Published online Nov 6, 2025. doi: 10.12998/wjcc.v13.i31.108942
Revised: May 31, 2025
Accepted: September 8, 2025
Published online: November 6, 2025
Processing time: 182 Days and 19.6 Hours
Kirsten rat sarcoma viral oncogene homolog (KRAS) is a commonly identified oncogenic driver in solid tumors, especially in non-small cell lung cancer. Until recently, KRAS was believed to be impossible to target because it lacks adenosine triphosphate-binding domains or other regions that allow specific small-molecule inhibitors to act. In this report, we described using KRAS at glycine 12 to cysteine (G12C) inhibitors as posterior line therapy in a patient with relapsed metastatic lung adenocarcinoma carrying KRAS G12C mutation.
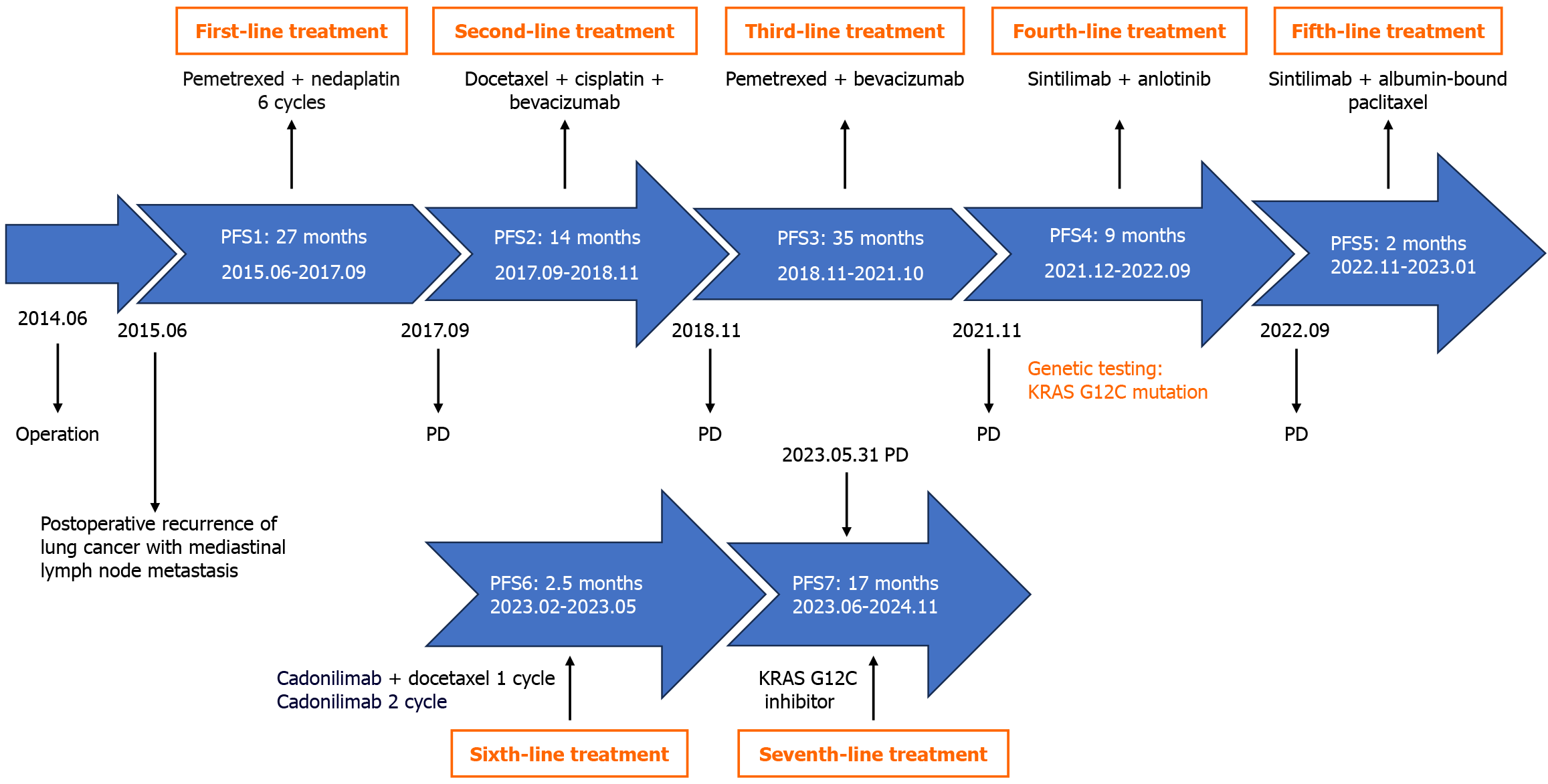
A 53-year-old Chinese man was treated with radical surgical resection for lung cancer in June 2014. Re-examination in June 2015 indicated postoperative rec
After multiple-line treatment including chemotherapy, targeted therapy, and immunotherapy, disease control was achieved in a case of advanced pulmonary adenocarcinoma carrying the KRAS G12C mutation by mutation-specific inhibitor therapy, and the adverse reactions to the therapy were tolerable.
