Published online Sep 16, 2025. doi: 10.12998/wjcc.v13.i26.107028
Revised: April 10, 2025
Accepted: May 29, 2025
Published online: September 16, 2025
Processing time: 132 Days and 4.6 Hours
Primary splenic lesions are rare and often detected incidentally through imaging, biopsy, or autopsy, typically without distinct clinical symptoms. Although ima
A 33-year-old male presented to the Department of Emergency with frank red blood hematemesis and a 1-week history of epigastric pain. On arrival, he was alert and hemodynamically stable. Contrast-enhanced abdominal computed to
This rare case of splenic hamartoma mimicking angiosarcoma highlights the importance of differential diagnosis in managing splenic tumors.
Core Tip: Splenic hamartomas are rare benign lesions that can closely mimic malignant tumors such as splenic angiosarcomas on imaging. We present a case initially suspected as splenic angiosarcoma with hepatic metastases, but histopathology after splenectomy confirmed splenic hamartoma. This rare case of splenic hamartoma mimicking angiosarcoma highlights the importance of differential diagnosis in managing splenic tumors.
- Citation: Song SB, Noh BG, Oh MH, Yoon M, Park YM, Seo HI, Hong SB, Kim S. Splenic hamartoma mimicking angiosarcoma: A case report. World J Clin Cases 2025; 13(26): 107028
- URL: https://www.wjgnet.com/2307-8960/full/v13/i26/107028.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v13.i26.107028
Splenic angiosarcoma is an exceptionally rare and aggressive malignancy originating from the endothelial cells of blood vessels in the spleen[1]. The annual incidence rate of primary splenic angiosarcoma is estimated to be between 0.14 and 0.25 cases per million individuals, with a peak incidence in patients aged 50-60 years. Its exact pathogenesis remains unknown, and unlike hepatic angiosarcomas, no established association exists between environmental and occupational exposure to carcinogens[2]. Considering its non-specific clinical presentation, imaging studies play a critical role in the initial evaluation. However, a definitive diagnosis is typically made through histopathological examination. In contrast, splenic hamartomas are hypervascular lesions composed of normal splenic white and red pulps. Cases of splenic ha
A 33-year-old male presented to the Department of Emergency with frank red blood hematemesis on the day of admission and a one-week history of epigastric pain and nausea.
The patient had no history of present illness.
The patient was diagnosed with a giant cell tumor in the left wrist and underwent seven operations. The patient had no other significant medical history.
The patient reported no relevant medical or family history.
The patient experienced a single episode of hematemesis, estimated to be approximately half a paper cup of bright red blood. Upon presentation, he appeared pale and reported persistent abdominal discomfort and nausea. Physical examination of the chest and abdomen revealed no remarkable findings. At the time of arrival to the Department of Emergency, the patient was alert and oriented, with stable vital signs: Blood pressure, 120/80 mmHg; heart rate, 98 beats per minute; respiratory rate, 16 breaths per minute; body temperature, 36.3 °C; and oxygen saturation, 98% on room air.
Biochemistry values upon admission as shown in Table 1. Arterial blood gas analysis and other laboratory tests performed at the time of admission revealed no abnormalities.
