Published online Jan 26, 2025. doi: 10.12998/wjcc.v13.i3.99558
Revised: October 9, 2024
Accepted: October 29, 2024
Published online: January 26, 2025
Processing time: 110 Days and 5.4 Hours
Wilson's disease (WD) is a rare metabolic disorder of copper accumulation in organs such as liver, brain, and cornea. Diagnoses and treatments are challenging in settings, where advanced diagnostic tests are unavailable, copper chelating agents are frequently scarce, healthcare professionals lack disease awareness, and medical follow-ups are limited. Prompt diagnoses and treatments help prevent complications, improve patients’ quality of life, and ensure a normal life expec
We present the cases of two siblings (19 and 27 years) from a consanguineous family in rural Ecuador, diagnosed as having WD during a family screening. The male patient, diagnosed at age 19 after his brother’s death from acute liver failure, presented with compensated cirrhosis, neurological symptoms, and bilateral Kayser-Fleischer rings. He developed progressive neurological deterioration during an irregular treatment with D-penicillamine due to medication shortages. His condition improved upon switching to trientine tetrahydrochloride, and his neurological symptoms improved over an 8-year period of follow-ups. The female patient, diagnosed at age 10, exhibited only biochemical alterations. Her treatment history was similar; however, she remained asymptomatic without disease progression over the same follow-up period. We discuss the potential influence of epigenetic mechanisms and modifier genes on the various phenotypes, emp
Our patients’ medical histories show how early diagnosis and treatment can prevent disease progression; and, how suboptimal treatments impact disease outcomes.
Core Tip: Diagnosing and treating Wilson disease (WD) is particularly challenging in developing countries due to limited resources. This case study of two siblings from Ecuador demonstrates why early diagnoses and consistent treatments are crucial. The male sibling showed severe symptoms, whereas an early intervention kept the female sibling asymptomatic. Switching from D-penicillamine to trientine tetrahydrochloride improved their condition. Our cases report emphasizes the need for an effective healthcare infrastructure and WD awareness to manage the condition under resource-limited settings, and our findings suggest that genetic and environmental factors contribute to the disease's variability.
- Citation: Carrera E, Alvarado J, Astudillo M, Pillajo G. Wilson's disease in two siblings from Ecuador: Two case reports. World J Clin Cases 2025; 13(3): 99558
- URL: https://www.wjgnet.com/2307-8960/full/v13/i3/99558.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v13.i3.99558
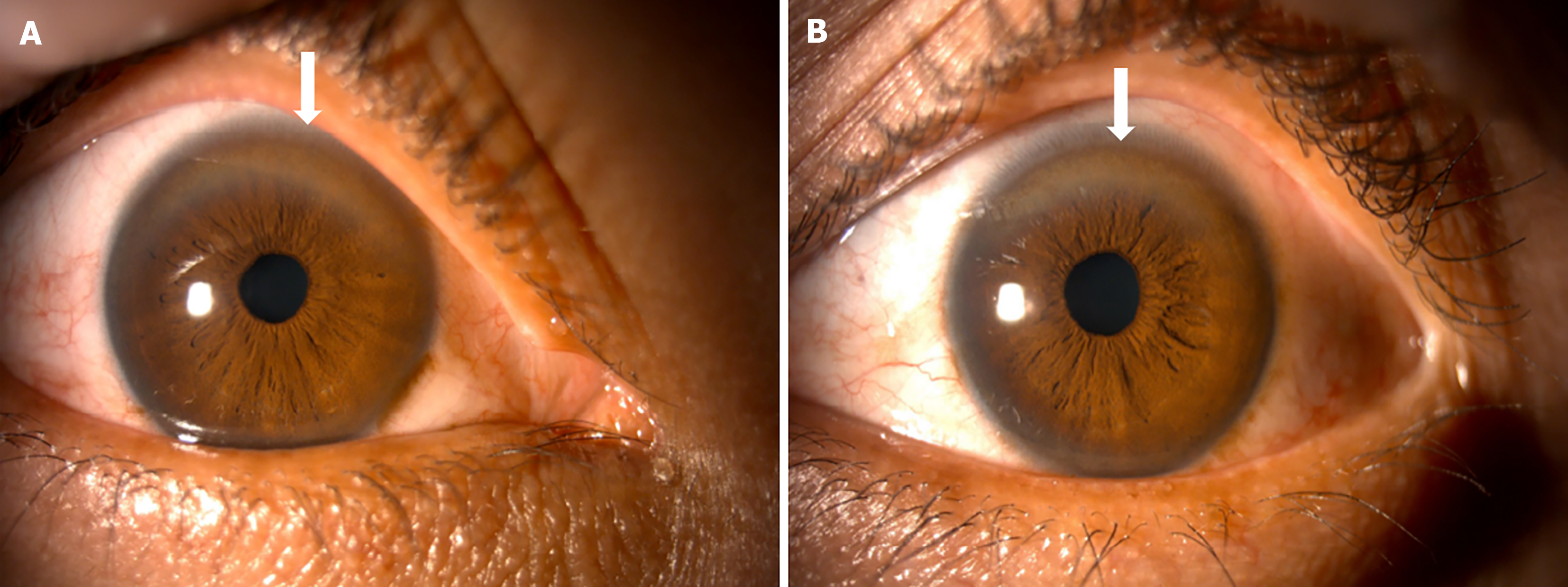
Wilson’s disease (WD) is caused by an inborn error of copper metabolism. The estimated prevalence in Europe, Asia, and the United States varies between 1:30000 to 1:50000 inhabitants. Certain regions, such as Sardinia (1:8700), Israel (1:16000), and Costa Rica (1:19000), exhibit a higher estimated prevalence due to increased frequencies of disease-causing mutations and consanguinity[1]. WD is a monogenic autosomal recessive disorder, with a genetic defect on chromosome 13q14.3. More than a thousand mutations of the ATP7B gene have been described, leading to alteration of the copper-transporting ATPase beta protein, and modifying copper homeostasis[2]. Hepatic manifestations of WD range from asymptomatic presentations to acute liver failure, a critical condition with high mortality. Findings may include hepatomegaly, steatosis, or incidental elevations in aminotransferases. Chronic hepatopathy signs are common at the time of diagnosis. Neurological manifestations are heterogeneous, frequent symptoms include dysarthria, gait disturbances, ataxia, dystonia, parkinsonism, and tremors. Psychiatric symptoms comprise personality and behavioral changes, depression, cognitive problems, and bipolar and psychotic disorders[3,4]. Kayser–Fleischer rings (K-F rings), observed upon slit lamp examination, are considered pathognomonic for WD; they result from copper deposition on Descemet's membrane, and are frequently observed as a brown or golden pigment[4-6]. Diagnostic and therapeutic delays are common, leading to neurological disability and hepatic complications. The diagnosis, on the basis of the Leipzig scoring criteria, involves both clinical and laboratory examination results. Brain magnetic resonance imaging (MRI) is valuable for visualizing copper deposition on damaged areas, often revealing symmetric hyperintense changes within the basal ganglia on T2-weighted (T2) sequences. The ‘face of the giant panda’ sign is another finding considered pathognomonic for WD[3-6]. WD requires a lifelong treatment with a combination of oral pharmacotherapy and a copper-restricted diet, recommended for both symptomatic and asymptomatic patients. Treatment with chelating agents, such as D-penicillamine (DPA) or trientine, is recommended initially for patients with active disease, and an oral chelator at a reduced dose or zinc may be indicated for maintenance therapy[4]. According to British guidelines, zinc salts, which inhibit the intestinal absorption of copper from the diet, have shown efficacy only in some cohorts; their use as monotherapy against WD is controversial, and they are not recommended in cirrhotic patients[6]. Liver transplantation is reserved for specific cases, such as patients with progressive chronic liver failure resistant to pharmacotherapy and acute liver failure[4].