Core Tip: Non-small cell lung cancer represents 85% of all pulmonary malignancies globally. Kirsten rat sarcoma viral oncogene homolog (KRAS) is among the most common mutations observed in malignant tumors, with roughly 13% of pulmonary adenocarcinomas carrying the KRAS at glycine 12 to cysteine (G12C) mutation. In this case, the initial therapeutic strategies for KRAS G12C-mutant lung adenocarcinomas were similar to those for driver mutation-negative non-small cell lung cancer. Following relapse, with KRAS G12C inhibitor administration, the patient’s post-relapse progression-free survival reached 17 months. Subsequently, the patient’s disease progressed, necessitating renewed treatment with radiotherapy, chemotherapy, and targeted therapy.
- Citation: Gan L, Shen JF, Yao MX, Chen ZG, Zhuang ZX. Kirsten rat sarcoma G12C inhibitor treatment for a patient with relapsed metastatic lung adenocarcinoma: A case report. World J Clin Cases 2025; 13(31): 108942
- URL: https://www.wjgnet.com/2307-8960/full/v13/i31/108942.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v13.i31.108942
Lung cancer, the second most generally diagnosed cancer and the foremost cause of cancer death in 2020, accounts for 11.4% of cancer diagnoses and 18.0% of mortality from malignancy. There are some 2.2 million lung cancer diagnoses resulting in 1.8 million annual deaths worldwide[1]. In men, lung cancer remains the highest cause of cancer mortality and morbidity. In women, lung cancer is the third-most common malignancy, after breast cancer and colorectal cancer, and the second leading cause of mortality after breast cancer. Approximately 25%-30% of non-small cell lung cancers (NSCLCs) have a mutated Kirsten rat sarcoma viral oncogene homolog (KRAS). The majority of KRAS mutations are found in exon 2, codons 12 and codons 13. The most common of these is KRAS at glycine 12 to cysteine (G12C) mutation, found in some 13% of NSCLCs[2]. Wankhede et al’s meta-analysis of 16 retrospective studies, including 10153 patients, found that this mutation is associated with poor survival in NSCLCs[3]. First of all, this study found that compared to that of subjects with different KRAS mutations, the presence of KRAS G12C mutation predicted worse disease-free survival. Next, those with KRAS G12C mutations were at a higher risk of all-cause mortality than those with KRAS wild-type tumors despite similar disease-free survival. Finally, tumors with KRAS G12C mutations had higher programmed cell death ligand 1 (PD-L1) expression (> 50%) than that observed in tumors bearing other KRAS mutations. In summary, the G12C mutation of KRAS confers a worse prognosis[3].
Clinical trials using sotorasib and adagrasib, inhibitors of KRAS G12C, highlighted their efficacy in cancers harboring the KRAS G12C mutation. Sotorasib, a specific inhibitor of KRAS G12C, was given accelerated regulatory approval in the United States in May 2021 to treat adults with KRAS G12C-mutant metastatic or locally advanced NSCLC following at least one systemic therapy previously[4]. A one-arm phase 2 study of sotorasib, termed the CodeBreak 100 trial evaluating 126 patients having pretreated KRAS G12C-mutant metastatic NSCLC, resulted in an objective response rate of 37.1% [95% confidence interval (CI): 28.6-46.2], 6.8 months (95%CI: 5.1-8.2) median progression-free survival (PFS), and 12.5 months (95%CI: 10.0 to not reached) median overall survival, with good tolerability and safety[5]. Adagrasib was initially approved on December 12, 2022, in the United States to treat the adults with KRAS G12C-mutant metastatic or locally advanced NSCLC having received at least one previous course of systemic treatment. Here, we report a patient with metastatic KRAS G12C-mutant NSCLC who received multiple courses of therapy, treated with an inhibitor of KRAS G12C mutation leading to PFS of 17 months. Although the patient in this case received targeted therapy with KRAS G12C inhibitors after six prior therapies, the patient’s therapeutic effect was still remarkable. KRAS G12C inhibitors not only can improve outcomes of patients with advanced lung cancer, but also enhance their quality of life. KRAS G12C inhibitors are the preferred option for treating lung adenocarcinoma with a KRAS G12C mutation.
A 62-year-old Chinese man having a history of NSCLC, treated with multiple lines of therapy, developed cough and bloody sputum production in early April 2023 and shortness of breath with exertion.
In June 2014, a 53-year-old Chinese man underwent radical resection of a lung tumor, and the postoperative pathology revealed lung adenocarcinoma. The patient did not receive adjuvant chemotherapy or adjuvant radiotherapy after the operation. Computed tomography (CT) examination in June 2015 indicated postoperative recurrence and lymph node (LN) metastasis. Subsequently, the patient received chemotherapy, targeted therapy and immunotherapy. In early May 2023, the patient developed a cough and bloody sputum production with shortness of breath upon exertion.
