MACE through its anti-inflammatory effects. Although it is now known that colchicine offers protective cardiovascular effects, the exact mechanism through which this occurs is still not well understood.
| Trial number | Trial name/year | Official title | Details | Outcome | Notes |
| ACTRN 12610000293066 | LoDoCo/2013 | Low-Dose Colchicine for Secondary Prevention of Cardiovascular Disease | n = 532; Patients diagnosed with stable, angiographically confirmed CAD who have maintained clinical stability for a minimum of six months were randomly assigned to receive either standard treatment in conjunction with 0.5 mg of daily colchicine or standard treatment alone | Colchicine had a lower composite outcome of OHCA, ACS, or non-cardioembolic ischemic stroke vs control (5.3% vs 16%; HR, 0.33; 95%CI: 0.18-0.59; P < 0.001) | The most common side effect was GI disturbance |
| NCT02551094 | COLCOT/2019 | Efficacy and Safety of Low-Dose Colchicine after Myocardial Infarction | n = 4745; Patients within 30 days post MI were randomized to either standard therapy plus placebo or standard therapy plus 0.5 mg daily colchicine | Lower incidence of MACE in the colchicine group (5.5% vs 7.1%; HR, 0.77; 95%CI: 0.61-0.96; P = 0.02) | The most common side effect was GI disturbance |
| ACTRN12615000861550 | COPS/2020 | Colchicine in Patients with Acute Coronary Syndrome: The Australian COPS Randomized Clinical Trial | n = 795; Patients presenting with ACS with evidence of CAD (angiographically managed with PCI or medical therapy) were randomized to receive either standard therapy plus colchicine (0.5 mg BID for the first month post ACS, then 0.5 mg daily for 11 months) or placebo plus standard therapy | There was no statistically significant difference between the two groups (HR, 0.65; 95%CI: 0.38-1.09; P = 0.1) in the primary outcome (i.e., a composite of all-cause mortality, ACS [STEMI/NSTEMI/UA], ischemia-driven urgent revascularization, and noncardioembolic ischemic stroke). However, the colchicine arm showed lower incidence of the primary outcome when all-cause mortality was replaced with CV mortality (5% vs 9.5%; HR, 0.51; 95%CI: 0.29-0.89; P = 0.019) | The most common side effect was GI disturbance |
| ACTRN12614000093684 | LoDoCo2/2020 | Colchicine in Patients with Chronic Coronary Disease | n = 5552; Patients with stable CAD (angiographically or CAC score ≥ 400 AU) who were clinically stable for ≥ 6 months were randomized to either standard therapy plus 0.5 mg daily colchicine or to standard therapy plus placebo. A total of 3,179 patients had ACS for a duration of 24 months or more prior to randomization | Colchicine demonstrated a reduced occurrence of cardiovascular death, spontaneous myocardial infarction, ischemic stroke, or ischemia-driven coronary revascularization compared to placebo (6.8% vs 9.6%; HR, 0.69; 95%CI: 0.57-0.83; P < 0.001) | The colchicine group experienced a higher occurrence of non-cardiovascular deaths, although this was not statistically significant (incidence, 0.7 compared to 0.5 events per 100 person-years; HR, 1.51; 95%CI: 0.99-2.31) |
| NCT03048825 | CLEAR SYNERGY/2024 | Colchicine in Acute Myocardial Infarction | n = 7062; Patients within 72 hours post MI were randomized to either standard therapy plus 0.5 mg daily colchicine or to standard therapy plus placebo | There was no significant difference in MACE outcomes observed between the two groups (9.1% compared to 9.3%; HR, 0.99; 95%CI: 0.85-1.16; P = 0.93). Additionally, there was no notable difference in all-cause mortality (4.6% compared to 5.1%; HR, 0.90; 95%CI: 0.73-1.12) | A higher incidence of diarrhea was reported in the colchicine group (6.6% vs 10.2%; P < 0.001) |
Perhaps the most recognized action of colchicine on inflammation includes its inhibitory effects on the function of neutrophils[78]. It is also known to affect the innate properties of the endothelium, SMCs, and macrophages, which reduce the interaction between platelets and neutrophils, mitigate plaque growth, improve stability, and lower the risk of thrombotic occlusion[59-64,68-70,79]. Bulnes et al[80] expanded on accumulating evidence from clinical and animal research that demonstrates the inhibitory effect of colchicine on the NLRP3 inflammasome. This protein complex, when activated in inflammatory cells, enhances the production of pro-atherosclerotic and highly inflammatory cytokines, specifically IL-18 and IL-1β. Crystals that develop due to the accumulation of cholesterol in plaque serve as a triggering factor for the activation of NLRP3. Another research article by Abideen et al[81] detailed an inverse relationship between the formation and expansion of cholesterol crystals in vitro and the dose of colchicine used, potentially elucidating the compound’s indirect effects on inhibiting NLRP3 activation, which ultimately may limit the risk of acute plaque rupture. In the study, in vitro tests conducted on rat and rabbit biological membranes, observed through scanning electron microscopy, demonstrated that cholesterol crystals could distort and protrude through the tissue due to their sharp geometric edges. This observation was found to be analogous to ex vivo results from human plaques and arterial tissues, which were prepared using vacuum dehydration for scanning electron microscopy, revealing cholesterol crystals that disrupt the plaque and penetrate the intimal surface. Abideen et al[81] and colleagues provided a novel mechanism suggesting that colchicine may reduce the risk of acute plaque rupture by altering cholesterol crystal production and slowing the rate of crystal formation.