Case 1: Referred to our hospital for follow-up care of WD, initially diagnosed in 2016 at another medical center.
Case 2: Referred to our hospital for follow-up care of WD, initially diagnosed in 2016 at another medical center.
Case 1: Case 1 was a 27-year-old male patient, born in Ducur, a rural community in the province of Cañar in Ecuador. The patient had mestizo ethnicity, he had finished high school and was working in construction at the time of writing this paper. In 2016, at the age of 19, he was diagnosed as having WD after a family screening prompted by the death of his older brother due to acute liver failure and neurological complications of WD. At the time of diagnosis, findings were consistent with compensated cirrhosis, he presented neurological disturbances accompanied by bilateral K-F rings (Figure 1).
Case 2: The patient was a 19-year-old woman born in Ducur, a rural community located in the province of Cañar in Ecuador. She had mestizo ethnicity and was an unmarried architecture college student at the time of writing this paper. She was diagnosed as having WD via a family screening in 2016, while asymptomatic at age 10.
Case 1: During school and before his diagnosis, the patient had experienced learning difficulties, but he was otherwise healthy.
Case 2: The patient has had no symptoms of WD.
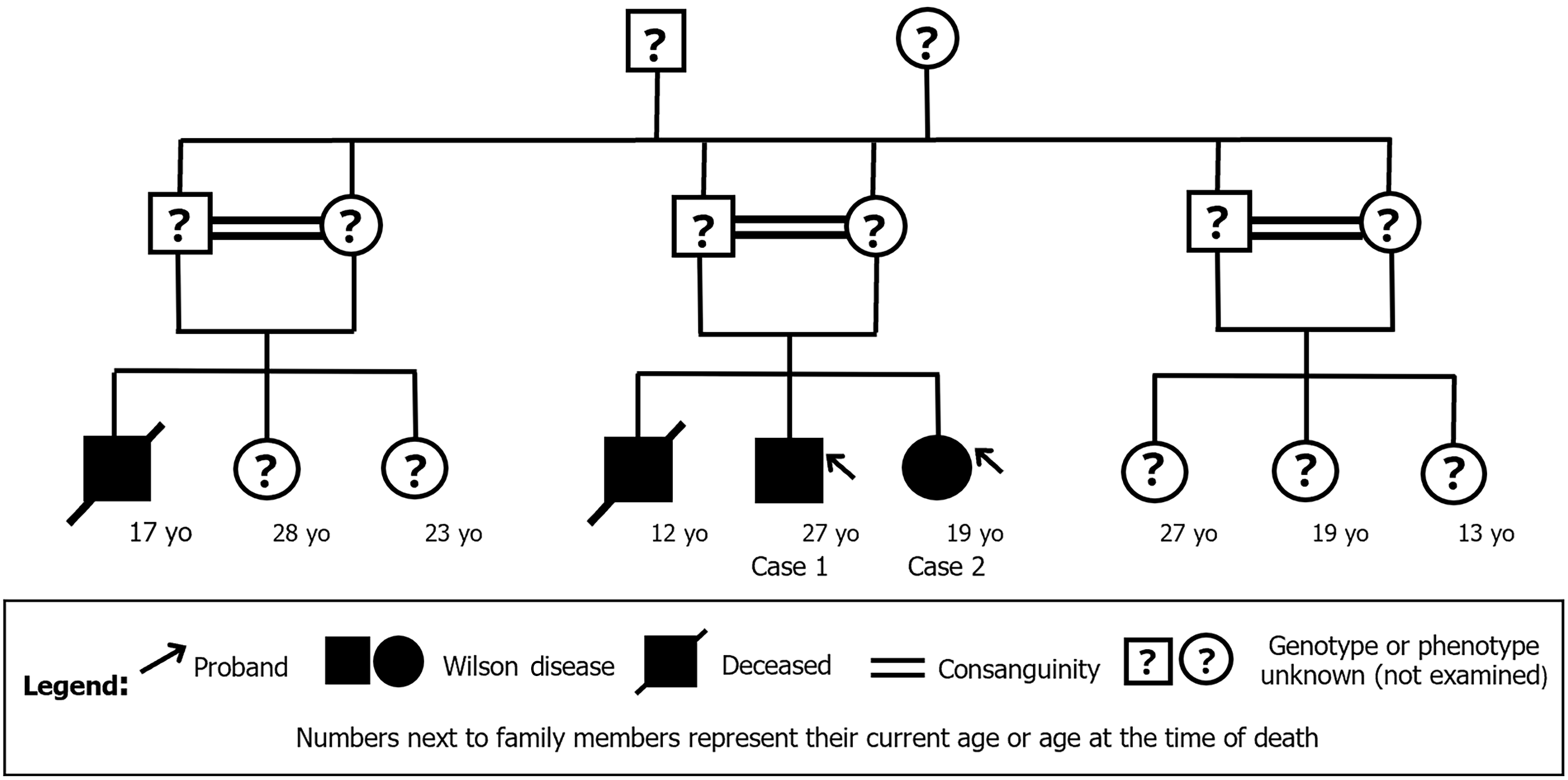
Case 1: The patient’s family history included a sister with WD (Case 2), alongside a brother and cousin who had died due to complications of the same disorder at the ages of 12 and 17, respectively. Additionally, the individual’s parents were siblings, indicating a consanguineous relationship. The cousin’s parents were also siblings. The affected individuals are illustrated in the family pedigree shown in Figure 2.