| Value | Unit | Reference range | Result | |
| White blood cell count | 6.99 | 109/μL | 3.8-11.0 | Normal |
| Neutrophil count | 85.7 | 109/μL | 1.5-7.0 | Elevated |
| Hemoglobin | 6.9 | g/dL | 13.5-17.5 | Low |
| Hematocrit | 23.1 | % | 39.0-53.0 | Low |
| Platelet count | 157 | 109/μL | 140-420 | Normal |
| AST | 26 | U/L | 0-40 | Normal |
| ALT | 45 | U/L | 0-40 | Slightly elevated |
| ALP | 103 | U/L | 40-129 | Normal |
| BUN | 16.6 | mg/dL | 6-26 | Normal |
| Creatinine | 0.89 | mg/dL | 0.4-1.2 | Normal |
| INR | 1.24 | 0.88-1.12 | Slightly elevated | |
| C-reactive protein | 0.05 | mg/dL | 0-0.5 | Normal |
| CEA | 0.8 | ng/mL | 0-5.0 | Normal |
| CA 19-9 | 4.85 | U/mL | 0-39 | Normal |
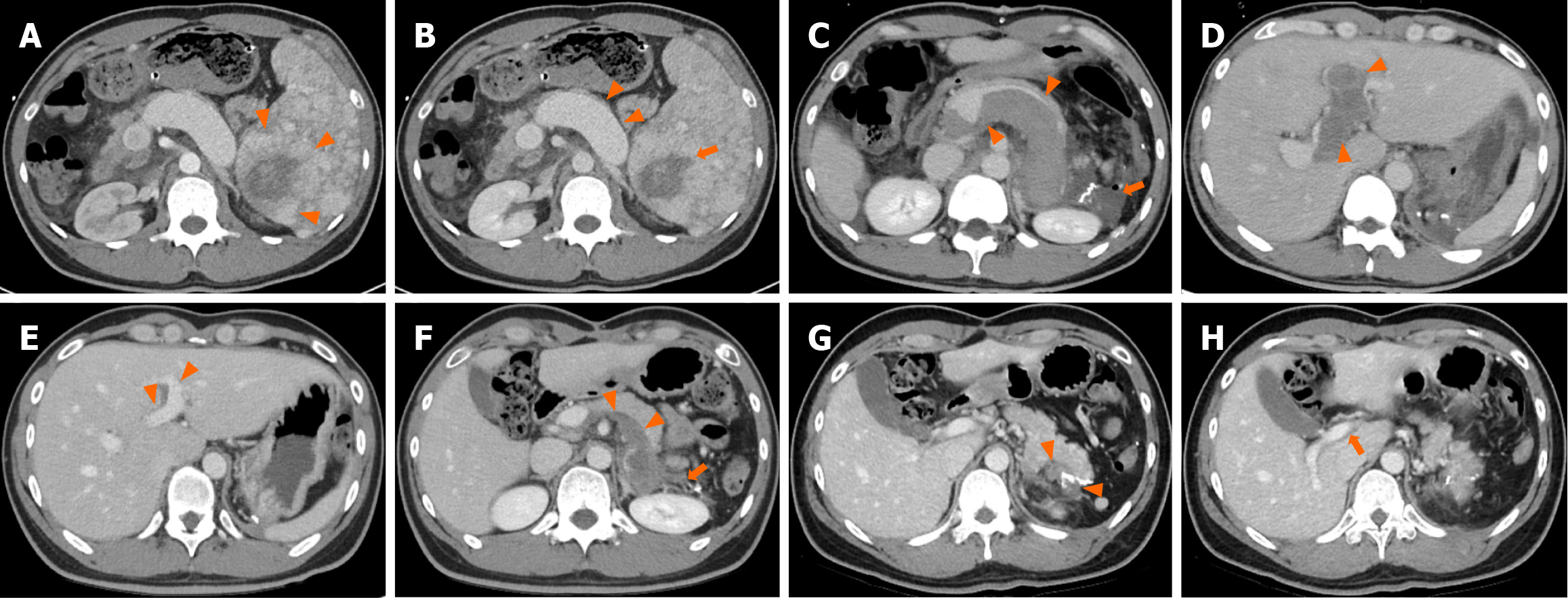
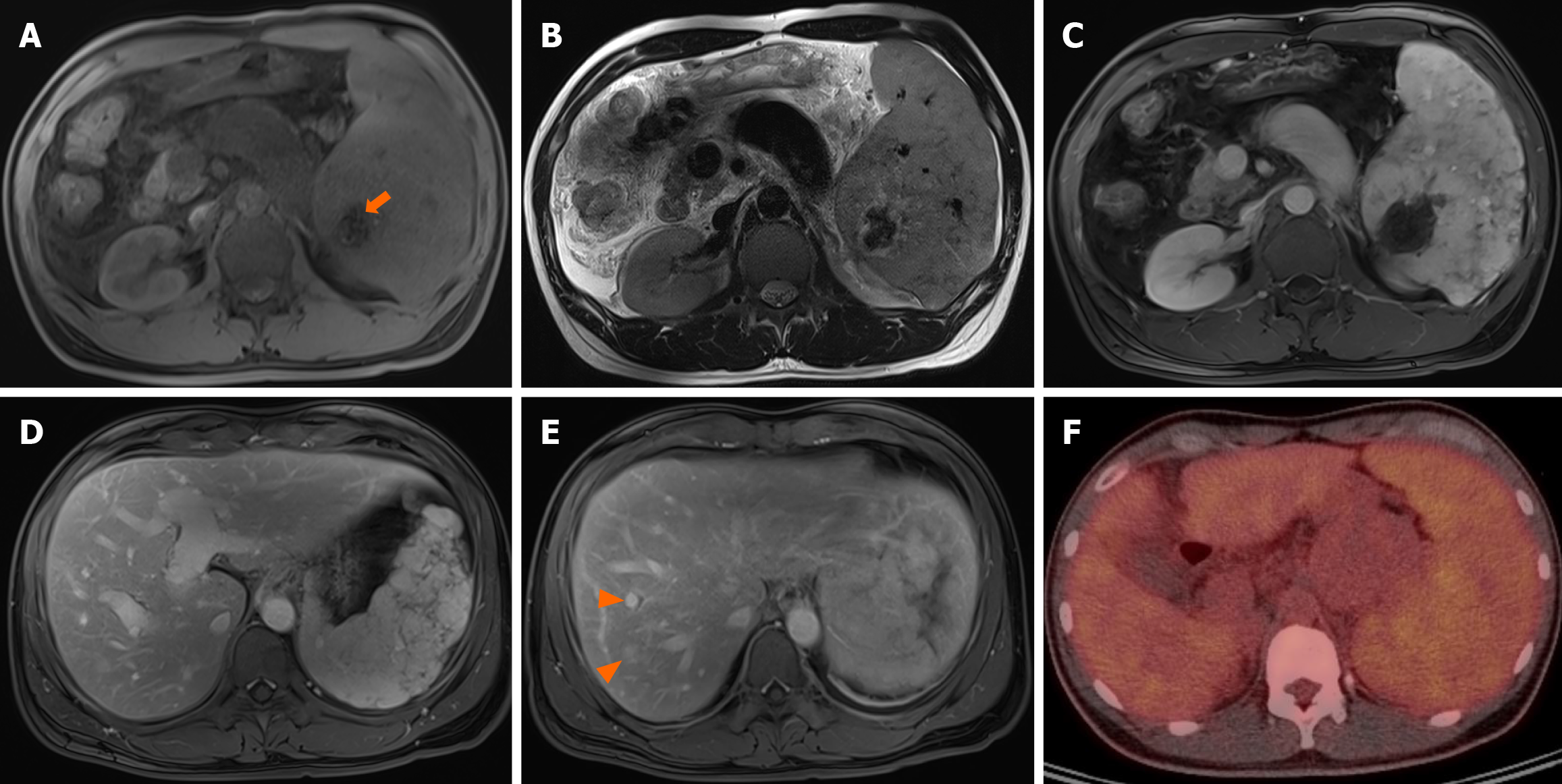
Contrast-enhanced abdominal computed tomography (CT) revealed approximately 8.2 cm × 6.2 cm sized diffuse nodular lesion in the spleen, containing a peripheral enhancing mass-like lesion (Figure 1A), indicative of splenic angiosarcoma. Necrosis was observed at the center of the splenic mass (Figure 1B). Multiple indeterminate lymph nodes were identified in the small bowel mesentery. Abdominal magnetic resonance imaging (MRI) revealed hemosiderosis in the central portion of the splenic mass, exhibiting low signal intensity on both T1-weighted imaging (T1WI) and T2WI (Figure 2A and B). On dynamic contrast-enhanced MRI, the splenic mass displayed iso-to-high signal intensity (Figure 2C and D). Additionally, contrast-enhanced MRI revealed multiple hypervascular masses in the right hepatic lobe (Figure 2E). Consequently, splenic angiosarcoma with hepatic metastasis was suspected. 18F-fluorodeoxyglucose positron emission tomography-CT demonstrated splenomegaly with mildly and diffusely increased metabolic activity, raising suspicion of hematologic malignancy (Figure 2F).
