Published online Sep 6, 2025. doi: 10.12998/wjcc.v13.i25.107907
Revised: April 15, 2025
Accepted: May 26, 2025
Published online: September 6, 2025
Processing time: 98 Days and 10.5 Hours
Lung cancer is the most frequent cause of cancer-related mortality worldwide. Nitric oxide (NO), prostaglandins (PGs), thromboxanes (TXs), and endothelins (ETs) participate in numerous physiological processes. These agents play an important role in lung carcinogenesis by regulating cancer cell proliferation, apoptosis, invasion, and angiogenesis. NO is a gaseous free radical with tumo
Core Tip: Lung cancer remains the leading cause of cancer-related deaths globally. Nitric oxide (NO), prostaglandins (PGs), thromboxanes (TXs), and endothelins (ETs) significantly influence lung carcinogenesis by modulating cancer cell proliferation, apoptosis, invasion, and angiogenesis. This study highlights the dual roles of NO, the opposing effects of arachidonic acid-derived PGs, the involvement of TXA2 and its metabolite TXB2, and the critical functions of ETs in lung cancer progression. The findings emphasize the complex interplay of these agents in tumor biology, offering insights for potential therapeutic targets.
- Citation: Demirel S, Sinag IN. Role of nitric oxide, prostaglandins, thromboxanes and endothelins in lung cancer: An overview. World J Clin Cases 2025; 13(25): 107907
- URL: https://www.wjgnet.com/2307-8960/full/v13/i25/107907.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v13.i25.107907
Lung cancer is one of the most commonly diagnosed cancers and the leading cause of cancer-related mortality in the world[1]. In 2024, it was estimated that there were 2001140 new cases of lung cancer and 611720 deaths in the United States[2]. The incidence rate was 27% higher in men than women between 2015 and 2019[3]. Lung cancer is a complex and heterogeneous disease due to genetic diversity in histological subtypes with clinical relevance[4]. The primary etiologic factor is tobacco smoking for lung cancer. Other etiologic factors are environmental and occupational exposures to agents, such as second-hand smoke, air pollution, radiation, asbestos, nickel, arsenic, and silica[5].
Lung cancer is classified into two main types: Small cell lung cancer (SCLC) and non- SCLC (NSCLC). SCLC arises from pulmonary neuroendocrine cells, representing 13% of all lung cancer cases[6,7]. NSCLC originates from lung epithelial cells, accounting for 87% of the cases[8,9]. NSCLC comprises the subtypes of adenocarcinoma, squamous cell carcinoma, and large cell carcinoma[10].
This review aims to provide a comprehensive overview of the roles of endogenous modulators-nitric oxide (NO), prostaglandins (PGs), thromboxanes (TXs), and endothelins (ETs)-in the molecular mechanisms underlying lung cancer. A search strategy was performed in various electronic databases, including PubMed, Scopus, Web of Science, EBSCO, and TR Dizin. The keywords included “lung cancer,” “nitric oxide,” “prostaglandin D2,” “prostaglandin I2,” “prostaglandin E2,” “8-iso-prostaglandin-F2a,” “thromboxane A2,” “thromboxane B2,” “endothelin-1,” and “endothelin-2.”
This section describes the biosynthesis, receptors, signaling pathways, and biological effects of NO, PGs (PGD2, PGI2, PGE2, and 8-iso-PGF2α), TXs (TXA2 and TXB2), and ETs (ET-1 and ET-2), with associated figures contributing to the understanding. The multifunctional endogenous modulators play essential roles in physiological processes and are used as biomarkers for various pathological conditions.
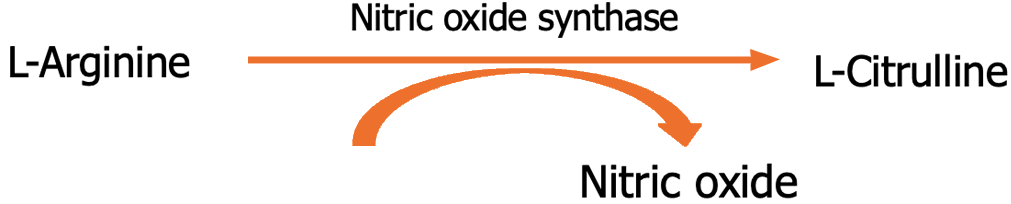
NO is an endogenously synthesized free radical and short-lived diatomic gas[11]. It is produced in endothelial cells, airway epithelial cells, kuffer cells, macrophages, and natural killer cells by NO synthase (NOS) isoforms, which convert L-arginine to L-citrulline[12] (Figure 1). NOS isoforms are categorized into two classes: Calcium/calmodulin-dependent and -independent[13]. Calcium/calmodulin-dependent neuronal NOS (nNOS or NOS1) and endothelial NOS (eNOS or NOS3) are constitutively expressed by neurons and endothelial cells, respectively[14]. In a calcium/calmodulin-independent manner, inducible NOS (iNOS or NOS2) expression is triggered by proinflammatory molecules[15]. NOS1 and NOS3 generate NO at nanomolar levels, while NOS2 at micromolar concentrations. NO regulates vascular function, metabolism, neurotransmission, and macrophage-mediated cytotoxicity[16,17].
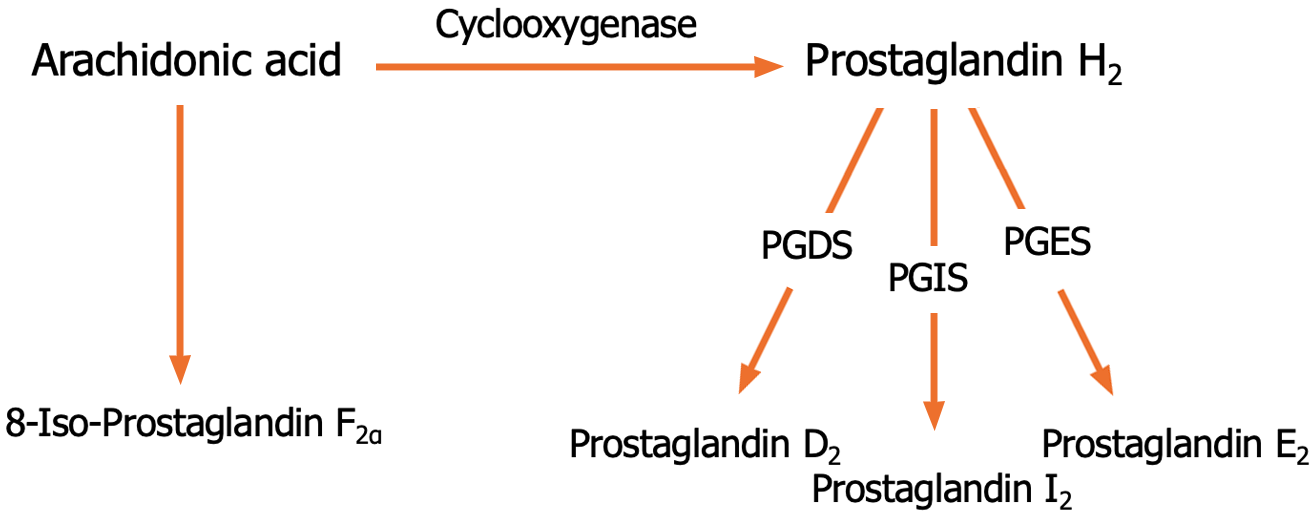
PGs are lipid autacoids that have hormone-like activities[18]. Cyclooxygenase (COX) has COX-1 and COX-2 isoforms, which catalyze the conversion of the cell membrane lipid derivative arachidonic acid into PGH2[19]. PGDS, PGIS, and PGES convert PGH2 to PGD2, PGI2, and PGE2, respectively (Figure 2). However, 8-iso-PGF2α is the product of a non-enzymatic peroxidation of arachidonic acid[20]. PGD2, PGI2, and PGE2 exhibit their activities via D1-2, IP, and EP1-4 receptors, respectively. 8-iso-PGF2α binds to the receptor of TXA2[21]. PGs play a role in various physiological and pathological processes, including vascular reactivity, platelet aggregation, and inflammation[22,23]. Moreover, 8-iso-PGF2α is a potential biomarker for oxidative stress and lipid peroxidation[24,25].