Case 2: The patient is the sister of Case 1 and they share the same family history. In 2013, laboratory test results inci
Case 1: An asymmetrical fine tremor of the upper right limb was observed during the admission physical examination. The rest of the neurological examination was unremarkable. Bilateral K-F rings were observed during a slit lamp examination. There were no signs of jaundice or hemorrhage. The abdomen was non-tender, and without ascites, hepatomegaly or splenomegaly. No pathological findings were found in other organs or systems.
Case 2: The admission physical examination was unremarkable. No jaundice, hemorrhagic signs, or abdominal disturbances were present.
Case 1: The initial assessment results revealed normal blood cell counts, as well as normal renal and liver function tests. Viral and autoimmune etiologies were ruled out. Other test results included the patient’s serum copper at 1.64 mg/L (reference value, 0.70-1.50 mg/L), 24-hour urinary copper excretion at 37 μg/L (reference value, 10-30 μg/L), ceruloplasmin at 0.10 g/L (reference value, 0.20-0.35 g/L), and serum non-ceruloplasmin bound copper at 1.32 mg/L (reference value, < 0.15 mg/L). The unavailability of certain diagnostic tests in the public healthcare system of Ecuador made it impossible for us to measure the total liver copper accumulation or assess hepatic copper deposition using rhodanine or orcein stains. Additionally, it was not possible to conduct genetic testing to detect potential ATP7B gene mutations.
Case 2: At the time of diagnosis, the ceruloplasmin levels were below 0.03 g/L (reference values 0.20-0.35 g/L) and the urinary copper content was 105 μg/24 hour (reference values 10-30 μg/24 hour). Other tests included total cholesterol (5.23 mmol/L), total triglycerides (2.41 mmol/L), albumin (54 g/L), AST (62 U/L), ALT (80 U/L), and normal complete blood counts and bilirubin levels. Viral and autoimmune etiologies were ruled out. The unavailability of certain diagnostic tests in the public healthcare system of Ecuador made it impossible for us to measure the total liver copper accumulation or assess hepatic copper deposition using rhodanine or orcein stains. Additionally, it was not possible to conduct genetic testing to detect potential ATP7B gene mutations.
Case 1: Hepatic ultrasound revealed echostructure alterations and a pseudonodular appearance, without signs of portal hypertension. The liver size was preserved, the right lobe measured 14.2 cm in its craniocaudal length, and the left lobe measured 8.5 cm; the surface had a discrete micronodular echostructure and mildly irregular borders, suggestive of chronic liver disease. The spleen was homogeneous and measured 12.8 cm × 7.6 cm × 3.6 cm, with a volume of 191 mL. The gallbladder was contracted, without dilation of the bile ducts. The kidneys had a normal size and echogenicity. The pancreas was not assessable due to gas interposition. No free fluid was present in the abdominal cavity.
According to the medical records, an initial brain MRI at the time of diagnosis revealed T2 hyperintense lesions in the basal ganglia consistent with WD. We were not able to obtain these images for this study.