Despite the considerable advancements made in comprehending inflammation and its contribution to the pathogenesis of atherosclerosis, there remain knowledge gaps, especially concerning the precise mechanism through which certain agents, like colchicine, mitigate MACE following an acute MI[82-86]. In clinical practice, the harmful impact of inflammation in CAD can be confirmed by randomizing patients to receive either anti-inflammatory agents or a placebo and monitoring the occurrence of cardiovascular events over time. The CANTOS trial was the first to validate the inflammatory hypothesis in relation to CAD, evaluating patients who had experienced a previous MI and exhibited a high-sensitivity CRP level of 2 mg or greater per liter. It is not surprising that the experimental group experienced advantages from the anti-inflammatory treatment. Conversely, the event rates in the placebo groups of both the CANTOS and CIRT trials were comparable, even though the CIRT trial did not include high-sensitivity CRP as a criterion for inclusion[72,87].
The key point to emphasize in the broader context is that there is no validated indicator of inflammation, including high-sensitivity CRP, deemed effective as a resource for clinical decision-making. This may be linked to chronic subclinical inflammation in individuals with previous cardiovascular incidents or other unidentified inflammatory disorders, and potentially colchicine due to its various effects, particularly its direct impact on blood vessels and platelets. More research is needed to ascertain if the advantages of colchicine are associated with the circulating levels of inflammatory biomarkers and baseline cardiovascular risk. Additional research is also needed to elucidate the exact mechanism by which colchicine exerts its anti-inflammatory effects in CAD. Furthermore, studies are required to determine the optimal timing of colchicine in the short term following a MI. It would also be intriguing to evaluate whether colchicine is advantageous in primary prevention (i.e., in patients without symptomatic CAD or previous cardiovascular events).
In summary, most patients with atherosclerotic cardiovascular disease possess residual inflammatory risk, which predisposes MACE, especially after an acute MI. These patients remain at significant risk despite effective cholesterol-lowering therapies. Colchicine, particularly through its suppressive action on the NLRP3 inflammasome, reduces inflammatory cytokine response in atherosclerosis, which ultimately decreases the risk of plaque progression and subsequent thrombosis. As such, anti-inflammatory therapy in the form of colchicine, particularly in patients with CAD, should be seriously considered by preventative cardiologists if there are no contraindications to treatment, such as severe renal or liver dysfunction.