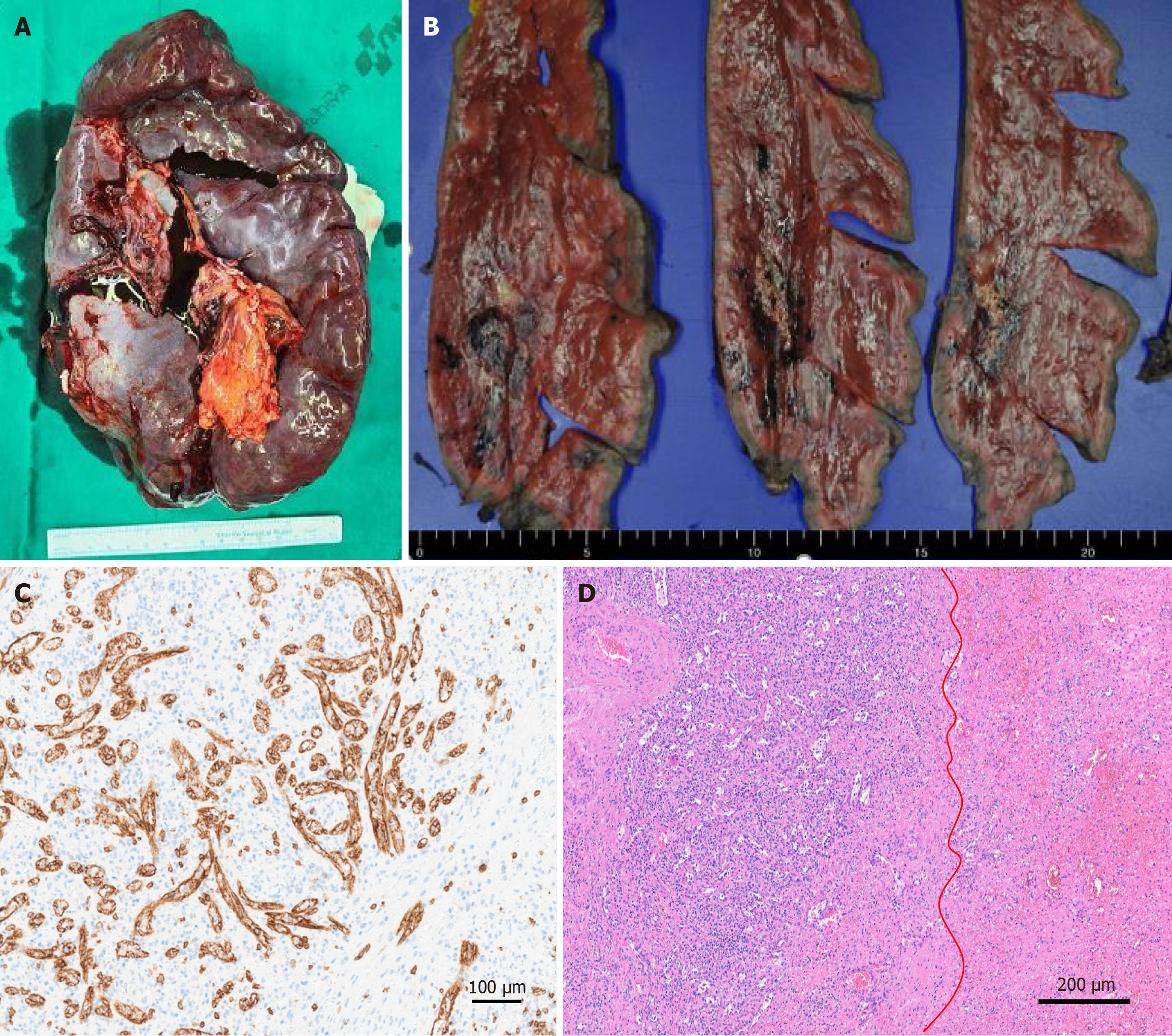
Endoscopic variceal ligation was performed preoperatively to evaluate and manage the esophageal varices that caused hematemesis. Hemostasis was achieved at six points. To identify malignancy, the patient underwent exploratory laparotomy with splenectomy and liver biopsy. A frozen liver biopsy performed at segments 2 and 4a revealed chronic inflammation, and no additional resection was deemed necessary. The resected spleen weighed 1205.8 g and measured 21.7 cm × 15.5 cm × 6.3 cm, with an intact capsule and no signs of congestion (Figure 3A). Gross examination revealed an ill-defined yellow-white solid mass with hemorrhage, measuring 4.5 cm × 2.5 cm × 2 cm (Figure 3B). The lesion size observed on CT was found to include a pseudo-lesion, as confirmed by the pathological findings. Immunohistopathological examination revealed CD8+ sinusoidal staining (Figure 3C) positive for CD34, smooth muscle actin, E-twenty-six-related gene, and CD68. The lesions were weakly positive for pan-cytokeratin antibody and negative for desmin, S100 protein, epithelial membrane antigen, CD21, and leukocyte common antigen. Microscopically, vascular proliferation composed of disorganized red pulp elements and hemorrhage were observed (Figure 3D), consistent with the diagnosis of splenic hamartoma.
Based on the histopathology results, the final diagnosis of splenic hamartoma was confirmed.
The patient experienced acute upper gastrointestinal bleeding due to prominent splenic and gastroesophageal varices, which were attributed to idiopathic portal flow reduction. Ceftriaxone and metronidazole were administered for antimicrobial prophylaxis. Considering the reduced risk of postoperative infection, antibiotics were discontinued on postope