Further evaluations in 2023 revealed characteristic T2 hyperintense lesions in the basal ganglia on a brain MRI. Fluid-attenuation inversion recovery (FLAIR) MRI with an axial section view at the level of the midbrain exhibited the classic ‘giant panda’ sign, where the mesencephalic tegmentum shows increased T2 signal intensity, highlighting the contrast of the substantia nigra, corresponding to the ‘bear ears’, as well as the red nuclei, corresponding to the ‘bear eyes’ (Figure3A). Figures 3B and C provide reference images of the giant panda (Ailuropoda melanoleuca) and the red panda (Ailurus fulgens), respectively. In Figure 3D, a FLAIR MRI with an axial section at the pons level shows the ‘small panda’ sign in the pontine tegmentum, where increased T2 signal intensity allows better differentiation of the medial longitudinal fasciculi and central tegmental tracts, representing the ‘eyes of the bear’, and the superior angle of the fourth ventricle represents the ‘nose of the bear’.
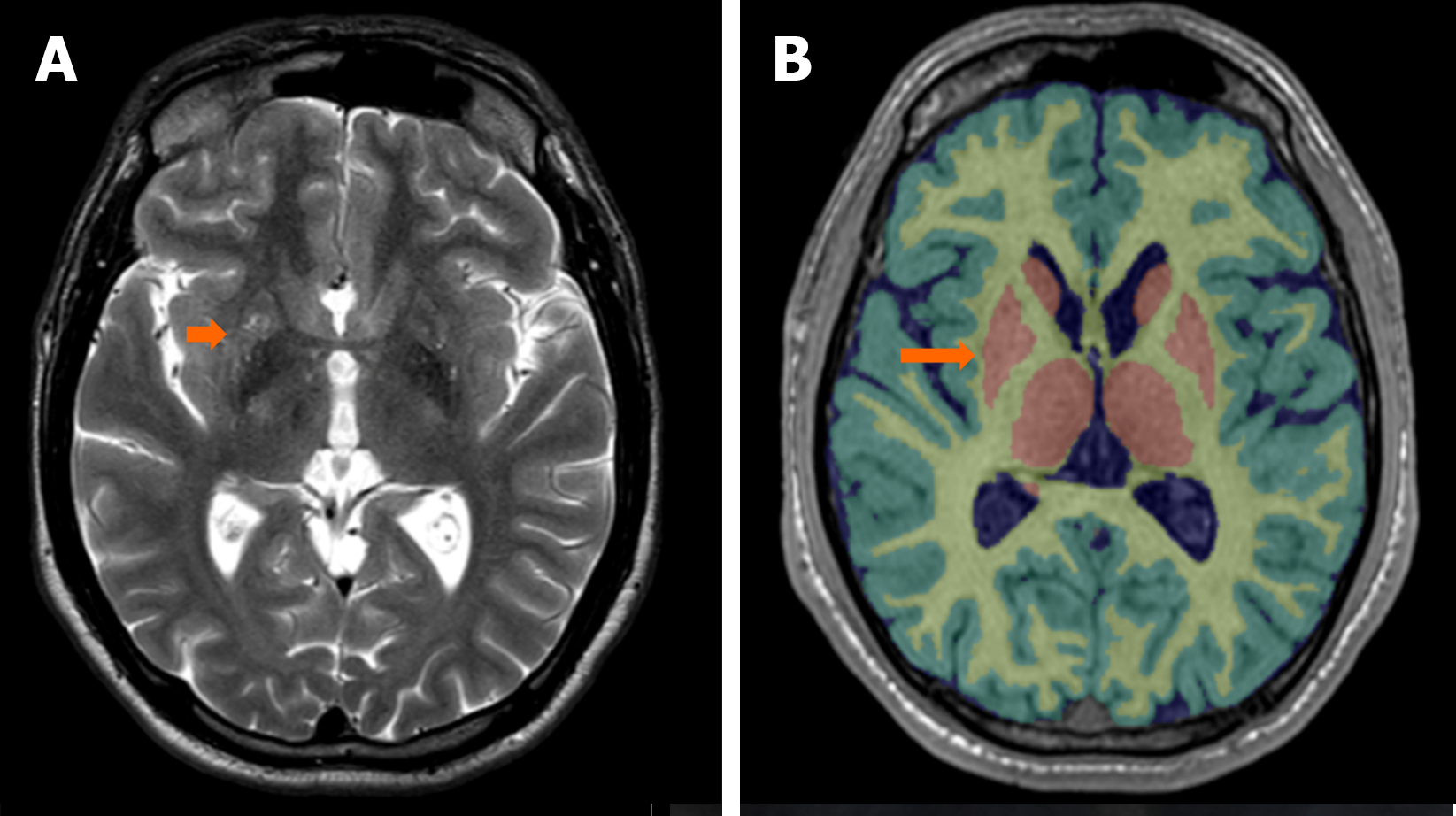
T2-turbo spin-echo MRI findings were consistent with multiple diffuse T2 hyperintensities in the lentiform and caudate nuclei (Figure 4A). An MRI with automated analysis of T1-3D sequence showed pathological findings in the right lentiform nucleus, a part of the basal nuclei. The basal nuclei presented a reduced volume corresponding to less than the 1st percentile for age and sex in our patient (Figure 4B).
In May 2024, the patient underwent an annual follow-up with abdominal ultrasound and color Doppler ultrasound. Relevant findings included a preserved liver size with lobulated margins and mild volume redistribution due to central atrophy of the left lobe. The liver maintained a hyperechoic echogenicity with a reticular morphology, interspersed with hypoechoic areas, and a heterogeneous echotexture giving it a micronodular appearance. There was no bile duct dilation. No focal lesions or ascites were observed. The color Doppler showed incipient signs of portal hypertension with splenic collateralization.
Case 2: The abdominal ultrasound images revealed a slight increase in the liver’s echogenicity without other pathological findings.
Case 1: A WD diagnosis was confirmed with a total score of 7 points on the Leipzig scoring system. In addition, a Child Pugh score of 5 corresponding to class A, was established.
Case 2: A WD diagnosis was confirmed with a total score of 4 points on the Leipzig scoring system. The history of affected family members also supported the final diagnosis.
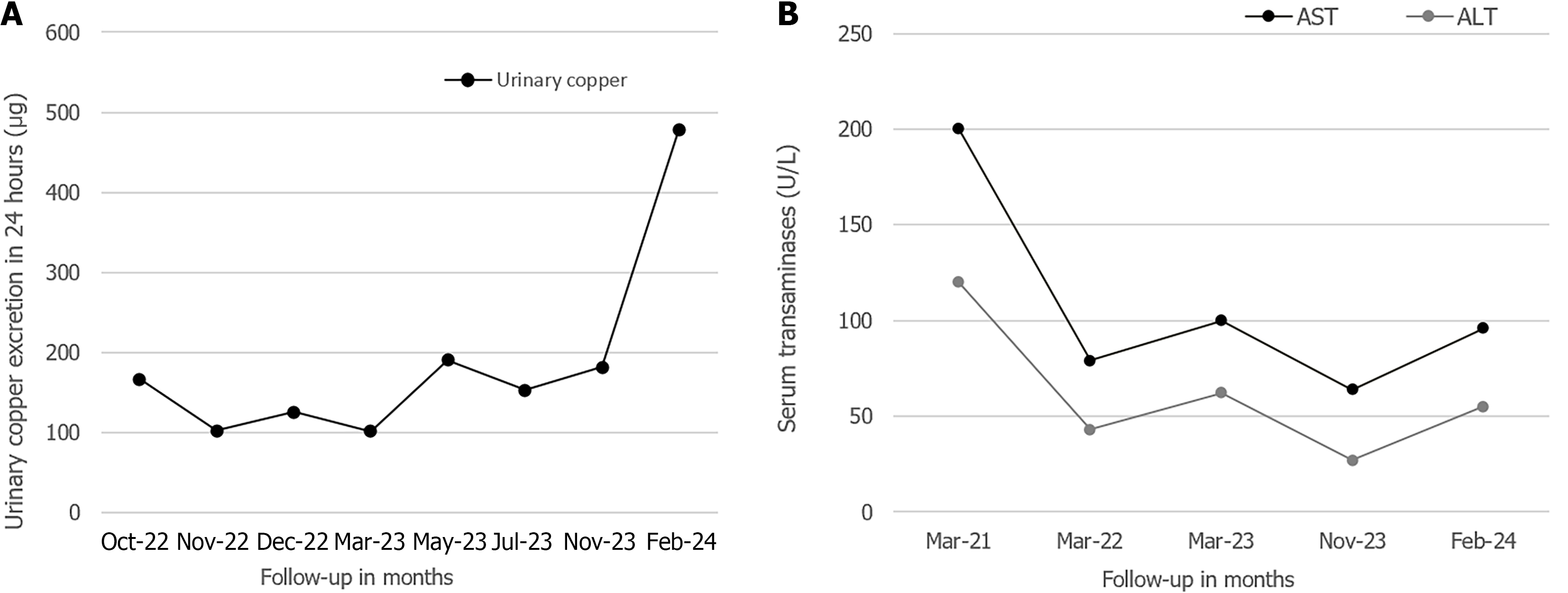
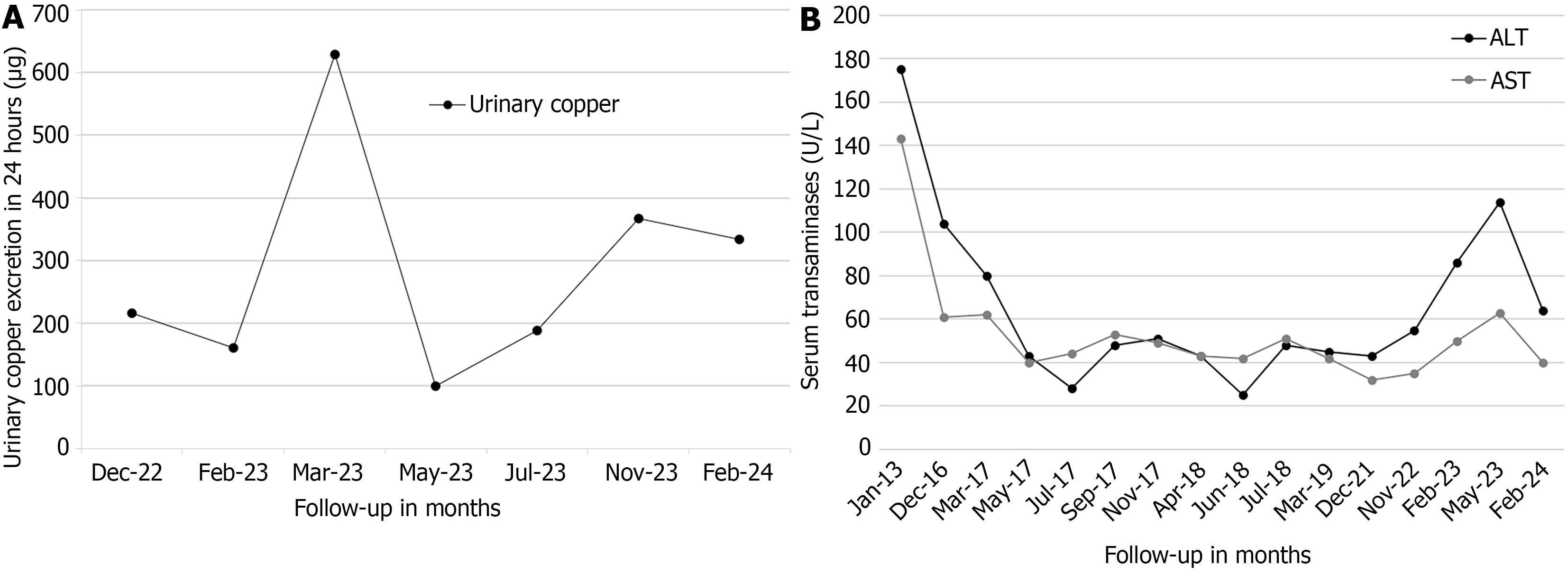
Case 1: Treatment with DPA was initiated six months post-diagnosis at a dose of 250 mg orally twice a day, administered irregularly until 2022 due to medication shortages in Ecuador and neighboring countries, a problem that disrupted treatment adherence. During the DPA treatment, no adverse effects were reported, and the liver cirrhosis remained compensated; however, the neurological symptoms worsened gradually. In September 2022, the copper chelator treatment was switched from DPA to trientine tetrahydrochloride (TETA 4HCl) to bypass the problem with the DPA scarcity. The treatment with TETA 4HCl started at an initial dose of 450 mg per day orally, divided into three doses, without reported adverse effects. This dosage was adjusted to achieve clinical stability and maintain urinary copper levels within the recommended therapeutic range of 200-500 μg/24 hour (Figure 5A). Between February and July 2023, the patient received a dose of 300 mg/day of TETA 4HCl, and the dosage was being maintained at 450 mg/day at the time of writing of this report.