The patient was treated with oral antihypertensive drugs to correct blood pressure elevation occurring during prior chemotherapy. Antihypertensive therapy was discontinued after the patient’s blood pressure normalized. The patient additionally had chronic renal insufficiency with elevated creatinine, and he was treated with oral JinShuiBao.
The patient had a several-decades history of smoking, but he quit smoking after being diagnosed with pulmonary adenocarcinoma in 2014. The patient denied drinking. The patient had no family history of malignant tumors or genetic diseases or infections. His Eastern Cooperative Oncology Group performance status was 1.
Physical examination revealed slightly reduced breath sounds on the right and palpable swollen LNs on the right side of the neck. His temperature was 36.5 °C, pulse 75 beats/minute, and blood pressure 118/68 mmHg.
The patient’s laboratory results were not abnormal. The patient’s biochemical tests revealed normal glutamic oxaloacetic transaminase, glutamic pyruvic transaminase levels, and total, direct, and indirect bilirubin levels. The patient’s renal studies found a slightly elevated creatinine level, and he had concomitant hypocalcemia. There were no laboratory findings consistent with infection with hepatitis A virus, hepatitis B virus, hepatitis C virus, or hepatitis E virus.
Studies had revealed tumor progression again in mid-November 2021. Tracheoscopy with biopsy was conducted on November 19, 2021. The biopsy showed: Transbronchial needle aspiration, LN 11 right; and the immunohistochemistry showed cytokeratin 7 (+), thyroid transcription factor-1 (+), synaptophysin (-), aspartyl peptidase napsin A (+), Ki-67 (+, 50%), chromogranin A (-), cytokeratin 5/cytokeratin 6 (-), and P40 (-). Based on these findings, of the patient was diagnosed with poorly differentiated pulmonary adenocarcinoma. Precise genetic testing revealed the presence of the KRAS G12C mutation (mutation abundance: 34.83%), a tumor mutation burden of 5.0 mutations/Mb, and microsatellite stability. With PD-L1 (22C3) testing, both the tumor proportion score and combined positive score were 1%.
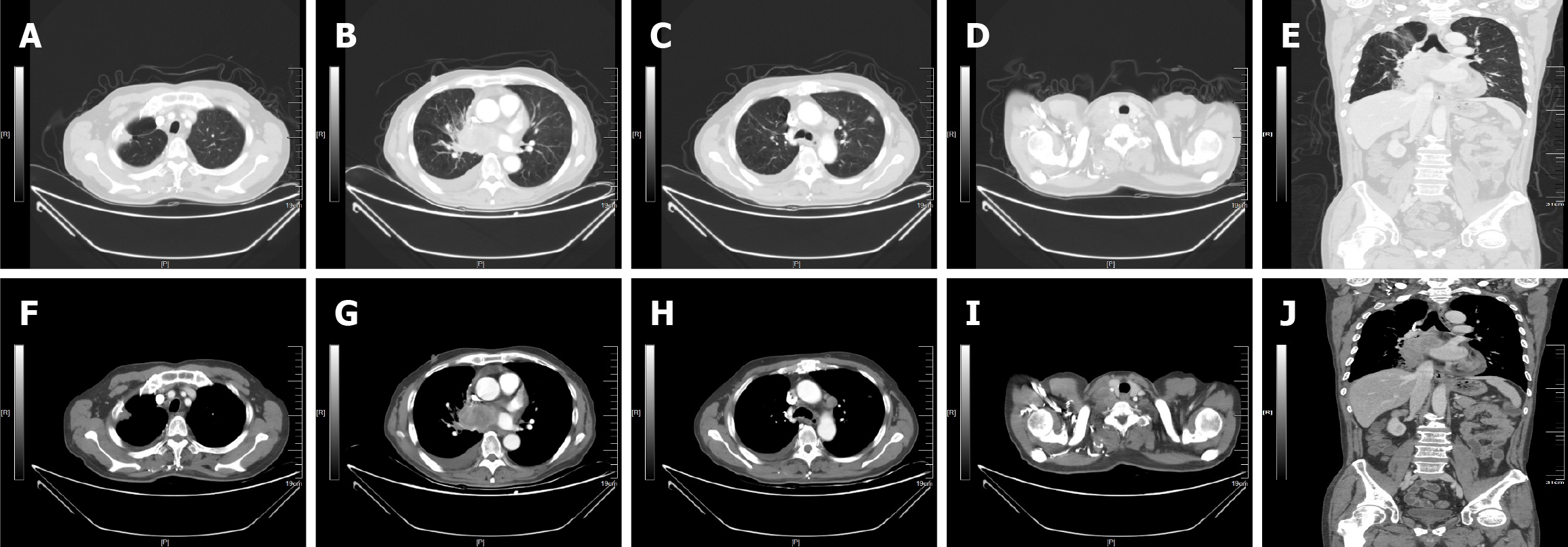
In May 31, 2023, enhanced CT revealed a mass film shadow near the right lung parenchyma with bronchial stenosis, a solid nodule and cavity in the left lung (anterior upper lobe), enlarged LNs (mediastinum, superior and inferior to right clavicle, and right lung parenchyma), scant fluid in the right thoracic cavity, and local nodular right pleural thickening (Figure 1).