| 1. | Mensah GA, Wei GS, Sorlie PD, Fine LJ, Rosenberg Y, Kaufmann PG, Mussolino ME, Hsu LL, Addou E, Engelgau MM, Gordon D. Decline in Cardiovascular Mortality: Possible Causes and Implications. Circ Res. 2017;120:366-380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 486] [Cited by in RCA: 615] [Article Influence: 68.3] [Reference Citation Analysis (0)] |
| 2. | Huffman MD, Lloyd-Jones DM, Ning H, Labarthe DR, Guzman Castillo M, O'Flaherty M, Ford ES, Capewell S. Quantifying options for reducing coronary heart disease mortality by 2020. Circulation. 2013;127:2477-2484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 50] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 3. | Short L, La VT, Patel M, Pai RG. Primary and Secondary Prevention of CAD: A Review. Int J Angiol. 2022;31:16-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 4. | Ralapanawa U, Sivakanesan R. Epidemiology and the Magnitude of Coronary Artery Disease and Acute Coronary Syndrome: A Narrative Review. J Epidemiol Glob Health. 2021;11:169-177. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 372] [Cited by in RCA: 378] [Article Influence: 75.6] [Reference Citation Analysis (0)] |
| 5. | Chong B, Jayabaskaran J, Jauhari SM, Chan SP, Goh R, Kueh MTW, Li H, Chin YH, Kong G, Anand VV, Wang JW, Muthiah M, Jain V, Mehta A, Lim SL, Foo R, Figtree GA, Nicholls SJ, Mamas MA, Januzzi JL, Chew NWS, Richards AM, Chan MY. Global burden of cardiovascular diseases: projections from 2025 to 2050. Eur J Prev Cardiol. 2025;32:1001-1015. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 238] [Article Influence: 238.0] [Reference Citation Analysis (1)] |
| 6. | Mocumbi AO. Cardiovascular Health Care in Low- and Middle-Income Countries. Circulation. 2024;149:557-559. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 56] [Reference Citation Analysis (0)] |
| 7. | Wurie HR, Cappuccio FP. Cardiovascular disease in low- and middle-income countries: an urgent priority. Ethn Health. 2012;17:543-550. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 31] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 8. | Ajoolabady A, Pratico D, Lin L, Mantzoros CS, Bahijri S, Tuomilehto J, Ren J. Inflammation in atherosclerosis: pathophysiology and mechanisms. Cell Death Dis. 2024;15:817. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 150] [Reference Citation Analysis (0)] |
| 9. | Gusev E, Sarapultsev A. Atherosclerosis and Inflammation: Insights from the Theory of General Pathological Processes. Int J Mol Sci. 2023;24:7910. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 153] [Reference Citation Analysis (0)] |
| 10. | Nguyen MT, Fernando S, Schwarz N, Tan JT, Bursill CA, Psaltis PJ. Inflammation as a Therapeutic Target in Atherosclerosis. J Clin Med. 2019;8:1109. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 81] [Cited by in RCA: 133] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 11. | Libby P. Inflammation in atherosclerosis. Arterioscler Thromb Vasc Biol. 2012;32:2045-2051. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1404] [Cited by in RCA: 1681] [Article Influence: 120.1] [Reference Citation Analysis (0)] |
| 12. | Jebari-Benslaiman S, Galicia-García U, Larrea-Sebal A, Olaetxea JR, Alloza I, Vandenbroeck K, Benito-Vicente A, Martín C. Pathophysiology of Atherosclerosis. Int J Mol Sci. 2022;23:3346. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 547] [Article Influence: 136.8] [Reference Citation Analysis (0)] |
| 13. | Lusis AJ. Atherosclerosis. Nature. 2000;407:233-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4039] [Cited by in RCA: 4194] [Article Influence: 161.3] [Reference Citation Analysis (0)] |
| 14. | Hartwig H, Silvestre Roig C, Daemen M, Lutgens E, Soehnlein O. Neutrophils in atherosclerosis. A brief overview. Hamostaseologie. 2015;35:121-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 39] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 15. | Gimbrone MA Jr, García-Cardeña G. Endothelial Cell Dysfunction and the Pathobiology of Atherosclerosis. Circ Res. 2016;118:620-636. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1448] [Cited by in RCA: 2568] [Article Influence: 256.8] [Reference Citation Analysis (0)] |