In addition, the patient had been administered a symptomatic treatment for dystonia and parkinsonism since August 2022 consisting of propranolol at a dose of 40 mg/day orally.
During the medical history, the patient denied using any additional medications and reported complying with the copper-restricted diet prescribed since the diagnosis.
Case 2: Treatment with DPA was initiated approximately one year after the diagnosis, at a dose of 250 mg once daily (administered irregularly due to DPA scarcity problems). In February 2023, the copper chelator treatment was switched from DPA to TETA 4HCl to bypass the DPA unavailability problem. The initial TETA 4CHl dose was 450 mg daily divided into three doses; no adverse effects were reported. The maintenance dose was established at 450 mg/day, adjusted as needed to keep the urinary copper content within therapeutic targets of 200-500 μg/24 hour (Figure 6A) and maintain clinical stability. During the medical history, the patient denied taking any additional medications to treat WD, and she reported complying with the copper-restricted diet prescribed since her diagnosis.
Case 1: After the diagnosis in 2016, the neurological symptoms worsened slowly but progressively. During the coronavirus disease 2019 (COVID-19) pandemic, the patient's remote rural location and transportation difficulties to the tertiary care medical center prevented him from attending regular medical appointments from 2020 to 2022. In August 2022, the patient presented to the hospital with a moderately disabling postural and resting tremor that affected all four limbs with a predominance in the right hand. He also presented with severe dysarthria, and communicated using signs. He denied gait disorders or dysphagia. Physical examination revealed complete bilateral K-F rings and normal facial movements. However, he displayed mandibular, lingual, and facial dystonia during speech accompanied by an antagonistic gesture with the left hand. His strength and muscle tone were preserved; however, discreet upper limb bradykinesia was noted.
From 2022 to the time of writing this report, the patient underwent regular monitoring examinations approximately every three months, for a total follow-up and treatment period of 8 years since the diagnosis. The patient had been on continuous TETA 4HCl therapy since 2022. At the time of writing this report, the clinical course of the disease had been favorable, the dysarthria was significantly improved, and the dystonia and tremor had subsided. His serum transaminase levels have gradually decreased since the start of treatment but remain above the normal range (Figure 5B). The hepatic cirrhosis remained compensated without signs of progression. A Child-Pugh score of 5 (class A) was calculated during the last assessment. The patient remained living in his family home with his mother and was unemployed at the time.