Based on the imaging results and previous surgical history, the patient was ultimately diagnosed with recurrent lung adenocarcinoma and multiple metastases (T1N3M1a, stage IV; Figure 1).
After post-surgical lung cancer recurrence, the patient had received multiple courses of treatment, including combined chemotherapy and immune-targeting combination therapy. Upon disease progression, the patient underwent tracheoscopy, and biopsy tissue was sent for genetic testing, identifying a KRAS G12C mutation. Given the patient’s previously diagnosed KRAS G12C mutation, the patient was treated with oral KRAS G12C inhibitors (GEC-255, 1000 mg daily) from June 7, 2023, until November 2024. The patient’s treatment process is presented in Figure 2.
During KRAS G12C inhibitor therapy, routine blood, biochemical, and electrolyte analyses were obtained. Routine hematology testing revealed no decrease in white blood cell or platelet count or hemoglobin level. While receiving oral medications, the hemoglobin level increased (Supplementary Table 1). However, there was transiently impaired hepatic function. After treatment with the hepatoprotective drugs polyene phosphatidylcholine and silymarin, the patient’s transaminase index normalized (Supplementary Table 2).
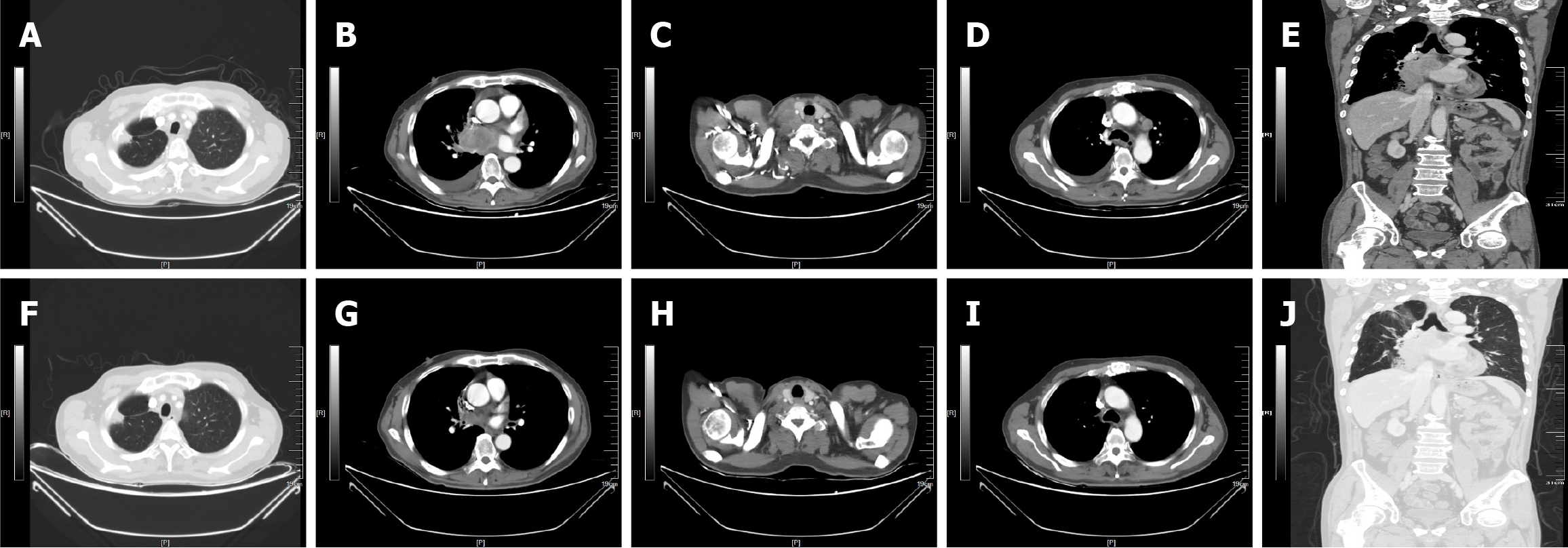
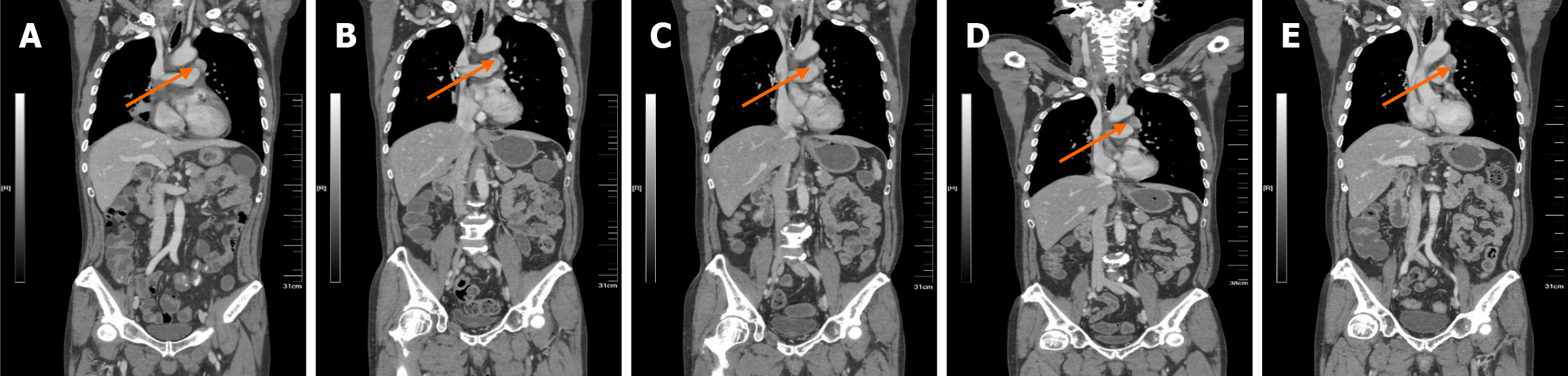
The patient exhibited a good therapeutic response after KRAS G12C inhibitor therapy, with a PFS of 17 months. After 6 weeks of oral KRAS G12C inhibitor treatment, enhanced CT revealed that, compared with the prior CT, the right lung parenchyma mass was significantly smaller; the right pleural effusion had resolved; and the right clavicular, mediastinal, and right hilar LNs were significantly reduced (Figure 3). With treatment, the patient initially experienced alternating bouts of diarrhea and constipation (grade 1, common terminology criteria for adverse events). After oral Bifidobacterium and montmorillonite powder administration, these symptoms could be tolerated. The most critical adverse reaction was nausea (grade 2, common terminology criteria for adverse events), which was relieved with oral ondansetron without need for dosage adjustment. Subsequent enhanced the chest, abdomen, and pelvis CT revealed partial remission (Supplementary Figure 1). On November 11, 2024, repeat enhanced CT indicated progressive enlargement of the left diaphragmatic angle LN (Figure 4). Subsequently, the patient underwent radiotherapy of the left diaphragmatic angle LN, which was significantly smaller by enhanced CT in February 2025.