| 16. | Hooglugt A, Klatt O, Huveneers S. Vascular stiffening and endothelial dysfunction in atherosclerosis. Curr Opin Lipidol. 2022;33:353-363. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 41] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 17. | Kirkgöz K. C-Reactive Protein in Atherosclerosis-More than a Biomarker, but not Just a Culprit. Rev Cardiovasc Med. 2023;24:297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 10] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 18. | Cederström S, Lundman P, Alfredsson J, Hagström E, Ravn-Fischer A, Söderberg S, Yndigegn T, Tornvall P, Jernberg T. Association between high-sensitivity C-reactive protein and coronary atherosclerosis in a general middle-aged population. Sci Rep. 2023;13:12171. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 16] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 19. | Mehta A, Blumenthal RS, Gluckman TJ, Feldman DI, Kohli P. High-sensitivity C-reactive Protein in Atherosclerotic Cardiovascular Disease: To Measure or Not to Measure? US Cardiol. 2025;19:e06. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 20. | Ma Z, Chen J, Jin K, Chen X. Colchicine and coronary heart disease risks: A meta-analysis of randomized controlled clinical trials. Front Cardiovasc Med. 2022;9:947959. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 24] [Reference Citation Analysis (0)] |
| 21. | Nidorf SM, Fiolet ATL, Mosterd A, Eikelboom JW, Schut A, Opstal TSJ, The SHK, Xu XF, Ireland MA, Lenderink T, Latchem D, Hoogslag P, Jerzewski A, Nierop P, Whelan A, Hendriks R, Swart H, Schaap J, Kuijper AFM, van Hessen MWJ, Saklani P, Tan I, Thompson AG, Morton A, Judkins C, Bax WA, Dirksen M, Alings M, Hankey GJ, Budgeon CA, Tijssen JGP, Cornel JH, Thompson PL; LoDoCo2 Trial Investigators. Colchicine in Patients with Chronic Coronary Disease. N Engl J Med. 2020;383:1838-1847. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 653] [Cited by in RCA: 1548] [Article Influence: 258.0] [Reference Citation Analysis (0)] |
| 22. | Samuel M, Berry C, Dubé MP, Koenig W, López-Sendón J, Maggioni AP, Pinto FJ, Roubille F, Tardif JC. Long-term trials of colchicine for secondary prevention of vascular events: a meta-analysis. Eur Heart J. 2025;46:2552-2563. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 29] [Article Influence: 29.0] [Reference Citation Analysis (10)] |
| 23. | Deftereos SG, Beerkens FJ, Shah B, Giannopoulos G, Vrachatis DA, Giotaki SG, Siasos G, Nicolas J, Arnott C, Patel S, Parsons M, Tardif JC, Kovacic JC, Dangas GD. Colchicine in Cardiovascular Disease: In-Depth Review. Circulation. 2022;145:61-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 119] [Reference Citation Analysis (0)] |
| 24. | Poredos P, Poredos AV, Gregoric I. Endothelial Dysfunction and Its Clinical Implications. Angiology. 2021;72:604-615. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 88] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 25. | Zhou H, Khan D, Hussain SM, Gerdes N, Hagenbeck C, Rana M, Cornelius JF, Muhammad S. Colchicine prevents oxidative stress-induced endothelial cell senescence via blocking NF-κB and MAPKs: implications in vascular diseases. J Inflamm (Lond). 2023;20:41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 19] [Reference Citation Analysis (0)] |
| 26. | Robinson PC, Terkeltaub R, Pillinger MH, Shah B, Karalis V, Karatza E, Liew D, Imazio M, Cornel JH, Thompson PL, Nidorf M. Consensus Statement Regarding the Efficacy and Safety of Long-Term Low-Dose Colchicine in Gout and Cardiovascular Disease. Am J Med. 2022;135:32-38. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 90] [Cited by in RCA: 74] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 27. | Chaldakov GN. Colchicine, a microtubule-disassembling drug, in the therapy of cardiovascular diseases. Cell Biol Int. 2018;42:1079-1084. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 29] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 28. | Yoshimatsu K, Yamaguchi A, Yoshino H, Koyanagi N, Kitoh K. Mechanism of action of E7010, an orally active sulfonamide antitumor agent: inhibition of mitosis by binding to the colchicine site of tubulin. Cancer Res. 1997;57:3208-3213. [PubMed] |
| 29. | Cirillo P, Conte S, Pellegrino G, Barra G, De Palma R, Sugraliyev A, Golino P, Cimmino G. Effects of colchicine on tissue factor in oxLDL-activated T-lymphocytes. J Thromb Thrombolysis. 2022;53:739-749. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 30. | Cimmino G, Loffredo FS, De Rosa G, Cirillo P. Colchicine in Athero-Thrombosis: Molecular Mechanisms and Clinical Evidence. Int J Mol Sci. 2023;24:2483. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 31. | Cimmino G, Conte S, Morello A, Pellegrino G, Marra L, Calì G, Golino P, Cirillo P. Colchicine inhibits the prothrombotic effects of oxLDL in human endothelial cells. Vascul Pharmacol. 2021;137:106822. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 32. | Meyer-Lindemann U, Mauersberger C, Schmidt AC, Moggio A, Hinterdobler J, Li X, Khangholi D, Hettwer J, Gräßer C, Dutsch A, Schunkert H, Kessler T, Sager HB. Colchicine Impacts Leukocyte Trafficking in Atherosclerosis and Reduces Vascular Inflammation. Front Immunol. 2022;13:898690. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 44] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 33. | Sukeishi A, Isami K, Hiyama H, Imai S, Nagayasu K, Shirakawa H, Nakagawa T, Kaneko S. Colchicine alleviates acute postoperative pain but delays wound repair in mice: roles of neutrophils and macrophages. Mol Pain. 2017;13:1744806917743680. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 22] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 34. | Blagov AV, Markin AM, Bogatyreva AI, Tolstik TV, Sukhorukov VN, Orekhov AN. The Role of Macrophages in the Pathogenesis of Atherosclerosis. Cells. 2023;12:522. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 96] [Article Influence: 32.0] [Reference Citation Analysis (0)] |
| 35. | Mijajlović MD, Bornstein NM, Aleksić V. Secondary stroke prevention beyond antiplatelets: The role of colchicine and GLP-1RA - an ounce of prevention is worth a pound of cure. Ther Adv Neurol Disord. 2025;18:17562864251326769. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 36. | Zhang FS, He QZ, Qin CH, Little PJ, Weng JP, Xu SW. Therapeutic potential of colchicine in cardiovascular medicine: a pharmacological review. Acta Pharmacol Sin. 2022;43:2173-2190. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 95] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 37. | Tang J, Li T, Xiong X, Yang Q, Su Z, Zheng M, Chen Q. Colchicine delivered by a novel nanoparticle platform alleviates atherosclerosis by targeted inhibition of NF-κB/NLRP3 pathways in inflammatory endothelial cells. J Nanobiotechnology. 2023;21:460. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 33] [Reference Citation Analysis (0)] |
| 38. | Jasper JR, Post SR, Desai KH, Insel PA, Bernstein D. Colchicine and cytochalasin B enhance cyclic AMP accumulation via postreceptor actions. J Pharmacol Exp Ther. 1995;274:937-942. [PubMed] |
| 39. | Martínez GJ, Robertson S, Barraclough J, Xia Q, Mallat Z, Bursill C, Celermajer DS, Patel S. Colchicine Acutely Suppresses Local Cardiac Production of Inflammatory Cytokines in Patients With an Acute Coronary Syndrome. J Am Heart Assoc. 2015;4:e002128. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 204] [Cited by in RCA: 221] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 40. | Vaidya K, Tucker B, Kurup R, Khandkar C, Pandzic E, Barraclough J, Machet J, Misra A, Kavurma M, Martinez G, Rye KA, Cochran BJ, Patel S. Colchicine Inhibits Neutrophil Extracellular Trap Formation in Patients With Acute Coronary Syndrome After Percutaneous Coronary Intervention. J Am Heart Assoc. 2021;10:e018993. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 94] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 41. | Aw KL, Koh A, Lee HL, Kudzinskas A, De Palma R. Colchicine for symptomatic coronary artery disease after percutaneous coronary intervention. Open Heart. 2022;9:e001887. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 42. | Zhang BC, Zhu WY, Wang SN, Zhu MM, Ma H, Dong L, Yang XX, Ma CR, Ma LK, Chen YL. Colchicine reduces neointima formation and VSMC phenotype transition by modulating SRF-MYOCD activation and autophagy. Acta Pharmacol Sin. 2025;46:951-963. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 43. | Brozovich FV, Nicholson CJ, Degen CV, Gao YZ, Aggarwal M, Morgan KG. Mechanisms of Vascular Smooth Muscle Contraction and the Basis for Pharmacologic Treatment of Smooth Muscle Disorders. Pharmacol Rev. 2016;68:476-532. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 386] [Cited by in RCA: 368] [Article Influence: 36.8] [Reference Citation Analysis (0)] |
| 44. | Touyz RM, Alves-Lopes R, Rios FJ, Camargo LL, Anagnostopoulou A, Arner A, Montezano AC. Vascular smooth muscle contraction in hypertension. Cardiovasc Res. 2018;114:529-539. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 232] [Cited by in RCA: 495] [Article Influence: 70.7] [Reference Citation Analysis (0)] |
| 45. | Skeyni A, Pradignac A, Matz RL, Terrand J, Boucher P. Cholesterol trafficking, lysosomal function, and atherosclerosis. Am J Physiol Cell Physiol. 2024;326:C473-C486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 17] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 46. | Paudel KR, Panth N, Kim DW. Circulating Endothelial Microparticles: A Key Hallmark of Atherosclerosis Progression. Scientifica (Cairo). 2016;2016:8514056. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 41] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 47. | Falk E. Pathogenesis of atherosclerosis. J Am Coll Cardiol. 2006;47:C7-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 723] [Cited by in RCA: 1106] [Article Influence: 55.3] [Reference Citation Analysis (0)] |