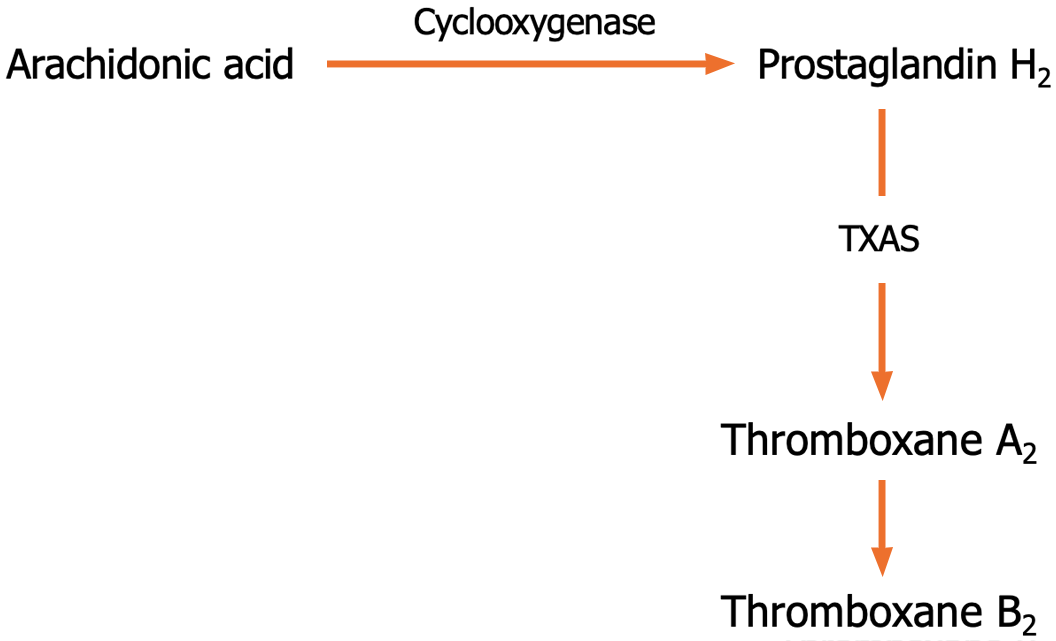
TXA2 is produced in endothelial cells, platelets, macrophages, and neutrophils through COXs and TXA2 synthase (TXA2S)[26]. TXA2 interacts with the TXA2-prostanoid receptor (TP) on the cell surface[27]. TP isoforms TPα and TPβ couple to G proteins[28]. TPα isoform is expressed in more tissues than TPβ[29]. TXA2 is spontaneously hydrolyzed non-enzymatically to the stable TXB2 metabolite[30] (Figure 3). TXA2 is involved in promoting vasoconstriction and platelet aggregation[31]. TXB2 leads to bronchoconstriction and inflammation[32,33].
ET peptides, ET-1 and ET-2, are encoded by two distinct genes[34]. ETs are 21-amino acid polypeptides that result from the cleavage of big ETs through ET-converting enzymes (ECEs)[35] (Figure 4). ET-1 is synthesized in various tissues, such as endothelial cells, smooth muscle cells, and neurons, whereas ET-2 is abundant in intestinal epithelial cells and ovaries[36]. ETs mediate biological processes by activating ETA and ETB receptors[37]. ET-1 and ET-2 act as constrictor agents in vascular and airway smooth muscle cells[38,39].
This section provides a comprehensive overview of the roles of endogenous modulators in the molecular mechanisms of lung cancer. The following studies present the effects and levels of NO, PGs, TXs, and ETs on lung carcinogenesis, with associated tables contributing to the understanding.
Table 1 summarizes 16 studies on the effects of NO in lung cancer models, including H460, LLC-1, H292, H23, A549, H1299, CL3, and H3255 cell lines, as well as patients. Results indicated that NO contributes to lung cancer development and progression via migratory, tumorigenic, anti-apoptotic, and metastatic effects. NO may promote growth and stemness maintenance and cause resistance to drugs and radiation. Moreover, the factor has apoptotic properties on lung cancer.
| Factor | Models | Effects | Ref. |
| Nitric oxide | H460 cell line | Migratory | [40] |
| LLC-1 cell line | Apoptotic | [41] | |
| Lung cancer patient | Tumorigenic | [42] | |
| Lung cancer tissue | Anti-apoptotic | [43] | |
| NCI-H292 cell line | Anti-apoptotic | [44] | |
| H23 cell line | Migratory | [45] | |
| Lung cancer patient | Tumorigenic | [46] | |
| H292 and H460 cell lines | Tumorigenic | [47] | |
| H23, H292, and H460 cell lines | Migratory | [48] | |
| Anoikis-resistant H460 cell line | Metastatic | [49] | |
| A549, H1299, and CL3 cell lines | Apoptotic | [50] | |
| Cisplatin-resistant A549 cells | Drug resistance | [51] | |
| KRAS mutation-positive mice | Growth | [52] | |
| A549 and H3255 cell lines | Radiation resistance | [53] | |
| - | Stemness maintenance | [54] | |
| LUAD patient | Tumorigenic | [55] |
Sanuphan et al[40] investigated the prolonged effects of NO treatment on H460 Large cell lung cancer cell motility. Results from the study suggested that NO exposure augmented tumor cell migration and invasion, which was related to the upregulation of caveolin-1 expression, possibly through the FAK-Akt cascade. Moreover, NO caused an increase in the filopodia formation and cell division cycle 42 protein levels.
Confino et al[41] examined the roles of gaseous NO on the Lewis lung carcinoma (LLC-1) cells. NO led to a decrease in cell viability in a time-dependent manner. However, gaseous NO developed resistance to the second LLC-1 vaccination in vivo. These results indicated that NO may inhibit local and distant metastases due to its non-carcinogenic properties.
Koçer et al[42] reported a positive correlation between the b allele and b/b genotype frequency of the intron four variable number of tandem repeat (VNTR) polymorphism in the eNOS gene and epidermoid lung cancer. In this regard, the eNOS gene-related intron 4 VNTR may be counted among genetic risk factors for lung cancer.
Chen et al[43] investigated the tobacco carcinogen NNK exposure on tissue samples from lung cancer cases. In NNK-treated cells, iNOS levels enhanced while decreasing caspase-3 levels. Therefore, iNOS may have anti-apoptotic activities.
Wongvaranon et al[44] showed that long-term NO exposure led to resistance against chemotherapeutic drugs, such as doxorubicin, cisplatin (CDDP), and etoposide, via caveolin-1, B-cell lymphoma-2, and Akt.
Chanvorachote et al[45] investigated the role of extended NO treatment on lung cancer H23 cells. NO exposure may elicit cell motility by inducing lamellipodia formation. NO exhibited a migratory effect and resistance to anoikis through epithelial-mesenchymal transition (EMT) and caveolin-1. Moreover, prolonged exposure to nontoxic concentrations of NO promoted EMT in H292 and H460 cells.
Peddireddy et al[46] detected a significant correlation between the a/a genotype frequency of the eNOS gene polymorphism and NSCLC in the South Indian population.
Yongsanguanchai et al[47] reported that NO promoted the expression of cancer stem cell markers and triggered migration and colony formation in H292 and H460 cells. Additionally, it enhanced resistance to anoikis.
Saisongkorh et al[48] found that the nontoxic NO doses increased the expression of integrin αv and β1 in H23, H292, and H460 cells. The migratory effect may be related to the protein kinase G/Akt signaling pathway.
Powan and Chanvorachote[49] demonstrated that NO led to E-cadherin-mediated cell aggregation in anoikis-resistant H460 cells.
Chao et al[50] examined survivin expression in NO-treated A549, H1299, and CL3 cells. Exogenous NO donors, sodium nitroprusside (SNP) and S-nitroso-N-acetyl-penicillamine (SNAP), and endogenous iNOS caused cell death by inhibiting survivin expression. The apoptotic effects of SNP and iNOS were concerned with the p38 MAPK pathway. SNAP inhibited cell growth while increasing G2/M phase fractions. SNAP led to a decrease in cyclin B1 levels; however, it did not significantly change cdc2 levels.
Li et al[51] found that iNOS enhanced CDDP resistance in A549 cells through the canonical Wnt/β-catenin signaling pathway.