NSCLC represents 85% of all lung cancer globally[6]. KRAS is one of the most frequently mutated oncogenes in malignant tumors, including some 25%-30% NSCLC in West, with most mutations arising in codon 12. The most abundant KRAS mutations are G12C (43%), glycine 12 to valine (18%), and glycine 12 to aspartic acid (11%)[7], with KRAS G12C occurring in roughly 13%-16% of pulmonary adenocarcinomas plus approximately 3% of colorectal adenocarcinomas[2,8], but less frequently in uterine, pancreatic, bladder, cervical, and ovarian cancers[9-11]. KRAS mutation is also strongly correlated with smoking; only 5% of these mutations occur in non-smokers[12]. Furthermore, the KRAS G12C mutation and KRAS at glycine 12 to valine mutation are more commonly observed in smokers, whereas the KRAS at glycine 12 to aspartic acid mutation is more often seen in non-smokers[7]. The current patient had a long smoking history. Increased PD-L1 expression is often observed with KRAS mutation in NSCLC due to the stimulation of downstream pathways[13,14].
In early-phase clinical trials, the small molecules adagrasib and sotorasib, dominant, selective, and irreversible inhibitors of KRAS G12C, exhibited encouraging activity in NSCLC[15-17]. In 2021, the Food and Drug Administration granted accelerated approval of the first KRAS G12C-inhibitor, sotorasib, for adults with NSCLC. This followed a phase 2 study of 126 patients with NSCLC bearing KRAS G12C previously treated with chemotherapy or immunotherapy[5]. A daily oral dose of sotorasib (960 mg), decreased disease burden in 37.1% of study subjects, with a median response of 11.1 months[4]. Subsequently, adagrasib was given breakthrough treatment designation by the United States Food and Drug Administration for patients with NSCLC with KRAS G12C mutation as second line treatment following systemic therapy. In a phase 1/phase 2 trial of 51 individuals with NSCLC with KRAS G12C mutation who had evaluable responses, subjects were given adagrasib, 600 mg twice daily[18,19]. Of them, 45% had a partial response, while 51% had stable disease. The most commonly observed adverse events were diarrhea, nausea, vomiting, fatigue, and elevated aminotransferase levels.