Case 2: From 2021 until the time of writing this report, the patient underwent regular medical follow-ups approximately every three months, for a total follow-up and treatment period of 8 years since the WD diagnosis. The patient had been on continuous TETA 4HCl therapy since 2022. She remained asymptomatic and without hepatic cirrhosis, neurological, or psychiatric manifestations. Variable transaminase elevations have been witnessed throughout the follow-up (Figure 6B). In 2023 she moved from her family home to the capital city and was attending college and displaying a strong academic performance at the time of this report. This patient achieved clinical stability without disease progression with her treatment.
In this paper, we report the cases of two siblings born of a consanguineous union, a 27-year-old man (Case 1) and a 19-year-old woman (Case 2) diagnosed as having WD. The two siblings had WD like another sibling and a cousin who died due to complications related to the same condition. Case 1 presented a compensated cirrhotic clinical course accompanied by bilateral K-F rings and severe neurological manifestations. These symptoms were gradually reversed with copper chelation therapy and the liver disease did not progress. Case 2 was asymptomatic, she only displayed biochemical alterations in serum transaminases and the copper profile, and her disease had shown no progression towards disability or cirrhosis due to timely treatment. Both cases met the treatment goals regarding urinary copper excretion, clinical improvement, and stability.
Diagnosing and treating WD remains challenging. Early recognition through clinical, biochemical, or genetic exa
Identifying correlations between mutations in the ATP7B gene and the WD phenotype has been challenging, with many studies failing to find a definitive association. Epigenetic mechanisms are hypothesized to contribute to the discordance between genotypes and phenotypes, potentially explaining the variability of symptoms in different patients. Identifying the specific factors that trigger epigenetic modifications is crucial for improving therapeutic approaches, preventing disability, and enhancing the patients' quality of life. Clinical presentations often do not correlate with the genetic defects, and siblings may exhibit different forms of the disease. Differences in diagnosis timing, nutrition, and lifestyle may contribute to the phenotypic variability as well[7]. Kegley et al[10] reported the case of one monozygotic twin who required an emergency liver transplant due to acute liver failure, while the other twin presented only mild liver disease. Członkowska et al[11] studied the cases of two pairs of monozygotic twins with discordant WD phenotypes, attributing the differences to epigenetic and environmental factors. Epigenetic differences seem more pronounced among siblings who are older, have different lifestyles, and have spent less time together[7]. Senzolo et al[12] studied the cases of homozygotic twins diagnosed as having WD who presented liver cirrhosis, both twins underwent liver transplantations with markedly different outcomes. One twin, initially presenting with neuropsychiatric alterations associated with drug abuse and mild dysarthria, improved significantly and remained asymptomatic. In contrast, the other twin initially presenting severe neuropsychiatric manifestations, got worse after post-transplantation, leading to his death two months later. Cheng et al[13] examined five pairs of twins with WD and phenotypic discordance despite carrying the same mutations. They also reported a case of triplets, one sister had definite WD and brain symptoms at the onset, the brother had subclinical WD, and the other sister was completely normal. Khan et al[14] performed a genetic analysis on a family affected by WD, consisting of consanguineous parents (cousins) and five children. Four of the children had manifest disease, and one was clinically normal; however, all the children exhibited a mutation in the ATP7B gene. Zhang et al[15], conducted a four-year follow-up on two siblings with WD (compound heterozygotes carrying a missense mutation in the ATP7B gene); the 17-year-old sibling presented neurological manifestations since early childhood that progressed to a severe impairment and had a K-F ring on her right cornea, whereas the 12-year-old sibling remained asymptomatic but displayed bilateral K-F rings and abnormalities in aminotransferases and copper profiles.
The potential reasons for the different clinical presentations of the two consanguineous siblings in our case report are multiple: The timing of the diagnosis and treatment initiation were a main difference, although the copper chelation therapies were administered irregularly for several years in both cases. Environmental factors such as lifestyles, nutritional habits, and living settings were also different between them, Case 1 lived in a rural community whereas Case 2 moved to a large city. The unavailability of diagnostic tests and resources within the Ecuadorian public healthcare system prevented us from performing genetic testing for ATP7B mutations in both siblings or their relatives. This limitation created several gaps in understanding the patients' conditions and in identifying familial patterns of inheritance. Without identification of the specific ATP7B gene mutation, a thorough analysis of potential correlations between the genotypes and phenotypes of Cases 1 and 2 were impossible, we cannot know whether epigenetics or other factors may have had a role in their differing clinical presentations.
The unavailability of ATP7B genetic testing has a negative impact on individuals with WD in Ecuador, as they cannot receive a genetic confirmation of their disease and cannot have prognoses based on their genetics, which in some cases may help predict their clinical course[16-18]. Another implication of this limitation is the inability to perform adequate family screenings, particularly for individuals who may be affected but are in the presymptomatic stages of the disease, where early diagnosis could improve their prognoses and quality of life.
Variations in drug responses further impact long-term outcomes in WD. Despite being classified as a monogenic disease, the potential influence of modifier genes remains a topic of interest, pending comprehensive whole-genome analyses in large cohorts of patients[7]. The incidence of neurological deterioration following initial DPA therapy ranges widely, from 30% to 75%. The discontinuation of DPA leads to improvements in most patients experiencing neurological worsening[8]. Chang et al[19] found a lower risk of clinical deterioration in patients treated with a combination of low-dose DPA and high-dose zinc. Copper chelators can be used to effectively manage WD, but zinc therapy is a viable alternative. Trientine, another copper chelator, may offer better tolerance. In practice, drug selection depends on patient affordability and the availability of trientine. Trientine has been used in patients experiencing neurological worsening following DPA therapy. The reported risks of trientine therapy vary between 10% and 15%, in contrast those of the DPA, which vary between 30% and 75%. The mechanisms leading to neurological deterioration likely involve copper mobilization following chelation therapy, resulting in increased free copper, elevated malondialdehyde, and decreased glutathione levels, as evidenced in experiments with animal models of WD. One hypothesis posits that free copper is rapidly released from the liver, crosses the blood-brain barrier, and induces brain damage. Another hypothesis suggests that chelators generate free copper within brain tissues. This is supported by the increased serum and brain tissue free copper levels observed within three days of initiating DPA treatments in animal models of WD. During this period, a decrease in protein-bound brain copper occurred alongside elevated levels of ATP7A (a neuronal copper transporter) and CTR1 (a copper uptake mediator) in the cortex and basal ganglia, indicating intracellular copper mobilization rather than blood-brain barrier influx[9]. At a subcellular level, ATP7A expression, crucial for biliary copper excretion in animal models, correlates with free copper levels in the cortex and basal ganglia. A third hypothesis, proposed by Miki[20], suggests that penicillamine-copper complexes, though non-toxic, could catalyze membrane oxidation due to changes in copper's redox potential (Cu2+ to Cu1+), thereby causing neurological symptoms. Other factors contributing to neu
In this study, in Case 1, DPA was initiated at age 19, resulting in a progressive worsening of his neurological disease. Once the treatment was switched to TETA 4HCl, his condition improved and he had remained stable until the time of writing this. Case 2 initiated DPA at 11 years old and was switched to TETA 4 HCl at 17 years old, she had remained asymptomatic until the time of writing.