Pershing et al[52] investigated the role of L-NAME, a NOS inhibitor, in mice with KRAS mutation-positive NSCLC. The findings showed that L-NAME reduced cell growth in vivo.
Saleem et al[53] reported that decreased NO levels augmented radiosensitivity in the hypoxic A549 and H3255 cells. Additionally, the reduction caused the downregulation of epidermal growth factor receptor (EGFR) and hypoxia-inducible factor-1α expressions, especially in H3255 cells.
Zhang et al[54] showed that NO-induced S-nitrosylation and deubiquitination of the Notch1 protein maintained cancer stem cell stemness.
Fujimoto et al[55] demonstrated a significant relationship between NOS activity and p53 mutation in patients with early-stage lung adenocarcinoma (LUAD).
Table 2 summarizes nine studies on NO levels in lung cancer models. NO levels may be either low or high in patients and tissues with lung cancer.
Kaynar et al[56] detected higher NO levels in the patients with SCLC and NSCLC than in the healthy subjects.
Muto et al[57] examined serum NO levels in bevacizumab plus chemotherapy-treated NSCLC patients. Results showed that NO concentration reduced after the second course of bevacizumab administration.
Yagcı et al[58] conducted a study on patients with lung cancer and found an enhancement in serum iNOS levels. According to the finding, the iNOS protein may be a biomarker candidate for lung cancer.
Gào et al[59] studied the impact of NO metabolites, nitrite and nitrate, on lung tumorigenesis. Urine samples from lung cancer patients revealed elevated levels of nitrite/nitrate.
Masri et al[60] detected higher levels of exhaled NO and nitrite in the lung cancer group than in the control group.
Liu et al[61] measured fractional exhaled NO levels using a NO analyzer in patients with various subtypes of lung cancer. The findings demonstrated that patients with SCLC and squamous cell carcinoma especially had higher levels of exhaled NO.
Speranza et al[62] indicated that the levels of iNOS mRNA and protein in lung cancer tissue were enhanced compared to normal lung tissue. The upregulation of iNOS may be associated with the development of lung cancer.
Sim et al[63] examined iNOS expression between pre-chemotherapy and post-chemotherapy groups with NSCLC. Results showed that iNOS levels decreased post-chemotherapy.
Ambs et al[64] suggested that Ca2+-dependent NOS isoforms, NOS1 and NOS3, diminished in tissue with NSCLC compared to healthy tissue. Ca2+-independent NOS2 expression was low in tumor and healthy tissues but significantly enhanced in squamous cell carcinomas.
Table 3 summarizes 20 studies on the effects of PGs in lung cancer models, including A549, PC-9, GLC82, and NCI-H157 cell lines, as well as patients and mice. In this regard, PGD2 and PGI2 exhibit anti-cancer effects, while PGE2 displays pro-tumor effects.
| Factor | Models | Effects | Ref. |
| Prostaglandin D2 | A549 cell line | Apoptosis | [65] |
| Prostaglandin I2 | A549 cell line | Anti-growth | [66] |
| LLC | Anti-metastatic | [67] | |
| LLC | Anti-metastatic | [68] | |
| Transgenic mice | Chemopreventive | [69] | |
| Prostaglandin E2 | NSCLC | Growth | [70] |
| A549 and PC-9 cell lines | Pro-tumorigenic | [71] | |
| A549 and GLC82 cell lines | Proliferative | [72] | |
| NSCLC cell line | Apoptosis resistance | [73] | |
| A549 cell line | Anti-apoptotic | [74] | |
| A549 cell line and lung cancer mice | Multidrug resistance | [75] | |
| Lung cancer patients | Tumorigenic | [76] | |
| A549 cell line | Migratory | [77] | |
| NSCLC cell line | Invasive | [78] | |
| A549 cell line | Tumorigenic | [79] | |
| NSCLC cell line | Proliferative | [80] | |
| NSCLC cells | Metastatic | [81] | |
| NCI-H157 cell line | Angiogenic | [82] | |
| NSCLC patients | Immunosuppressive | [83] | |
| A549 cell line | Migratory | [84] |
Wang and Mak[65] demonstrated that PGD2 promoted cell death in A549 cells via the intrinsic apoptotic pathway.
Fukumoto et al[66] showed that PGI2 induced negative growth control of A549 by stimulating PPARδ.
Cuneo et al[67] investigated the effect of PGI2 on LLC metastasis. PGI2 blunted metastasis formation and LLC adherence to endothelial cells.
Karmali et al[68] examined the impact of nafazatrom, a stimulator of PGI2 release, on LLC. Treatment with nafazatrom diminished lung weight. Moreover, the administration enhanced survival time in mice.
Keith et al[69] reported that the PGI2 synthase overexpression reduced lung tumor incidence and multiplicity in transgenic mice.
Zhong et al[70] showed that PGE2 led to upregulating the α7 nAChR gene in NSCLC via the EP4/JNK/PI3K/PKA/c-Jun pathway. PGE2 led to NSCLC cell growth by increasing α7 nAChR expression.
Filippelli et al[71] reported that PGE2 may exhibit pro-tumorigenic effects via the EP1-ERK5 signaling pathway.
Bazzani et al[72] presented that PGE2 caused A549 and GLC82 cell proliferation by promoting the nuclear translocation of EGFR.
Krysan et al[73] demonstrated that PGE2 induced apoptosis resistance through c-myc-mediated upregulation of miR-17-92, which blunted PTEN expression.
Li et al[74] investigated the roles of EP3, a PGE2 receptor, on NSCLC progression. In A549 cells, EP3/TGFβ/Smad signaling inhibition triggered apoptosis and reduced viability, migration, and invasion.
Maeng et al[75] found that COX-2 upregulation enhanced the expression of MRP4 in A549 cells and a lung cancer mouse models via the mPGES-1/PGE2 cascade.
Liang et al[76] assessed the roles of SNPs in COX/PGE2 pathway-related genes in lung cancer patients. PTGS2-rs4648298, PTGS2-rs2745557, PTGER2-rs2075797, and PTGIS-rs6125671 were related to an increased risk of lung cancer.
Kim et al[77] reported that the PGE2/EP4/β-arrestin1/c-SRC signaling pathway mediated the migration of A549 cells.
Dohadwala et al[78] displayed that PGE2 promoted the expression of CD44 and MMP-2, which caused invasion in NSCLC cells.
Hanaka et al[79] revealed that mPGES-1 knockdown inhibited the clonogenicity and tumorigenicity of A549 cells.
Krysan et al[80] investigated the impacts of EGFR inhibitors on PGE2-mediated NSCLC cell proliferation. The findings showed that PGE2 stimulated the proliferation of NSCLC cells in an EGFR-independent manner. The proliferative effect was related to the MAPK-Erk signal transduction.
Wang et al[81] demonstrated that PGE2 had a metastatic effect on NSCLC cells through USP9X/PGES.
Casibang et al[82] found that PGE2 enhanced the mRNA expression of vascular endothelial growth factor (VEGF) mRNA in NCI-H157 by activating adenylyl cyclase. According to the results, PGE2 may promote angiogenesis.
Baratelli et al[83] reported that COX-2/PGE2 signaling increased the immunosuppressive activity of CD4+CD25bright regulatory T cells (Treg) mediated by TGF-β in NSCLC patients.
Bai et al[84] revealed that PGE2 and its EP1 receptor targeted the downstream signaling of NF-κB. The prostanoid increased β1-integrin expression, contributing to the migration of A549 cells.
Table 4 summarizes 16 studies on PG levels in lung cancer models. PGD2 and PGI2 levels were generally decreased, while PGE2 and 8-iso-PGF2α levels were elevated in lung cancer.
| Factor | Models | Levels | Ref. |
| Prostaglandin D2 | Lung cancer tissue | Low | [85] |
| NSCLC | Low | [86] | |
| Prostaglandin I2 | Lung cancer patient | Low | [87] |
| neoplastic cell | Low | [88] | |
| Lung cancer patient | High | [89] | |
| Lung tumor tissue | Low | [90] | |
| Prostaglandin E2 | lung cancer tissue | High | [91] |
| NSCLC cell line | High | [92] | |
| Lung cancer tissue | High | [85] | |
| Lung cancer patient | high | [93] | |
| NSCLC patient | High | [94] | |
| 8-Iso-prostaglandin F2α | Lung cancer patient | High | [95] |
| Lung cancer patient | High | [96] | |
| NSCLC patient | High | [97] | |
| Lung cancer patient | High | [98] | |
| NSCLC patient | High | [99] |
McLemore et al[85] detected lower PGD2 levels in lung cancer tissue than in normal lung tissue.