Although the recent advancement of specific inhibitors of KRAS G12C has provided better outcomes for patients with malignant tumors bearing KRAS mutations, only a fraction of patients responds to them, and tumors invariably become resistant over time. Therefore, it is of vital importance to identify the causes of drug resistance developing on therapy, to aid development of novel therapeutic approaches, and determine innovative treatment-sensitive pathways suitable for drug targeting. There are multiple mechanisms by which tumors acquire resistance to KRAS G12C inhibitors, including on-target and off-target resistance. On-target mechanisms include secondary KRAS mutations in codon 12, in addition to mutations of codons 13 and 61 and in drug-binding sites. Acquired off-target resistance arises via effector signaling cascade (e.g., mitogen-activated protein kinase 1) activation, development of oncogenic rearrangement (e.g., echinoderm microtubule-associated protein-like 4-anaplastic lymphoma kinase, coiled-coil domain containing 176-rearranged during transfection), gain in copy number (e.g., MET amplification), or mutations in additional growth-promoting and survival pathways (e.g., neuroblastoma RAS viral oncogene homolog, fibroblast growth factor receptor 3, phosphatase and tensin homolog)[20].
Subsequently, the CodeBreak 200 phase 3 trial demonstrated the effectiveness of daily oral sotorasib (960 mg) together with conventional docetaxel chemotherapy (75 mg/m2 once every 3 weeks) in subjects with KRAS G12C-mutant progressive NSCLC following platinum-based treatment plus programmed cell death protein 1 or PD-L1 inhibition[21]. After a 17.7 months median follow-up, subjects receiving sotorasib had better median PFS compared with docetaxel (5.6 months vs 4.7 months). Huang et al’s case report described a male patient with advanced pulmonary sarcomatoid carcinoma with a BUB1 mitotic checkpoint serine/threonine kinase b-anaplastic lymphoma kinase rearrangement plus KRAS G12C mutation[22]. Successful disease control was achieved in this patient using the combination of sintilimab and anlotinib, and PFS exceeded 20 months[22]. In a therapeutic study of subjects with cancers containing KRAS G12C mutations (27 patients with NSCLC, 10 with colorectal cancer, and 1 with appendiceal cancer) given adagrasib alone, DNA sequencing of tumor samples or circulating tumor DNA identified 17 of 38 patients in whom adagrasib resistance arose through various mechanisms, including new mutations or amplifications in KRAS, downstream receptor tyrosine kinase/RAS signaling pathway activation without directly altering KRAS, and transformation from adenocarcinoma to squamous cell carcinoma[23]. Iska and Alley[24] reported a case suggesting efficacy for sotorasib as up-front treatment for advanced NSCLC with G12C KRAS mutation, in that the patient had a complete response by imaging.
In our case, the patient completed six lines of treatment containing chemotherapy, targeted treatment, and immunotherapy, and KRAS G12C inhibitor therapy was then given, leading to PFS of 17 months. We recommended to repeat the genetic testing, but the patient declined, so the cause of drug resistance remains unknown. Wankhede et al[3] studies have shown that PD-L1 is highly expressed in lung cancer patients with KRAS G12C mutations. Therefore, when patients progress following KRAS G12C inhibitors, combination immune checkpoint inhibitors may be a new therapeutic strategy, which requires further investigation.
The patient received targeted therapy with KRAS G12C inhibitors, resulting in a PFS of 17 months. During follow-up, left diaphragmatic angle LN enlargement was found, which was treated with radiotherapy. In summary, this case indicates that KRAS inhibitors can achieve disease control in individuals with KRAS G12C-mutant NSCLC after progression following multiagent treatment. In addition, during oral KRAS inhibitor therapy, the primary adverse reaction was gastrointestinal distress, which could be tolerated with symptomatic management. This approach offers a new treatment strategy for such patients.