The patient was discharged on POD15. A 3-month follow-up CT performed on an outpatient basis showed substantial improvement in thrombosis (Figure 1E and F). Subsequently, CT scans were conducted at 6-month intervals, and the patient continued to show good progress without any issues requiring readmission. At the 1-year postoperative follow-up, portal venous phase CT confirmed substantial resolution of the resection site hematoma. Complete resolution of the extrahepatic and intrahepatic portal vein thrombosis was observed, with no evidence of recurrence or other abnormalities (Figure 1G and H). Three months postoperatively, the patient secured employment and has since been leading a normal life.
A splenic hamartoma is a benign tumor composed of an overgrowth of mature splenic tissue. The reported incidence is approximately three cases per 200000 splenectomies, making it a very rare condition. It is more common in women, with 20% of cases diagnosed among children[5]. Most patients are asymptomatic; however, 15% experience symptoms related to hypersplenism such as abdominal pain and cytopenia[4,5]. Fever and malaise are observed more frequently in chil
Splenic lesions are difficult to diagnose based solely on imaging, and biopsies are often limited due to the risk of rupture and potential dissemination of cancer cells. This rare case of splenic hamartoma mimicking angiosarcoma underscores the importance of differential diagnosis and expands the clinical perspective of physicians managing splenic tumors.