The intermittence in treatment in patients with WD represents a significant health risk. This unfavorable situation is experienced not only by patients in Ecuador, where this medication is unavailable, but has also been documented in Peru by Huanco-condori et al[21]. The cost and difficulties associated with importing medication from abroad do not ensure proper adherence. The discontinuation of DPA in WD patients results in rapid clinical deterioration, often fatal, but replacing DPA with trientine can prevent this adverse clinical event[22]. Patients with WD, who discontinue treatment altogether, risk the development of intractable hepatic decompensation. Failure to comply with therapy is associated with clinical worsening, elevation of hepatic enzymes, and increased urinary copper excretion, leading to a significant progression of liver disease and liver failure within 1-12 months after treatment discontinuation, resulting in death or the urgent need for liver transplantation. Patients with neurological manifestations may experience recurrence or the emergence of new symptoms within a few months of discontinuing the treatment. This condition may be irreversible in patients who suspend treatment and later resume it[4,22-24].
In Ecuador, there is only one reported case of WD involving a 20-year-old male who exhibited cirrhotic evolution, neurological involvement, and bilateral K-F rings. The patient received copper chelation therapy with a favorable outcome; however, he developed fever as an adverse effect within 48 hours of initiating the therapy. The patient described in that study shares a similar family history with cases 1 and 2, as three of his sisters died of WD. It is worth noting that the described patient is from Cuenca, a city located near Cañar province, where Cases 1 and 2 were born[25].
We are aware of the limitations inherent to our case report. Some limitations are unavoidable in resource-limited settings. The patients at our center often come from remote regions, and they lack prompt access to healthcare services. In first-level healthcare facilities, physicians face challenges after identifying a patient with clinical manifestations suggestive of WD, because the referral to second- or third-level institutions is limited. In Ecuador the public healthcare system is overburdened, aggravated by a shortage of healthcare professionals, inadequate infrastructure and equipment, and limited resources that restrict access to healthcare services. Most cases of WD are diagnosed by specialists in gastroenterology or hepatology, and these physicians are available only in second- and third-level healthcare facilities. Ecuador is a relatively small country with a limited number of second- and third-level healthcare facilities located in the principal cities. Diagnostic challenges are exacerbated by the unavailability of diagnostics tests. Specific genetic testing for the ATP7B mutation is not performed within the public healthcare system due to a lack of financial resources. This issue limits the acquisition of the necessary equipment and hinders the recruitment of qualified medical personnel to process samples and interpret results. Additionally, the absence of established protocols and training for genetic testing further complicates the implementation of such services. This situation is not limited to patients with WD; it applies to the majority of individuals suffering from rare diseases in the country. If a patient with WD requires this type of genetic testing, requested by their physician or healthcare team, the biological sample must be sent abroad for processing and result reporting. The patients are forced to pay for the tests themselves, but many cannot afford to do this.
Likewise, biochemical tests such as ceruloplasmin levels, urinary and serum copper quantification, as well as histochemical stains like rhodanine or orcein, and slit-lamp examinations, are only available in a few tertiary care centers. The quantification of ceruloplasmin poses another limitation, as enzymatic quantification is unavailable in the country, likely due to the technical difficulties of performing the standard copper oxidase method. Therefore, immunoassays are used, but they may lead to overestimation of ceruloplasmin concentrations because they detect both apoceruloplasmin and holoceruloplasmin[4].
The lack of resources in the public health system results in frequently late diagnoses of individuals in advanced stages of the disease. This results in a profound negative impact on these patients in Ecuador. Patra[26] described similar limitations in India, emphasizing the diagnostic challenges of WD, despite being a common clinical entity in childhood, only 6%-21% of cases of chronic liver disease in children in developing countries are attributed to the disease. Hepatic copper estimation is available only in few centers in Ecuador. This adds to the inherent limitations of liver biopsy, an invasive procedure in which false negatives are common due to the uneven distribution of copper in hepatic tissues, especially in the later stages of the disease The COVID-19 pandemic posed a significant barrier to proper medical follow-ups, as well as the availability of copper-chelating medications, preventing adequate treatment adherence and disease control. Finally, inherent limitations of the healthcare system's resources reduced the abundance of clinical data, including neuroimaging results, preventing us from being able to illustrate the clinical cases more accurately.
In conclusion, our report emphasizes the crucial importance of early WD diagnoses and treatments to prevent severe complications and improve patient prognoses. Our cases illustrate the diverse clinical presentations of this disease and the effectiveness of copper-chelating agents to manage the disease. Family screenings are always recommended, in particular for those born from consanguineous unions, to detect the disease during its early stages. Improving access to treatment is important in many Latin American countries. Further research is needed to better understand the clinical characteristics and management of WD in Latin America. Our case reports offer evidence-based ideas for the clinical practice.