He et al[86] reported that PGD2 synthase, responsible for PGD2 production, was downregulated in NSCLC.
Dumanskiy et al[87] measured PGI2 at low concentrations in patients with lung cancer.
Dwyer-Nield et al[88] showed that PGI2 expression was lower in murine neoplastic cells than in non-neoplastic cells.
Xin et al[89] presented that serum levels of PGI2 were elevated in lung cancer patients compared to normal subjects. However, levels of PGI2 mRNA decreased after cancer treatment. PGI2 levels were associated with distant metastasis.
Nana-Sinkam et al[90] detected lower levels of PGI2 synthase mRNA and protein in lung tumor tissue than in normal lung tissue.
Liu et al[91] displayed that PGE2 levels were higher in lung tumor tissue than in normal lung tissue.
Zhu et al[92] reported that NSCLC cells highly expressed PGE2 compared to SCLC cells.
McLemore et al[85] found that lung cancer tissue produced more PGE2 than normal lung tissue.
Honegger et al[93] indicated higher PGE2 levels in lung cancer patients than in the normal controls.
Hidalgo et al[94] determined elevated plasma PGE2 levels in NSCLC patients compared to the control group.
Gào et al[95] detected a correlation between urinary 8-iso-PGF2α levels and lung cancer incidence.
Ma et al[96] showed higher blood 8-iso-PGF2α levels in patients with lung cancer than in patients with benign lung nodules and patients with chronic obstructive pulmonary disease/asthma.
Stathopoulos et al[97] demonstrated that chemotherapy treatment declined 8-iso-PGF2α levels in NSCLC patients.
Dalaveris et al[98] determined elevated serum levels of 8-iso-PGF2α in lung cancer patients compared to healthy controls. Serum 8-iso-PGF2α levels were higher in patients with advanced stage than in patients with locoregional stage.
Chan et al[99] found elevated 8-iso-PGF2α levels in exhaled breath condensate (EBC) of NSCLC patients compared to smokers.
Table 5 summarizes five studies on the effects of TXs in lung cancer cell lines, including NCI-H23, A549, H157, and PC-9. TXA2 induces anti-apoptosis, angiogenesis, invasion, cell growth, and proliferation.
Huang et al[100] investigated the role of TXA2 on LUAD cells. TXA2S blocker furegrelate and TP inhibitor SQ29548 hindered NCI-H23 and A549 cell proliferation. Similarly, TXA2 modulators PTXA2 and BM567 inhibited the cell proliferation and arrested the NNK-induced G2/M phase cell cycle. PTXA2 exhibited its inhibitory effects by downregulating cyclinB1 and CDK1 expressions. The expression of survivin, an anti-apoptotic protein, was reduced by NS398 and BM567 inhibitors. The TXA2 agonist U46619 stimulated NCI-H23 cell proliferation inhibited by COX-2 siRNA.
Li and Tai[101] reported that TXA2 could promote monocyte chemoattractant protein-1 (MCP-1) release from A549 cells, leading to the invasion of lung cancer cells.
Li and Tai[102] demonstrated that TP agonist I-BOP led to a proliferation in H157 cells by inducing Nurr1 gene expression.
Wei et al[103] showed increased tumor growth and angiogenesis in nude mice inoculated with A549-TPα cells. However, the activated TPα signaling cascade could induce VEGF expression.
Fujimura et al[104] examined the effects of TP antagonists, S-1452 and ONO-NT-126, on CDDP-induced apoptosis in PC-9 and PC-9/CDDP cells. TXA2 blockage enhanced the cell death by inducing ICH-1 L protein expression.
Table 6 summarizes four studies on TX levels in lung cancer models. Both TXA2 and TXB2 levels were elevated in lung cancer.
Huang et al[100] determined that COX-2 Levels were abundant in NCI-H23, NCI-H460, and A549 cells compared with normal cells. The specific COX-1 inhibitor SC560 did not significantly suppress TXA2 production, while the COX-2 inhibitor NS398 caused a decrease in A549 and NCI-H23 cells.
Wei et al[105] detected that I-BOP enhanced TXB2 levels in A549 cells with transfected TPα. The stimulatory activity of I-BOP was concerned with COX-2 upregulation.
Chen et al[106] found higher TXB2 and anti-apoptotic protein Bcl-2 Levels in NSCLC tissue than in normal tissue.
Cathcart et al[107] measured elevated TXB2 levels in NSCLC serum samples compared to control serum samples.
Table 7 summarizes three studies on the effects of TXs in A549 and SPC-A1 cancer cell lines. ET-1 is involved in tumor cell proliferation and invasion, and ET-2 exerts anti-apoptotic effects.
Zhang et al[108] reported that RNA interference-induced ET-1 gene silencing suppressed proliferation and invasion in A549 Lung carcinoma cells. The combination with Endostar, a modified human recombinant endostatin, potentiated those effects. ET-1 silencing increased E-cadherin while decreasing pro-angiogenic VEGF.
Zhang et al[109] showed that ET-1 promoted SPC-A1 cell proliferation by activating the ET-A receptor. However, the ET-1/ET-A receptor signaling enhanced intracellular Ca2+ levels via the phosphoinositol/Ca2+ pathway and voltage-dependent Ca2+ channel. Intracellular Ca2+ could contribute to the mitogenic property of ET-1.
Suprapto et al[110] demonstrated that silencing ET-2 reduced proliferation, migration, and invasion and facilitated apoptosis in A549 lung cancer cells. Additionally, ET-2 ablation enhanced E-cadherin while decreasing survivin and X-linked inhibitor of apoptosis.
Table 8 summarizes five studies on ET levels in lung cancer models. Both ET-1 and ET-2 Levels were elevated in lung cancer.
Boldrini et al[111] detected that ET-1, ET-A, and ECE-1 mRNA expressions were more elevated in NSCLC tissue than in control tissue. On the contrary, higher ET-B receptor mRNA expression was in control tissue compared to NSCLC tissue.
Ahmed et al[112] showed that ET-1 mRNA was highly expressed by COR-L88 and A549 cells, comparable to human umbilical vein endothelial cells.
Arun et al[113] determined higher plasma big ET-1 levels in NSCLC patients than in healthy subjects.
Chen et al[114] assessed ET-1 and cancer embryo antigen (CEA) levels in patients with NSCLC using EBC, a non-invasive method. ET-1 and CEA levels were higher in serum and EBC of NSCLC patients than in control subjects. However, ET-1 and CEA levels were more elevated in serum and EBC of patients with stages III and IV lung cancer compared to patients with stages I and II lung cancer.
Suprapto et al[110] presented that ET-2 expression in LUAD tissue was higher than in normal lung tissue.
Lung cancer is one of the most frequent cancers globally. Endogenous modulators, such as NO, PGs, TXs, and ETs, are involved in the molecular mechanisms of lung carcinogenesis. This study reviewed 76 studies investigating the effects and levels of NO, PGs, TXs, and ETs on lung cancer. In this context, NO induces lung tumorigenesis. PGD2 and PGI2 were low concentrations, whereas PGE2 and 8-iso-PGF2α were high concentrations in lung cancer. TXA2 may contribute to tumor development and progression. Lung cancer cells expressed TXB2 at high levels. ET-1 and ET-2 exhibit neoplastic effects. Therefore, the endogenous modulators may serve as potential biomarkers for predicting lung carcinogenesis.
However, the variability in the levels and effects of endogenous modulators across lung cancer models may undermine diagnostic accuracy and generalizability. Sample heterogeneity poses a critical barrier to the clinical translation of these endogenous modulators for lung cancer. Further in vivo and in vitro preclinical studies are needed to validate NO, PGD2, PGI2, PGE2, 8-iso-PGF2α, TXA2, TXB2, ET-1, and ET-2 as predictive biomarkers in a real-world setting.