| 48. | Milutinović A, Šuput D, Zorc-Pleskovič R. Pathogenesis of atherosclerosis in the tunica intima, media, and adventitia of coronary arteries: An updated review. Bosn J Basic Med Sci. 2020;20:21-30. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 72] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 49. | Ning L, Wang C, Fan X, Ding X, Wang Y, Zhang Y, Wang J, Yue S. Role of colchicine-induced microtubule depolymerization in hyperalgesia via TRPV4 in rats with chronic compression of the dorsal root ganglion. Neurol Res. 2014;36:70-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 13] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 50. | Fitzgerald DJ, Roy L, Catella F, FitzGerald GA. Platelet activation in unstable coronary disease. N Engl J Med. 1986;315:983-989. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 904] [Cited by in RCA: 842] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 51. | Lievens D, von Hundelshausen P. Platelets in atherosclerosis. Thromb Haemost. 2011;106:827-838. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 175] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 52. | Menche D, Israel A, Karpatkin S. Platelets and microtubules. Effect of colchicine and D2O on platelet aggregation and release induced by calcium ionophore A23187. J Clin Invest. 1980;66:284-291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 45] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 53. | Saji K, Fukumoto Y, Suzuki J, Fukui S, Nawata J, Shimokawa H. Colchicine, a microtubule depolymerizing agent, inhibits myocardial apoptosis in rats. Tohoku J Exp Med. 2007;213:139-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 28] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 54. | Cimmino G, Tarallo R, Conte S, Morello A, Pellegrino G, Loffredo FS, Calì G, De Luca N, Golino P, Trimarco B, Cirillo P. Colchicine reduces platelet aggregation by modulating cytoskeleton rearrangement via inhibition of cofilin and LIM domain kinase 1. Vascul Pharmacol. 2018;111:62-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 49] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 55. | Wang J, Miller DD, Li W. Molecular interactions at the colchicine binding site in tubulin: An X-ray crystallography perspective. Drug Discov Today. 2022;27:759-776. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 65] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 56. | Massarotti A, Coluccia A, Silvestri R, Sorba G, Brancale A. The tubulin colchicine domain: a molecular modeling perspective. ChemMedChem. 2012;7:33-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 137] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 57. | Taylor EW. The Mechanism Of Colchicine Inhibition Of Mitosis. I. Kinetics Of Inhibition And The Binding Of H3-Colchicine. J Cell Biol. 1965;25:SUPPL:145-SUPPL:160. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 299] [Cited by in RCA: 294] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 58. | Caperta AD, Delgado M, Ressurreição F, Meister A, Jones RN, Viegas W, Houben A. Colchicine-induced polyploidization depends on tubulin polymerization in c-metaphase cells. Protoplasma. 2006;227:147-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 36] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 59. | Angelidis C, Kotsialou Z, Kossyvakis C, Vrettou AR, Zacharoulis A, Kolokathis F, Kekeris V, Giannopoulos G. Colchicine Pharmacokinetics and Mechanism of Action. Curr Pharm Des. 2018;24:659-663. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 122] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 60. | Roberge CJ, Gaudry M, Gilbert C, Malawista SE, de Médicis R, Lussier A, Poubelle PE, Naccache PH. Paradoxical effects of colchicine on the activation of human neutrophilis by chemotactic factors and inflammatory microcrystal. J Leukoc Biol. 1996;59:864-871. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 22] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 61. | Popa-Nita O, Marois L, Paré G, Naccache PH. Crystal-induced neutrophil activation: X. Proinflammatory role of the tyrosine kinase Tec. Arthritis Rheum. 2008;58:1866-1876. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 30] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 62. | Casey A, Quinn S, McAdam B, Kennedy M, Sheahan R. Colchicine-regeneration of an old drug. Ir J Med Sci. 2023;192:115-123. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 63. | Leung YY, Yao Hui LL, Kraus VB. Colchicine--Update on mechanisms of action and therapeutic uses. Semin Arthritis Rheum. 2015;45:341-350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 405] [Cited by in RCA: 666] [Article Influence: 60.5] [Reference Citation Analysis (0)] |