| 1. | Damouny M, Mansour S, Khuri S. Primary Angiosarcoma of the Spleen: An Aggressive Neoplasm. World J Oncol. 2022;13:337-342. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 2. | Li R, Li M, Zhang LF, Liu XM, Hu TZ, Xia XJ, Chi JS, Jiang XX, Xu CX. Clinical Characteristics and Prognostic Factors of Primary Splenic Angiosarcoma: A Retrospective Clinical Analysis from China. Cell Physiol Biochem. 2018;49:1959-1969. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 3. | Lee H, Maeda K. Hamartoma of the spleen. Arch Pathol Lab Med. 2009;133:147-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 48] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 4. | Obeidat KA, Afaneh MW, Al-Domaidat HM, Al-Qazakzeh HI, AlQaisi FJ. Splenic Hamartoma: A Case Report and Literature Review. Am J Case Rep. 2022;23:e937195. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 5. | Silverman ML, LiVolsi VA. Splenic hamartoma. Am J Clin Pathol. 1978;70:224-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 84] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 6. | Gao Y, Qian B, Zhang X, Liu H, Han T. Prophylactic antibiotics on patients with cirrhosis and upper gastrointestinal bleeding: A meta-analysis. PLoS One. 2022;17:e0279496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 7. | Bikdeli B, Zahedi Tajrishi F, Sadeghipour P, Talasaz AH, Fanikos J, Lippi G, Siegal DM, Eikelboom JW, Monreal M, Jimenez D, Connors JM, Ageno W, Barnes GD, Piazza G, Angiolillo DJ, Parikh SA, Kirtane AJ, Lopes RD, Bhatt DL, Weitz JI, Mehran R, Krumholz HM, Goldhaber SZ, Lip GYH. Efficacy and Safety Considerations With Dose-Reduced Direct Oral Anticoagulants: A Review. JAMA Cardiol. 2022;7:747-759. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 39] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 8. | Valizadeh P, Jannatdoust P, Tahamtan M, Ghorani H, Dorcheh SS, Farnoud K, Salahshour F. Diagnostic performance of different imaging modalities for splenic malignancies: A comparative meta-analysis. Eur J Radiol Open. 2024;12:100566. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 9. | Wang JH, Ma XL, Ren FY, Zuo CJ, Tian JM, Wang ZF, Zheng JM. Multi-modality imaging findings of splenic hamartoma: a report of nine cases and review of the literature. Abdom Imaging. 2013;38:154-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 31] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 10. | Borch WR, Aguilera NS, Brissette MD, O'Malley DP, Auerbach A. Practical Applications in Immunohistochemistry: An Immunophenotypic Approach to the Spleen. Arch Pathol Lab Med. 2019;143:1093-1105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 11. | Sim J, Ahn HI, Han H, Jun YJ, Rehman A, Jang SM, Jang K, Paik SS. Splenic hamartoma: A case report and review of the literature. World J Clin Cases. 2013;1:217-219. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 14] [Cited by in RCA: 16] [Article Influence: 1.2] [Reference Citation Analysis (1)] |
| 12. | Zhang X, Zhong X, Lin X, Li X, Tian H, Chang B, Wang Y, Tong J, Wang N, Li D, Jin X, Huang D, Wang Y, Cui H, Guan L, Li Y. Tuberous Sclerosis Complex With Multiple Organ Tumors: Case Report and Literature Review. Front Oncol. 2022;12:916016. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 13. | Hayes TC, Britton HA, Mewborne EB, Troyer DA, Saldivar VA, Ratner IA. Symptomatic splenic hamartoma: case report and literature review. Pediatrics. 1998;101:E10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 33] [Article Influence: 1.2] [Reference Citation Analysis (1)] |
| 14. | Hsu CW, Lin CH, Yang TL, Chang HT. Splenic inflammatory pseudotumor mimicking angiosarcoma. World J Gastroenterol. 2008;14:6421-6424. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 12] [Cited by in RCA: 12] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 15. | Akkucuk S, Aydogan A, Gokce H, Davran R, Karcioglu M. Splenic hematoma mimicking angiosarcoma: a case report. Case Rep Oncol Med. 2012;2012:183458. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/