| 1. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 68390] [Article Influence: 13678.0] [Reference Citation Analysis (201)] |
| 2. | Thai AA, Solomon BJ, Sequist LV, Gainor JF, Heist RS. Lung cancer. Lancet. 2021;398:535-554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 402] [Cited by in RCA: 1684] [Article Influence: 336.8] [Reference Citation Analysis (0)] |
| 3. | Wankhede D, Bontoux C, Grover S, Hofman P. Prognostic Role of KRAS G12C Mutation in Non-Small Cell Lung Cancer: A Systematic Review and Meta-Analysis. Diagnostics (Basel). 2023;13:3043. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 4. | Lee A. Sotorasib: A Review in KRAS G12C Mutation-Positive Non-small Cell Lung Cancer. Target Oncol. 2022;17:727-733. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 27] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 5. | Skoulidis F, Li BT, Dy GK, Price TJ, Falchook GS, Wolf J, Italiano A, Schuler M, Borghaei H, Barlesi F, Kato T, Curioni-Fontecedro A, Sacher A, Spira A, Ramalingam SS, Takahashi T, Besse B, Anderson A, Ang A, Tran Q, Mather O, Henary H, Ngarmchamnanrith G, Friberg G, Velcheti V, Govindan R. Sotorasib for Lung Cancers with KRAS p.G12C Mutation. N Engl J Med. 2021;384:2371-2381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 941] [Cited by in RCA: 1285] [Article Influence: 257.0] [Reference Citation Analysis (0)] |
| 6. | Duma N, Santana-Davila R, Molina JR. Non-Small Cell Lung Cancer: Epidemiology, Screening, Diagnosis, and Treatment. Mayo Clin Proc. 2019;94:1623-1640. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 747] [Cited by in RCA: 1502] [Article Influence: 214.6] [Reference Citation Analysis (0)] |
| 7. | Ricciuti B, Leonardi GC, Metro G, Grignani F, Paglialunga L, Bellezza G, Baglivo S, Mencaroni C, Baldi A, Zicari D, Crinò L. Targeting the KRAS variant for treatment of non-small cell lung cancer: potential therapeutic applications. Expert Rev Respir Med. 2016;10:53-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 56] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 8. | Garcia BNC, van Kempen LC, Kuijpers CCHJ, Schuuring E, Willems SM, van der Wekken AJ. Prevalence of KRAS p.(G12C) in stage IV NSCLC patients in the Netherlands; a nation-wide retrospective cohort study. Lung Cancer. 2022;167:1-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 26] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 9. | Kim D, Xue JY, Lito P. Targeting KRAS(G12C): From Inhibitory Mechanism to Modulation of Antitumor Effects in Patients. Cell. 2020;183:850-859. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 181] [Article Influence: 30.2] [Reference Citation Analysis (0)] |
| 10. | Nassar AH, Adib E, Kwiatkowski DJ. Distribution of KRAS (G12C) Somatic Mutations across Race, Sex, and Cancer Type. N Engl J Med. 2021;384:185-187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 151] [Article Influence: 30.2] [Reference Citation Analysis (0)] |
| 11. | Arbour KC, Rizvi H, Plodkowski AJ, Hellmann MD, Knezevic A, Heller G, Yu HA, Ladanyi M, Kris MG, Arcila ME, Rudin CM, Lito P, Riely GJ. Treatment Outcomes and Clinical Characteristics of Patients with KRAS-G12C-Mutant Non-Small Cell Lung Cancer. Clin Cancer Res. 2021;27:2209-2215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 102] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 12. | Xie M, Xu X, Fan Y. KRAS-Mutant Non-Small Cell Lung Cancer: An Emerging Promisingly Treatable Subgroup. Front Oncol. 2021;11:672612. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 46] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 13. | Chen N, Fang W, Lin Z, Peng P, Wang J, Zhan J, Hong S, Huang J, Liu L, Sheng J, Zhou T, Chen Y, Zhang H, Zhang L. KRAS mutation-induced upregulation of PD-L1 mediates immune escape in human lung adenocarcinoma. Cancer Immunol Immunother. 2017;66:1175-1187. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 135] [Cited by in RCA: 245] [Article Influence: 27.2] [Reference Citation Analysis (0)] |
| 14. | Liu C, Zheng S, Jin R, Wang X, Wang F, Zang R, Xu H, Lu Z, Huang J, Lei Y, Mao S, Wang Y, Feng X, Sun N, Wang Y, He J. The superior efficacy of anti-PD-1/PD-L1 immunotherapy in KRAS-mutant non-small cell lung cancer that correlates with an inflammatory phenotype and increased immunogenicity. Cancer Lett. 2020;470:95-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 226] [Article Influence: 32.3] [Reference Citation Analysis (0)] |