| 64. | D'Amario D, Cappetta D, Cappannoli L, Princi G, Migliaro S, Diana G, Chouchane K, Borovac JA, Restivo A, Arcudi A, De Angelis A, Vergallo R, Montone RA, Galli M, Liuzzo G, Crea F. Colchicine in ischemic heart disease: the good, the bad and the ugly. Clin Res Cardiol. 2021;110:1531-1542. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 34] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 65. | Chan YH, Ramji DP. Atherosclerosis: Pathogenesis and Key Cellular Processes, Current and Emerging Therapies, Key Challenges, and Future Research Directions. Methods Mol Biol. 2022;2419:3-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 66. | Insull W Jr. The pathology of atherosclerosis: plaque development and plaque responses to medical treatment. Am J Med. 2009;122:S3-S14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 339] [Cited by in RCA: 377] [Article Influence: 22.2] [Reference Citation Analysis (0)] |
| 67. | Björkegren JLM, Lusis AJ. Atherosclerosis: Recent developments. Cell. 2022;185:1630-1645. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 424] [Cited by in RCA: 758] [Article Influence: 189.5] [Reference Citation Analysis (0)] |
| 68. | Ali M, Girgis S, Hassan A, Rudick S, Becker RC. Inflammation and coronary artery disease: from pathophysiology to Canakinumab Anti-Inflammatory Thrombosis Outcomes Study (CANTOS). Coron Artery Dis. 2018;29:429-437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 41] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 69. | Guo X, Ma L. Inflammation in coronary artery disease-clinical implications of novel HDL-cholesterol-related inflammatory parameters as predictors. Coron Artery Dis. 2023;34:66-77. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 27] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 70. | Medina-Leyte DJ, Zepeda-García O, Domínguez-Pérez M, González-Garrido A, Villarreal-Molina T, Jacobo-Albavera L. Endothelial Dysfunction, Inflammation and Coronary Artery Disease: Potential Biomarkers and Promising Therapeutical Approaches. Int J Mol Sci. 2021;22:3850. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 190] [Cited by in RCA: 378] [Article Influence: 75.6] [Reference Citation Analysis (0)] |
| 71. | Tardif JC, Kouz S, Waters DD, Bertrand OF, Diaz R, Maggioni AP, Pinto FJ, Ibrahim R, Gamra H, Kiwan GS, Berry C, López-Sendón J, Ostadal P, Koenig W, Angoulvant D, Grégoire JC, Lavoie MA, Dubé MP, Rhainds D, Provencher M, Blondeau L, Orfanos A, L'Allier PL, Guertin MC, Roubille F. Efficacy and Safety of Low-Dose Colchicine after Myocardial Infarction. N Engl J Med. 2019;381:2497-2505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1228] [Cited by in RCA: 2227] [Article Influence: 318.1] [Reference Citation Analysis (0)] |
| 72. | Ridker PM, Everett BM, Thuren T, MacFadyen JG, Chang WH, Ballantyne C, Fonseca F, Nicolau J, Koenig W, Anker SD, Kastelein JJP, Cornel JH, Pais P, Pella D, Genest J, Cifkova R, Lorenzatti A, Forster T, Kobalava Z, Vida-Simiti L, Flather M, Shimokawa H, Ogawa H, Dellborg M, Rossi PRF, Troquay RPT, Libby P, Glynn RJ; CANTOS Trial Group. Antiinflammatory Therapy with Canakinumab for Atherosclerotic Disease. N Engl J Med. 2017;377:1119-1131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4997] [Cited by in RCA: 7117] [Article Influence: 790.8] [Reference Citation Analysis (0)] |
| 73. | Nidorf SM, Eikelboom JW, Budgeon CA, Thompson PL. Low-dose colchicine for secondary prevention of cardiovascular disease. J Am Coll Cardiol. 2013;61:404-410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 551] [Cited by in RCA: 807] [Article Influence: 62.1] [Reference Citation Analysis (0)] |
| 74. | Tong DC, Quinn S, Nasis A, Hiew C, Roberts-Thomson P, Adams H, Sriamareswaran R, Htun NM, Wilson W, Stub D, van Gaal W, Howes L, Collins N, Yong A, Bhindi R, Whitbourn R, Lee A, Hengel C, Asrress K, Freeman M, Amerena J, Wilson A, Layland J. Colchicine in Patients With Acute Coronary Syndrome: The Australian COPS Randomized Clinical Trial. Circulation. 2020;142:1890-1900. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 286] [Article Influence: 47.7] [Reference Citation Analysis (0)] |