Future therapeutic approaches should target the endogenous modulators in lung cancer treatment. In this regard, a combination with PGD2/PGI2 agonists can enhance the tumor-suppressive effects, while a combination with NO/PGE2/8-iso-PGF2α/TXA2/TXB2/ET-1/ET-2 antagonists can hinder tumor-promoting signaling cascades. Moreover, these combinatorial strategies could be integrated into targeted therapies and immunotherapy to modulate anti-tumor and pro-tumor pathways more effectively.
| 1. | Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I, Jemal A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2024;74:229-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5690] [Cited by in RCA: 12876] [Article Influence: 6438.0] [Reference Citation Analysis (8)] |
| 2. | Siegel RL, Giaquinto AN, Jemal A. Cancer statistics, 2024. CA Cancer J Clin. 2024;74:12-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2279] [Cited by in RCA: 6132] [Article Influence: 3066.0] [Reference Citation Analysis (4)] |
| 3. | Ratnakaram K, Yendamuri S, Groman A, Kalvapudi S. Sex-Based Differences in Lung Cancer Incidence: A Retrospective Analysis of Two Large US-Based Cancer Databases. Cancers (Basel). 2024;16. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 4. | Cooper WA, Lam DC, O'Toole SA, Minna JD. Molecular biology of lung cancer. J Thorac Dis. 2013;5 Suppl 5:S479-S490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 145] [Reference Citation Analysis (0)] |
| 5. | Bade BC, Dela Cruz CS. Lung Cancer 2020: Epidemiology, Etiology, and Prevention. Clin Chest Med. 2020;41:1-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 455] [Cited by in RCA: 1302] [Article Influence: 217.0] [Reference Citation Analysis (0)] |
| 6. | Cheng CY, Nikitin AY. Neuroendocrine cells: potential cells of origin for small cell lung carcinoma. Cell Cycle. 2011;10:3629-3630. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 7. | Raso MG, Bota-Rabassedas N, Wistuba II. Pathology and Classification of SCLC. Cancers (Basel). 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 114] [Article Influence: 22.8] [Reference Citation Analysis (2)] |
| 8. | Kumar A, Kumar A. Non-small-cell lung cancer-associated gene mutations and inhibitors. Adv Cancer Biol Metastasis. 2022;6:100076. [DOI] [Full Text] |
| 9. | Zhang X, Lei G, Zhao K, Zhang X, Dang C. CircTADA2A up-regulates MAPK8 by targeting MiR-214-3p and recruiting EIF4A3 to promote the invasion and migration of non-small cell lung cancer cells. Histol Histopathol. 2023;38:837-848. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 10. | Niemira M, Collin F, Szalkowska A, Bielska A, Chwialkowska K, Reszec J, Niklinski J, Kwasniewski M, Kretowski A. Molecular Signature of Subtypes of Non-Small-Cell Lung Cancer by Large-Scale Transcriptional Profiling: Identification of Key Modules and Genes by Weighted Gene Co-Expression Network Analysis (WGCNA). Cancers (Basel). 2019;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 159] [Cited by in RCA: 193] [Article Influence: 27.6] [Reference Citation Analysis (0)] |
| 11. | Lancaster JR Jr. Nitric oxide: a brief overview of chemical and physical properties relevant to therapeutic applications. Future Sci OA. 2015;1:FSO59. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 46] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 12. | Andrabi SM, Sharma NS, Karan A, Shahriar SMS, Cordon B, Ma B, Xie J. Nitric Oxide: Physiological Functions, Delivery, and Biomedical Applications. Adv Sci (Weinh). 2023;10:e2303259. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 72] [Cited by in RCA: 303] [Article Influence: 101.0] [Reference Citation Analysis (0)] |
| 13. | Salerno JC, Harris DE, Irizarry K, Patel B, Morales AJ, Smith SM, Martasek P, Roman LJ, Masters BS, Jones CL, Weissman BA, Lane P, Liu Q, Gross SS. An autoinhibitory control element defines calcium-regulated isoforms of nitric oxide synthase. J Biol Chem. 1997;272:29769-29777. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 197] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 14. | Costa ED, Rezende BA, Cortes SF, Lemos VS. Neuronal Nitric Oxide Synthase in Vascular Physiology and Diseases. Front Physiol. 2016;7:206. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 69] [Cited by in RCA: 100] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 15. | Reddy TP, Glynn SA, Billiar TR, Wink DA, Chang JC. Targeting Nitric Oxide: Say NO to Metastasis. Clin Cancer Res. 2023;29:1855-1868. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 42] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 16. | Lundberg JO, Weitzberg E. Nitric oxide signaling in health and disease. Cell. 2022;185:2853-2878. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 492] [Reference Citation Analysis (0)] |
| 17. | Regulski M, Tully T. Molecular and biochemical characterization of dNOS: a Drosophila Ca2+/calmodulin-dependent nitric oxide synthase. Proc Natl Acad Sci U S A. 1995;92:9072-9076. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 183] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 18. | Ricciotti E, FitzGerald GA. Prostaglandins and inflammation. Arterioscler Thromb Vasc Biol. 2011;31:986-1000. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2847] [Cited by in RCA: 2842] [Article Influence: 189.5] [Reference Citation Analysis (12)] |
| 19. | Wang Q, Morris RJ, Bode AM, Zhang T. Prostaglandin Pathways: Opportunities for Cancer Prevention and Therapy. Cancer Res. 2022;82:949-965. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 57] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 20. | Mozzini C, Girelli D, Cominacini L, Soresi M. An Exploratory Look at Bicuspid Aortic Valve (Bav) Aortopathy: Focus on Molecular and Cellular Mechanisms. Curr Probl Cardiol. 2021;46:100425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 21. | Thatcher TH, Peters-Golden M. From Biomarker to Mechanism? F2-isoprostanes in Pulmonary Fibrosis. Am J Respir Crit Care Med. 2022;206:530-532. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 22. | Beccacece L, Abondio P, Bini C, Pelotti S, Luiselli D. The Link between Prostanoids and Cardiovascular Diseases. Int J Mol Sci. 2023;24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 34] [Reference Citation Analysis (0)] |
| 23. | Harris SG, Padilla J, Koumas L, Ray D, Phipps RP. Prostaglandins as modulators of immunity. Trends Immunol. 2002;23:144-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 872] [Cited by in RCA: 877] [Article Influence: 36.5] [Reference Citation Analysis (0)] |
| 24. | van 't Erve TJ, Lih FB, Kadiiska MB, Deterding LJ, Eling TE, Mason RP. Reinterpreting the best biomarker of oxidative stress: The 8-iso-PGF(2α)/PGF(2α) ratio distinguishes chemical from enzymatic lipid peroxidation. Free Radic Biol Med. 2015;83:245-251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 103] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 25. | Woo SD, Park HS, Yang EM, Ban GY, Park HS. 8-Iso-prostaglandin F2α as a biomarker of type 2 low airway inflammation and remodeling in adult asthma. Ann Allergy Asthma Immunol. 2024;133:73-80.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 26. | Conti P, Caraffa A, Gallenga CE, Ross R, Kritas SK, Frydas I, Younes A, Di Emidio P, Ronconi G, Toniato E. IL-1 induces throboxane-A2 (TxA2) in COVID-19 causing inflammation and micro-thrombi: inhibitory effect of the IL-1 receptor antagonist (IL-1Ra). J Biol Regul Homeost Agents. 2020;34:1623-1627. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 31] [Reference Citation Analysis (0)] |
| 27. | Ekambaram P, Lambiv W, Cazzolli R, Ashton AW, Honn KV. The thromboxane synthase and receptor signaling pathway in cancer: an emerging paradigm in cancer progression and metastasis. Cancer Metastasis Rev. 2011;30:397-408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 275] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 28. | Sasaki M, Miyosawa K, Ohkubo S, Nakahata N. Physiological significance of thromboxane A(2) receptor dimerization. J Pharmacol Sci. 2006;100:263-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 26] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 29. | Ashton AW, Zhang Y, Cazzolli R, Honn KV. The Role and Regulation of Thromboxane A(2) Signaling in Cancer-Trojan Horses and Misdirection. Molecules. 2022;27. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 20] [Reference Citation Analysis (0)] |