| 15. | Canon J, Rex K, Saiki AY, Mohr C, Cooke K, Bagal D, Gaida K, Holt T, Knutson CG, Koppada N, Lanman BA, Werner J, Rapaport AS, San Miguel T, Ortiz R, Osgood T, Sun JR, Zhu X, McCarter JD, Volak LP, Houk BE, Fakih MG, O'Neil BH, Price TJ, Falchook GS, Desai J, Kuo J, Govindan R, Hong DS, Ouyang W, Henary H, Arvedson T, Cee VJ, Lipford JR. The clinical KRAS(G12C) inhibitor AMG 510 drives anti-tumour immunity. Nature. 2019;575:217-223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 843] [Cited by in RCA: 1647] [Article Influence: 235.3] [Reference Citation Analysis (1)] |
| 16. | Hallin J, Engstrom LD, Hargis L, Calinisan A, Aranda R, Briere DM, Sudhakar N, Bowcut V, Baer BR, Ballard JA, Burkard MR, Fell JB, Fischer JP, Vigers GP, Xue Y, Gatto S, Fernandez-Banet J, Pavlicek A, Velastagui K, Chao RC, Barton J, Pierobon M, Baldelli E, Patricoin EF 3rd, Cassidy DP, Marx MA, Rybkin II, Johnson ML, Ou SI, Lito P, Papadopoulos KP, Jänne PA, Olson P, Christensen JG. The KRAS(G12C) Inhibitor MRTX849 Provides Insight toward Therapeutic Susceptibility of KRAS-Mutant Cancers in Mouse Models and Patients. Cancer Discov. 2020;10:54-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 495] [Cited by in RCA: 974] [Article Influence: 139.1] [Reference Citation Analysis (0)] |
| 17. | Hong DS, Fakih MG, Strickler JH, Desai J, Durm GA, Shapiro GI, Falchook GS, Price TJ, Sacher A, Denlinger CS, Bang YJ, Dy GK, Krauss JC, Kuboki Y, Kuo JC, Coveler AL, Park K, Kim TW, Barlesi F, Munster PN, Ramalingam SS, Burns TF, Meric-Bernstam F, Henary H, Ngang J, Ngarmchamnanrith G, Kim J, Houk BE, Canon J, Lipford JR, Friberg G, Lito P, Govindan R, Li BT. KRAS(G12C) Inhibition with Sotorasib in Advanced Solid Tumors. N Engl J Med. 2020;383:1207-1217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1267] [Cited by in RCA: 1297] [Article Influence: 216.2] [Reference Citation Analysis (0)] |
| 18. | Ou SI, Jänne PA, Leal TA, Rybkin II, Sabari JK, Barve MA, Bazhenova L, Johnson ML, Velastegui KL, Cilliers C, Christensen JG, Yan X, Chao RC, Papadopoulos KP. First-in-Human Phase I/IB Dose-Finding Study of Adagrasib (MRTX849) in Patients With Advanced KRAS(G12C) Solid Tumors (KRYSTAL-1). J Clin Oncol. 2022;40:2530-2538. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 242] [Cited by in RCA: 224] [Article Influence: 56.0] [Reference Citation Analysis (0)] |
| 19. | Jänne PA, Riely GJ, Gadgeel SM, Heist RS, Ou SI, Pacheco JM, Johnson ML, Sabari JK, Leventakos K, Yau E, Bazhenova L, Negrao MV, Pennell NA, Zhang J, Anderes K, Der-Torossian H, Kheoh T, Velastegui K, Yan X, Christensen JG, Chao RC, Spira AI. Adagrasib in Non-Small-Cell Lung Cancer Harboring a KRAS(G12C) Mutation. N Engl J Med. 2022;387:120-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 663] [Article Influence: 165.8] [Reference Citation Analysis (0)] |
| 20. | Di Federico A, Ricciotti I, Favorito V, Michelina SV, Scaparone P, Metro G, De Giglio A, Pecci F, Lamberti G, Ambrogio C, Ricciuti B. Resistance to KRAS G12C Inhibition in Non-small Cell Lung Cancer. Curr Oncol Rep. 2023;25:1017-1029. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 29] [Reference Citation Analysis (0)] |
| 21. | de Langen AJ, Johnson ML, Mazieres J, Dingemans AC, Mountzios G, Pless M, Wolf J, Schuler M, Lena H, Skoulidis F, Yoneshima Y, Kim SW, Linardou H, Novello S, van der Wekken AJ, Chen Y, Peters S, Felip E, Solomon BJ, Ramalingam SS, Dooms C, Lindsay CR, Ferreira CG, Blais N, Obiozor CC, Wang Y, Mehta B, Varrieur T, Ngarmchamnanrith G, Stollenwerk B, Waterhouse D, Paz-Ares L; CodeBreaK 200 Investigators. Sotorasib versus docetaxel for previously treated non-small-cell lung cancer with KRAS(G12C) mutation: a randomised, open-label, phase 3 trial. Lancet. 2023;401:733-746. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 383] [Article Influence: 127.7] [Reference Citation Analysis (0)] |
| 22. | Huang N, Qu T, Zhang C, Li J. Case report: Successful treatment of advanced pulmonary sarcomatoid carcinoma with BUBIB-ALK rearrangement and KRAS G12C mutation by sintilimab combined with anlotinib. Front Oncol. 2024;14:1269148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 23. | Awad MM, Liu S, Rybkin II, Arbour KC, Dilly J, Zhu VW, Johnson ML, Heist RS, Patil T, Riely GJ, Jacobson JO, Yang X, Persky NS, Root DE, Lowder KE, Feng H, Zhang SS, Haigis KM, Hung YP, Sholl LM, Wolpin BM, Wiese J, Christiansen J, Lee J, Schrock AB, Lim LP, Garg K, Li M, Engstrom LD, Waters L, Lawson JD, Olson P, Lito P, Ou SI, Christensen JG, Jänne PA, Aguirre AJ. Acquired Resistance to KRAS(G12C) Inhibition in Cancer. N Engl J Med. 2021;384:2382-2393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 661] [Cited by in RCA: 861] [Article Influence: 172.2] [Reference Citation Analysis (0)] |
| 24. | Iska S, Alley EW. Sotorasib as First-Line Treatment for Advanced KRAS G12C-Mutated Non-Small Cell Lung Carcinoma: A Case Report. Case Rep Oncol. 2023;16:177-181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/