| 1. | Sandahl TD, Laursen TL, Munk DE, Vilstrup H, Weiss KH, Ott P. The Prevalence of Wilson's Disease: An Update. Hepatology. 2020;71:722-732. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 154] [Article Influence: 25.7] [Reference Citation Analysis (0)] |
| 2. | Dev S, Kruse RL, Hamilton JP, Lutsenko S. Wilson Disease: Update on Pathophysiology and Treatment. Front Cell Dev Biol. 2022;10:871877. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 38] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 3. | Shribman S, Poujois A, Bandmann O, Czlonkowska A, Warner TT. Wilson's disease: update on pathogenesis, biomarkers and treatments. J Neurol Neurosurg Psychiatry. 2021;92:1053-1061. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 88] [Article Influence: 17.6] [Reference Citation Analysis (1)] |
| 4. | Schilsky ML, Roberts EA, Bronstein JM, Dhawan A, Hamilton JP, Rivard AM, Washington MK, Weiss KH, Zimbrean PC. A multidisciplinary approach to the diagnosis and management of Wilson disease: Executive summary of the 2022 Practice Guidance on Wilson disease from the American Association for the Study of Liver Diseases. Hepatology. 2023;77:1428-1455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 103] [Article Influence: 34.3] [Reference Citation Analysis (1)] |
| 5. | Członkowska A, Litwin T, Dusek P, Ferenci P, Lutsenko S, Medici V, Rybakowski JK, Weiss KH, Schilsky ML. Wilson disease. Nat Rev Dis Primers. 2018;4:21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 605] [Cited by in RCA: 622] [Article Influence: 77.8] [Reference Citation Analysis (0)] |
| 6. | Shribman S, Marjot T, Sharif A, Vimalesvaran S, Ala A, Alexander G, Dhawan A, Dooley J, Gillett GT, Kelly D, McNeill A, Warner TT, Wheater V, Griffiths W, Bandmann O; British Association for the Study of the Liver Rare Diseases Special Interest Group. Investigation and management of Wilson's disease: a practical guide from the British Association for the Study of the Liver. Lancet Gastroenterol Hepatol. 2022;7:560-575. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 45] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 7. | Samadzadeh S, Kruschel T, Novak M, Kallenbach M, Hefter H. Different Response Behavior to Therapeutic Approaches in Homozygotic Wilson's Disease Twins with Clinical Phenotypic Variability: Case Report and Literature Review. Genes (Basel). 2022;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 8. | Kumar M, Murugan TP, Lionel AP, Thomas MM, Mannam P, Yoganathan S. Management of Children and Adolescents with Wilson Disease and Neurological Worsening Following D-Penicillamine Therapy: A Single Centre Experience. Ann Indian Acad Neurol. 2022;25:698-702. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 9. | Ghosh U, Sen Sarma M, Samanta A. Challenges and dilemmas in pediatric hepatic Wilson's disease. World J Hepatol. 2023;15:1109-1126. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 10. | Kegley KM, Sellers MA, Ferber MJ, Johnson MW, Joelson DW, Shrestha R. Fulminant Wilson's disease requiring liver transplantation in one monozygotic twin despite identical genetic mutation. Am J Transplant. 2010;10:1325-1329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 53] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 11. | Członkowska A, Gromadzka G, Chabik G. Monozygotic female twins discordant for phenotype of Wilson's disease. Mov Disord. 2009;24:1066-1069. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 68] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 12. | Senzolo M, Loreno M, Fagiuoli S, Zanus G, Canova D, Masier A, Russo FP, Sturniolo GC, Burra P. Different neurological outcome of liver transplantation for Wilson's disease in two homozygotic twins. Clin Neurol Neurosurg. 2007;109:71-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 31] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 13. | Cheng N, Wang X, Yu X, Zhou Z, Gao M, Rao R, Hu J, Yang R, Han Y. [Clinical and genetic study of Wilson's disease in affected twins and siblings]. Zhonghua Yi Xue Yi Chuan Xue Za Zhi. 2013;30:261-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 14. | Khan HN, Wasim M, Ayesha H, Awan FR. Molecular genetic diagnosis of Wilson disease by ARMS-PCR in a Pakistani family. Mol Biol Rep. 2018;45:2585-2591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 15. | Zhang QJ, Xu LQ, Wang C, Hu W, Wang N, Chen WJ. Four-year follow-up of a Wilson disease pedigree complicated with epilepsy and hypopituitarism: Case report with a literature review. Medicine (Baltimore). 2016;95:e5331. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 16. | Stapelbroek JM, Bollen CW, van Amstel JK, van Erpecum KJ, van Hattum J, van den Berg LH, Klomp LW, Houwen RH. The H1069Q mutation in ATP7B is associated with late and neurologic presentation in Wilson disease: results of a meta-analysis. J Hepatol. 2004;41:758-763. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 103] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 17. | Xue Z, Chen H, Yu L, Jiang P. A Systematic Review and Meta-Analysis of the R778L Mutation in ATP7B With Wilson Disease in China. Pediatr Neurol. 2023;145:135-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 18. | Ferenci P, Roberts EA. Defining Wilson disease phenotypes: from the patient to the bench and back again. Gastroenterology. 2012;142:692-696. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 18] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 19. | Chang H, Xu A, Chen Z, Zhang Y, Tian F, Li T. Long-term effects of a combination of D-penicillamine and zinc salts in the treatment of Wilson's disease in children. Exp Ther Med. 2013;5:1129-1132. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 18] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 20. | Miki M. Penicillamine as antioxidant. Methods Enzymol. 1994;234:542-547. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 21. | Huanco-condori J, Tomateo-torvisco JD, Cruzado L. Enfermedad de Wilson: A propósito de un caso neuropsiquiátrico de diagnóstico tardío. Revista Ecuatoriana de Neurologia. 2022;31:97-102. [DOI] [Full Text] |
| 22. | Roberts EA, Schilsky ML; American Association for Study of Liver Diseases (AASLD). Diagnosis and treatment of Wilson disease: an update. Hepatology. 2008;47:2089-2111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 884] [Cited by in RCA: 839] [Article Influence: 46.6] [Reference Citation Analysis (0)] |
| 23. | Walshe JM, Dixon AK. Dangers of non-compliance in Wilson's disease. Lancet. 1986;1:845-847. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 72] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 24. | Socha P, Janczyk W, Dhawan A, Baumann U, D'Antiga L, Tanner S, Iorio R, Vajro P, Houwen R, Fischler B, Dezsofi A, Hadzic N, Hierro L, Jahnel J, McLin V, Nobili V, Smets F, Verkade HJ, Debray D. Wilson's Disease in Children: A Position Paper by the Hepatology Committee of the European Society for Paediatric Gastroenterology, Hepatology and Nutrition. J Pediatr Gastroenterol Nutr. 2018;66:334-344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 221] [Cited by in RCA: 160] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 25. | Paola GGE, Elizabeth LDC, Fernando OCD. Enfermedad de Wilson: reporte de caso. Wilson´s disease: a case report. Revista de la Facultad de Ciencias Médicas Universidad de Cuenca. 2019;37:53-62. [DOI] [Full Text] |
| 26. | Patra PK. Wilson's disease and diagnostic conundrum in a low income country. Pan Afr Med J. 2017;26:201. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/