| 30. | Hubbard WC, Alley MC, McLemore TL, Boyd MR. Evidence for thromboxane biosynthesis in established cell lines derived from human lung adenocarcinomas. Cancer Res. 1988;48:2674-2677. [PubMed] |
| 31. | Szczuko M, Kozioł I, Kotlęga D, Brodowski J, Drozd A. The Role of Thromboxane in the Course and Treatment of Ischemic Stroke: Review. Int J Mol Sci. 2021;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 55] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 32. | Friedman LS, Fitzpatrick TM, Bloom MF, Ramwell PW, Rose JC, Kot PA. Cardiovascular and pulmonary effects of thromboxane B2 in the dog. Circ Res. 1979;44:748-751. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 26] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 33. | Gibson RJ, Bowen JM. Biomarkers of regimen-related mucosal injury. Cancer Treat Rev. 2011;37:487-493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 36] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 34. | Irani S, Salajegheh A, Smith RA, Lam AK. A review of the profile of endothelin axis in cancer and its management. Crit Rev Oncol Hematol. 2014;89:314-321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 38] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 35. | Barton M, Yanagisawa M. Endothelin: 20 years from discovery to therapy. Can J Physiol Pharmacol. 2008;86:485-498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 229] [Cited by in RCA: 223] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 36. | Bartáková A, Nováková M. Secondary Metabolites of Plants as Modulators of Endothelium Functions. Int J Mol Sci. 2021;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 37. | Davenport AP, Hyndman KA, Dhaun N, Southan C, Kohan DE, Pollock JS, Pollock DM, Webb DJ, Maguire JJ. Endothelin. Pharmacol Rev. 2016;68:357-418. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 616] [Cited by in RCA: 599] [Article Influence: 59.9] [Reference Citation Analysis (0)] |
| 38. | Mattoli S, Soloperto M, Marini M, Fasoli A. Levels of endothelin in the bronchoalveolar lavage fluid of patients with symptomatic asthma and reversible airflow obstruction. J Allergy Clin Immunol. 1991;88:376-384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 123] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 39. | Guimarães CL, Calixto JB, Rae GA. Potent constrictor actions of endothelin-1, endothelin-2, and endothelin-3 in rat isolated portal vein. Hypertension. 1992;19:II79-II86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 40. | Sanuphan A, Chunhacha P, Pongrakhananon V, Chanvorachote P. Long-term nitric oxide exposure enhances lung cancer cell migration. Biomed Res Int. 2013;2013:186972. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 32] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 41. | Confino H, Dirbas FM, Goldshtein M, Yarkoni S, Kalaora R, Hatan M, Puyesky S, Levi Y, Malka L, Johnson M, Chaisson S, Monson JM, Avniel A, Lisi S, Greenberg D, Wolf I. Gaseous nitric oxide tumor ablation induces an anti-tumor abscopal effect. Cancer Cell Int. 2022;22:405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 42. | Koçer C, Benlier N, Balci SO, Pehlivan S, Şanli M, Nacak M. The role of endothelial nitric oxide synthase gene polymorphisms in patients with lung cancer. Clin Respir J. 2020;14:948-955. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 43. | Chen GG, Lee TW, Xu H, Yip JH, Li M, Mok TS, Yim AP. Increased inducible nitric oxide synthase in lung carcinoma of smokers. Cancer. 2008;112:372-381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 44. | Wongvaranon P, Pongrakhananon V, Chunhacha P, Chanvorachote P. Acquired resistance to chemotherapy in lung cancer cells mediated by prolonged nitric oxide exposure. Anticancer Res. 2013;33:5433-5444. [PubMed] |
| 45. | Chanvorachote P, Pongrakhananon V, Chunhacha P. Prolonged nitric oxide exposure enhances anoikis resistance and migration through epithelial-mesenchymal transition and caveolin-1 upregulation. Biomed Res Int. 2014;2014:941359. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 18] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 46. | Peddireddy V, Badabagni SP, Gundimeda SD, Mundluru HP. Association of eNOS and ACE gene polymorphisms and plasma nitric oxide with risk of non-small cell lung cancer in South India. Clin Respir J. 2018;12:207-217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 47. | Yongsanguanchai N, Pongrakhananon V, Mutirangura A, Rojanasakul Y, Chanvorachote P. Nitric oxide induces cancer stem cell-like phenotypes in human lung cancer cells. Am J Physiol Cell Physiol. 2015;308:C89-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 42] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 48. | Saisongkorh V, Maiuthed A, Chanvorachote P. Nitric oxide increases the migratory activity of non-small cell lung cancer cells via AKT-mediated integrin αv and β1 upregulation. Cell Oncol (Dordr). 2016;39:449-462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 49. | Powan P, Chanvorachote P. Nitric oxide mediates cell aggregation and mesenchymal to epithelial transition in anoikis-resistant lung cancer cells. Mol Cell Biochem. 2014;393:237-245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 50. | Chao JI, Kuo PC, Hsu TS. Down-regulation of survivin in nitric oxide-induced cell growth inhibition and apoptosis of the human lung carcinoma cells. J Biol Chem. 2004;279:20267-20276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 86] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 51. | Li Y, Ma C, Shi X, Wen Z, Li D, Sun M, Ding H. Effect of nitric oxide synthase on multiple drug resistance is related to Wnt signaling in non-small cell lung cancer. Oncol Rep. 2014;32:1703-1708. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 52. | Pershing NL, Yang CFJ, Xu M, Counter CM. Treatment with the nitric oxide synthase inhibitor L-NAME provides a survival advantage in a mouse model of Kras mutation-positive, non-small cell lung cancer. Oncotarget. 2016;7:42385-42392. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 53. | Saleem W, Suzuki Y, Mobaraki A, Yoshida Y, Noda S, Saitoh JI, Nakano T. Reduction of nitric oxide level enhances the radiosensitivity of hypoxic non-small cell lung cancer. Cancer Sci. 2011;102:2150-2156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 30] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 54. | Zhang T, Lei J, Zheng M, Wen Z, Zhou J. Nitric oxide facilitates the S-nitrosylation and deubiquitination of Notch1 protein to maintain cancer stem cells in human NSCLC. J Cell Mol Med. 2024;28:e70203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 55. | Fujimoto H, Sasaki J, Matsumoto M, Suga M, Ando Y, Iggo R, Tada M, Saya H, Ando M. Significant correlation of nitric oxide synthase activity and p53 gene mutation in stage I lung adenocarcinoma. Jpn J Cancer Res. 1998;89:696-702. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 38] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 56. | Kaynar H, Meral M, Turhan H, Keles M, Celik G, Akcay F. Glutathione peroxidase, glutathione-S-transferase, catalase, xanthine oxidase, Cu-Zn superoxide dismutase activities, total glutathione, nitric oxide, and malondialdehyde levels in erythrocytes of patients with small cell and non-small cell lung cancer. Cancer Lett. 2005;227:133-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 90] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 57. | Muto S, Takagi H, Owada Y, Inoue T, Watanabe Y, Yamaura T, Fukuhara M, Okabe N, Matsumura Y, Hasegawa T, Osugi J, Hoshino M, Higuchi M, Shio Y, Suzuki H. Serum Nitric Oxide as a Predictive Biomarker for Bevacizumab in Non-small Cell Lung Cancer Patients. Anticancer Res. 2017;37:3169-3174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 58. | Muto E, Özbayer C, Ak G, Kurt H, Metintaş S, Metintaş M. Elevated Serum Levels of Inducible Nitric Oxide Synthase, Monocyte Chemoattractant Protein-1, And Cyclooxygenase-2 In Patients with Lung Cancer. Osmangazi J Med. 2024;46. [DOI] [Full Text] |