| 75. | Jolly SS, d'Entremont MA, Lee SF, Mian R, Tyrwhitt J, Kedev S, Montalescot G, Cornel JH, Stanković G, Moreno R, Storey RF, Henry TD, Mehta SR, Bossard M, Kala P, Layland J, Zafirovska B, Devereaux PJ, Eikelboom J, Cairns JA, Shah B, Sheth T, Sharma SK, Tarhuni W, Conen D, Tawadros S, Lavi S, Yusuf S; CLEAR Investigators. Colchicine in Acute Myocardial Infarction. N Engl J Med. 2025;392:633-642. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 161] [Article Influence: 161.0] [Reference Citation Analysis (0)] |
| 76. | Zhang RS, Weber BN, Araiza-Garaygordobil D, Garshick MS. Colchicine for the Prevention of Cardiovascular Disease: Potential Global Implementation. Curr Cardiol Rep. 2024;26:423-434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 8] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 77. | Zhou Y, Liu Y, Zeng R, Qiu W, Zhao Y, Zhou Y. Early long-term low-dosage colchicine and major adverse cardiovascular events in patients with acute myocardial infarction: a systematic review and meta-analysis. Front Cardiovasc Med. 2023;10:1194605. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 78. | Tercan H, van Broekhoven A, Bahrar H, Opstal T, Cossins BC, Rother N, Rodwell L, Bekkering S, El Messaoudi S, Riksen NP, Cornel JH. The Effect of Low-Dose Colchicine on the Phenotype and Function of Neutrophils and Monocytes in Patients with Chronic Coronary Artery Disease: A Double-Blind Randomized Placebo-Controlled Cross-Over Study. Clin Pharmacol Ther. 2024;116:1325-1333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 79. | Paschke S, Weidner AF, Paust T, Marti O, Beil M, Ben-Chetrit E. Technical advance: Inhibition of neutrophil chemotaxis by colchicine is modulated through viscoelastic properties of subcellular compartments. J Leukoc Biol. 2013;94:1091-1096. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 59] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 80. | Bulnes JF, González L, Velásquez L, Orellana MP, Venturelli PM, Martínez G. Role of inflammation and evidence for the use of colchicine in patients with acute coronary syndrome. Front Cardiovasc Med. 2024;11:1356023. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 81. | Abideen ZU, Pathak DR, Sabanci R, Manu M, Abela GS. The effect of colchicine on cholesterol crystal formation, expansion and morphology: a potential mechanism in atherosclerosis. Front Cardiovasc Med. 2024;11:1345521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 82. | Madanchi M, Young M, Tersalvi G, Maria Cioffi G, Attinger-Toller A, Cuculi F, Kurmann R, Bossard M. The impact of colchicine on patients with acute and chronic coronary artery disease. Eur J Intern Med. 2024;125:1-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 13] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 83. | Shaikh S, Hamza M, Neppala S, Singh S, Upreti P, Umer AM, Manish KC, Pandya K, Bahar Y, Sattar Y, Alraies MC. Colchicine for secondary prevention in patients with acute coronary syndrome: A systematic review and meta-analysis. Int J Cardiol. 2025;425:133045. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 11] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 84. | Bonaventura A, Potere N, Liberale L, Kraler S, Weber BN, Abbate A. Colchicine in Coronary Artery Disease: Where Do We Stand? J Cardiovasc Pharmacol. 2025;85:243-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 8] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 85. | Younas A, Awan Z, Khan T, Mehta S, Munir A, Raja HAA, Jain H, Raza A, Sehar A, Ahmed R, Nashwan AJ. The effect of colchicine on myocardial infarction: An updated systematic review and meta-analysis of randomized controlled trials. Curr Probl Cardiol. 2025;50:102878. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 17] [Article Influence: 17.0] [Reference Citation Analysis (1)] |
| 86. | Diaz-Arocutipa C, Benites-Meza JK, Chambergo-Michilot D, Barboza JJ, Pasupuleti V, Bueno H, Sambola A, Hernandez AV. Efficacy and Safety of Colchicine in Post-acute Myocardial Infarction Patients: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Front Cardiovasc Med. 2021;8:676771. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 87. | Ridker PM, Everett BM, Pradhan A, MacFadyen JG, Solomon DH, Zaharris E, Mam V, Hasan A, Rosenberg Y, Iturriaga E, Gupta M, Tsigoulis M, Verma S, Clearfield M, Libby P, Goldhaber SZ, Seagle R, Ofori C, Saklayen M, Butman S, Singh N, Le May M, Bertrand O, Johnston J, Paynter NP, Glynn RJ; CIRT Investigators. Low-Dose Methotrexate for the Prevention of Atherosclerotic Events. N Engl J Med. 2019;380:752-762. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 711] [Cited by in RCA: 1019] [Article Influence: 145.6] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/