| 59. | Gào X, Xuan Y, Benner A, Anusruti A, Brenner H, Schöttker B. Nitric Oxide Metabolites and Lung Cancer Incidence: A Matched Case-Control Study Nested in the ESTHER Cohort. Oxid Med Cell Longev. 2019;2019:6470950. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 60. | Masri FA, Comhair SA, Koeck T, Xu W, Janocha A, Ghosh S, Dweik RA, Golish J, Kinter M, Stuehr DJ, Erzurum SC, Aulak KS. Abnormalities in nitric oxide and its derivatives in lung cancer. Am J Respir Crit Care Med. 2005;172:597-605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 85] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 61. | Liu PF, Zhao DH, Qi Y, Wang JG, Zhao M, Xiao K, Xie LX. The clinical value of exhaled nitric oxide in patients with lung cancer. Clin Respir J. 2018;12:23-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 24] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 62. | Speranza L, De Lutiis MA, Shaik YB, Felaco M, Patruno A, Tetè S, Tetè A, Mastrangelo F, Madhappan B, Castellani ML, Conti F, Vecchiet J, Theoharides TC, Conti P, Grilli A. Localization and activity of iNOS in normal human lung tissue and lung cancer tissue. Int J Biol Markers. 2007;22:226-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 63. | Sim SH, Ahn YO, Yoon J, Kim TM, Lee SH, Kim DW, Heo DS. Influence of chemotherapy on nitric oxide synthase, indole-amine-2,3-dioxygenase and CD124 expression in granulocytes and monocytes of non-small cell lung cancer. Cancer Sci. 2012;103:155-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 21] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 64. | Ambs S, Bennett WP, Merriam WG, Ogunfusika MO, Oser SM, Khan MA, Jones RT, Harris CC. Vascular endothelial growth factor and nitric oxide synthase expression in human lung cancer and the relation to p53. Br J Cancer. 1998;78:233-239. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 93] [Cited by in RCA: 95] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 65. | Wang JJ, Mak OT. Induction of apoptosis in non-small cell lung carcinoma A549 cells by PGD₂ metabolite, 15d-PGJ₂. Cell Biol Int. 2011;35:1089-1096. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 66. | Fukumoto K, Yano Y, Virgona N, Hagiwara H, Sato H, Senba H, Suzuki K, Asano R, Yamada K, Yano T. Peroxisome proliferator-activated receptor delta as a molecular target to regulate lung cancer cell growth. FEBS Lett. 2005;579:3829-3836. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 55] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 67. | Cuneo KC, Fu A, Osusky KL, Geng L. Effects of vascular endothelial growth factor receptor inhibitor SU5416 and prostacyclin on murine lung metastasis. Anticancer Drugs. 2007;18:349-355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 68. | Karmali RA, Choi K, Otter G, Schmid F. Eicosanoids and metastasis: experimental aspects in Lewis lung carcinoma. Cancer Biochem Biophys. 1986;9:97-104. [PubMed] |
| 69. | Keith RL, Miller YE, Hoshikawa Y, Moore MD, Gesell TL, Gao B, Malkinson AM, Golpon HA, Nemenoff RA, Geraci MW. Manipulation of pulmonary prostacyclin synthase expression prevents murine lung cancer. Cancer Res. 2002;62:734-740. [PubMed] |
| 70. | Zhong X, Fan Y, Ritzenthaler JD, Zhang W, Wang K, Zhou Q, Roman J. Novel link between prostaglandin E2 (PGE2) and cholinergic signaling in lung cancer: The role of c-Jun in PGE2-induced α7 nicotinic acetylcholine receptor expression and tumor cell proliferation. Thorac Cancer. 2015;6:488-500. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 23] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 71. | Filippelli A, Ciccone V, Del Gaudio C, Simonis V, Frosini M, Tusa I, Menconi A, Rovida E, Donnini S. ERK5 mediates pro-tumorigenic phenotype in non-small lung cancer cells induced by PGE2. Biochim Biophys Acta Mol Cell Res. 2024;1871:119810. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 72. | Bazzani L, Donnini S, Finetti F, Christofori G, Ziche M. PGE2/EP3/SRC signaling induces EGFR nuclear translocation and growth through EGFR ligands release in lung adenocarcinoma cells. Oncotarget. 2017;8:31270-31287. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 44] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 73. | Krysan K, Kusko R, Grogan T, O'Hearn J, Reckamp KL, Walser TC, Garon EB, Lenburg ME, Sharma S, Spira AE, Elashoff D, Dubinett SM. PGE2-driven expression of c-Myc and oncomiR-17-92 contributes to apoptosis resistance in NSCLC. Mol Cancer Res. 2014;12:765-774. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 34] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 74. | Li L, Lv Y, Yan D. Inhibition of Ep3 attenuates migration and promotes apoptosis of non-small cell lung cancer cells via suppression of TGF-β/Smad signaling. Oncol Lett. 2018;16:5645-5654. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 75. | Maeng HJ, Lee WJ, Jin QR, Chang JE, Shim WS. Upregulation of COX-2 in the lung cancer promotes overexpression of multidrug resistance protein 4 (MRP4) via PGE2-dependent pathway. Eur J Pharm Sci. 2014;62:189-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 76. | Liang X, Wang J, Liu Y, Wei L, Tian F, Sun J, Han G, Wang Y, Ding C, Guo Z. Polymorphisms of COX/PEG2 pathway-related genes are associated with the risk of lung cancer: A case-control study in China. Int Immunopharmacol. 2022;108:108763. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 77. | Kim JI, Lakshmikanthan V, Frilot N, Daaka Y. Prostaglandin E2 promotes lung cancer cell migration via EP4-betaArrestin1-c-Src signalsome. Mol Cancer Res. 2010;8:569-577. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 125] [Cited by in RCA: 130] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 78. | Dohadwala M, Batra RK, Luo J, Lin Y, Krysan K, Pold M, Sharma S, Dubinett SM. Autocrine/paracrine prostaglandin E2 production by non-small cell lung cancer cells regulates matrix metalloproteinase-2 and CD44 in cyclooxygenase-2-dependent invasion. J Biol Chem. 2002;277:50828-50833. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 206] [Cited by in RCA: 210] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 79. | Hanaka H, Pawelzik SC, Johnsen JI, Rakonjac M, Terawaki K, Rasmuson A, Sveinbjörnsson B, Schumacher MC, Hamberg M, Samuelsson B, Jakobsson PJ, Kogner P, Rådmark O. Microsomal prostaglandin E synthase 1 determines tumor growth in vivo of prostate and lung cancer cells. Proc Natl Acad Sci U S A. 2009;106:18757-18762. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 84] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 80. | Krysan K, Reckamp KL, Dalwadi H, Sharma S, Rozengurt E, Dohadwala M, Dubinett SM. Prostaglandin E2 activates mitogen-activated protein kinase/Erk pathway signaling and cell proliferation in non-small cell lung cancer cells in an epidermal growth factor receptor-independent manner. Cancer Res. 2005;65:6275-6281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 195] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 81. | Wang T, Jing B, Sun B, Liao Y, Song H, Xu D, Guo W, Li K, Hu M, Liu S, Ling J, Kuang Y, Feng Y, Zhou BP, Deng J. Stabilization of PTGES by deubiquitinase USP9X promotes metastatic features of lung cancer via PGE(2) signaling. Am J Cancer Res. 2019;9:1145-1160. [PubMed] |
| 82. | Casibang M, Purdom S, Jakowlew S, Neckers L, Zia F, Ben-Av P, Hla T, You L, Jablons DM, Moody TW. Prostaglandin E2 and vasoactive intestinal peptide increase vascular endothelial cell growth factor mRNAs in lung cancer cells. Lung Cancer. 2001;31:203-212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 51] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 83. | Baratelli F, Lee JM, Hazra S, Lin Y, Walser TC, Schaue D, Pak PS, Elashoff D, Reckamp K, Zhang L, Fishbein MC, Sharma S, Dubinett SM. PGE(2) contributes to TGF-beta induced T regulatory cell function in human non-small cell lung cancer. Am J Transl Res. 2010;2:356-367. [PubMed] |
| 84. | Bai X, Yang Q, Shu W, Wang J, Zhang L, Ma J, Xia S, Zhang M, Cheng S, Wang Y, Leng J. Prostaglandin E2 upregulates β1 integrin expression via the E prostanoid 1 receptor/nuclear factor κ-light-chain-enhancer of activated B cells pathway in non-small-cell lung cancer cells. Mol Med Rep. 2014;9:1729-1736. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 85. | McLemore TL, Hubbard WC, Litterst CL, Liu MC, Miller S, McMahon NA, Eggleston JC, Boyd MR. Profiles of prostaglandin biosynthesis in normal lung and tumor tissue from lung cancer patients. Cancer Res. 1988;48:3140-3147. [PubMed] |
| 86. | He LP, Chen YF, Yang J. [Investigation on the role and mechanism of prostagland in D2 synthase in non-small cell lung cancer]. Zhonghua Yi Xue Za Zhi. 2017;97:3022-3027. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 87. | Dumanskiy YV, Stoliarova OY, Syniachenko OV, Iegudina ED. Endothelial dysfunction of vessels at lung cancer. Exp Oncol. 2015;37:277-280. [PubMed] |
| 88. | Dwyer-Nield LD, Srebernak MC, Barrett BS, Ahn J, Cosper P, Meyer AM, Kisley LR, Bauer AK, Thompson DC, Malkinson AM. Cytokines differentially regulate the synthesis of prostanoid and nitric oxide mediators in tumorigenic versus non-tumorigenic mouse lung epithelial cell lines. Carcinogenesis. 2005;26:1196-1206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 89. | Xin C, Chu L, Zhang L, Geng D, Wang Y, Sun D, Sui P, Zhao X, Gong Z, Sui M, Zhang W. Expression of Cytosolic Phospholipase A2 (cPLA2)-Arachidonic Acid (AA)-Cyclooxygenase-2 (COX-2) Pathway Factors in Lung Cancer Patients and Its Implication in Lung Cancer Early Detection and Prognosis. Med Sci Monit. 2019;25:5543-5551. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 90. | Nana-Sinkam P, Golpon H, Keith RL, Oyer RJ, Sotto-Santiago S, Moore MD, Franklin W, Nemenoff RA, Geraci MW. Prostacyclin in human non-small cell lung cancers. Chest. 2004;125:141S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 91. | Liu L, Ge D, Ma L, Mei J, Liu S, Zhang Q, Ren F, Liao H, Pu Q, Wang T, You Z. Interleukin-17 and prostaglandin E2 are involved in formation of an M2 macrophage-dominant microenvironment in lung cancer. J Thorac Oncol. 2012;7:1091-1100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 106] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 92. | Zhu YM, Azahri NS, Yu DC, Woll PJ. Effects of COX-2 inhibition on expression of vascular endothelial growth factor and interleukin-8 in lung cancer cells. BMC Cancer. 2008;8:218. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 34] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 93. | Honegger AE, Hofer EL, Barañao RI, Mackinlay TA, Mackinlay DA, Bullorsky EO, Bordenave RH, Chasseing NA. Interleukin-1 beta, transforming growth factor beta 1, prostaglandin E2, and fibronectin levels in the conditioned mediums of bone marrow fibroblast cultures from lung and breast cancer patients. Ann Hematol. 2002;81:80-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 94. | Hidalgo GE, Zhong L, Doherty DE, Hirschowitz EA. Plasma PGE-2 levels and altered cytokine profiles in adherent peripheral blood mononuclear cells in non-small cell lung cancer (NSCLC). Mol Cancer. 2002;1:5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 95. | Gào X, Brenner H, Holleczek B, Cuk K, Zhang Y, Anusruti A, Xuan Y, Xu Y, Schöttker B. Urinary 8-isoprostane levels and occurrence of lung, colorectal, prostate, breast and overall cancer: Results from a large, population-based cohort study with 14 years of follow-up. Free Radic Biol Med. 2018;123:20-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 96. | Ma L, Sun D, Xiu G, Lazarus P, Vachani A, Penning TM, Whitehead AS, Muscat JE. Quantification of Plasma 8-Isoprostane by High-Performance Liquid Chromatography with Tandem Mass Spectrometry in a Case-Control Study of Lung Cancer. Int J Environ Res Public Health. 2022;19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 97. | Stathopoulos D, Loukides S, Syrigos K. 8-Isoprostane in exhaled breath condensate of patients with non-small cell lung cancer: the effect of chemotherapy. Anticancer Res. 2014;34:5143-5145. [PubMed] |
| 98. | Dalaveris E, Kerenidi T, Katsabeki-Katsafli A, Kiropoulos T, Tanou K, Gourgoulianis KI, Kostikas K. VEGF, TNF-alpha and 8-isoprostane levels in exhaled breath condensate and serum of patients with lung cancer. Lung Cancer. 2009;64:219-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 75] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 99. | Chan HP, Tran V, Lewis C, Thomas PS. Elevated levels of oxidative stress markers in exhaled breath condensate. J Thorac Oncol. 2009;4:172-178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 49] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 100. | Huang RY, Li SS, Guo HZ, Huang Y, Zhang X, Li MY, Chen GG, Zeng X. Thromboxane A2 exerts promoting effects on cell proliferation through mediating cyclooxygenase-2 signal in lung adenocarcinoma cells. J Cancer Res Clin Oncol. 2014;140:375-386. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 101. | Li X, Tai HH. Activation of thromboxane A2 receptor (TP) increases the expression of monocyte chemoattractant protein -1 (MCP-1)/chemokine (C-C motif) ligand 2 (CCL2) and recruits macrophages to promote invasion of lung cancer cells. PLoS One. 2013;8:e54073. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 44] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 102. | Li X, Tai HH. Activation of thromboxane A(2) receptors induces orphan nuclear receptor Nurr1 expression and stimulates cell proliferation in human lung cancer cells. Carcinogenesis. 2009;30:1606-1613. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 58] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 103. | Wei J, Yan W, Li X, Ding Y, Tai HH. Thromboxane receptor alpha mediates tumor growth and angiogenesis via induction of vascular endothelial growth factor expression in human lung cancer cells. Lung Cancer. 2010;69:26-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 36] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 104. | Fujimura M, Kasahara K, Shirasaki H, Heki U, Iwasa K, Ueda A, Matsuda T. Up-regulation of ICH-1L protein by thromboxane A2 antagonists enhances cisplatin-induced apoptosis in non-small-cell lung-cancer cell lines. J Cancer Res Clin Oncol. 1999;125:389-394. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 105. | Wei J, Yan W, Li X, Chang WC, Tai HH. Activation of thromboxane receptor alpha induces expression of cyclooxygenase-2 through multiple signaling pathways in A549 human lung adenocarcinoma cells. Biochem Pharmacol. 2007;74:787-800. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 106. | Chen GG, Lee TW, Yip JH, Xu H, Lee IK, Mok TS, Warner TD, Yim AP. Increased thromboxane B(2) levels are associated with lipid peroxidation and Bcl-2 expression in human lung carcinoma. Cancer Lett. 2006;234:193-198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 107. | Cathcart MC, Gately K, Cummins R, Kay E, O'Byrne KJ, Pidgeon GP. Examination of thromboxane synthase as a prognostic factor and therapeutic target in non-small cell lung cancer. Mol Cancer. 2011;10:25. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 41] [Article Influence: 2.7] [Reference Citation Analysis (3)] |
| 108. | Zhang ZY, Chen LL, Xu W, Sigdel K, Jiang XT. Effects of silencing endothelin-1 on invasion and vascular formation in lung cancer. Oncol Lett. 2017;13:4390-4396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 109. | Zhang WM, Zhou J, Ye QJ. Endothelin-1 enhances proliferation of lung cancer cells by increasing intracellular free Ca2+. Life Sci. 2008;82:764-771. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 34] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 110. | Suprapto RP, Suzuki Y, Nagano T, Hirata KI, Emoto N. The loss of endothelin-2 exhibits an anticancer effect in A549 human lung adenocarcinoma cell line. Can J Physiol Pharmacol. 2022;100:818-827. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 111. | Boldrini L, Gisfredi S, Ursino S, Faviana P, Lucchi M, Melfi F, Mussi A, Basolo F, Fontanini G. Expression of endothelin-1 is related to poor prognosis in non-small cell lung carcinoma. Eur J Cancer. 2005;41:2828-2835. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 40] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 112. | Ahmed SI, Thompson J, Coulson JM, Woll PJ. Studies on the expression of endothelin, its receptor subtypes, and converting enzymes in lung cancer and in human bronchial epithelium. Am J Respir Cell Mol Biol. 2000;22:422-431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 75] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 113. | Arun C, DeCatris M, Hemingway DM, London NJ, O'Byrne KJ. Endothelin-1 is a novel prognostic factor in non-small cell lung cancer. Int J Biol Markers. 2004;19:262-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 114. | Chen JL, Lv XD, Ma H, Chen JR, Huang JA. Detection of cancer embryo antigen and endothelin-1 in exhaled breath condensate: A novel approach to investigate non-small cell lung cancer. Mol Clin Oncol. 2016;5:124-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/