Published online Sep 6, 2025. doi: 10.12998/wjcc.v13.i25.107484
Revised: April 14, 2025
Accepted: May 21, 2025
Published online: September 6, 2025
Processing time: 104 Days and 18.1 Hours
Mild cognitive impairment (MCI) and subjective cognitive decline (SCD) are risk indicators for dementia and require ongoing management. Traditional Korean medicine (TKM) commonly employs acupuncture and herbal medicine for cognitive impairment; yet, clinical research on acupotomy is lacking. Although most TKM treatments occur in primary care, the research is largely hospital-based. This registry was established to systematically collect real-world data on the clinical progress, efficacy, and safety of TKM with acupotomy for patients with MCI or SCD in primary care. It is hypothesized that TKM with acupotomy improves cognitive function and is safe for these patients.
To establish an MCI or SCD registry of patients receiving TKM, including acupotomy, to analyze its clinical efficacy and safety.
This observational registry study will be conducted across 22 medical institutions; approximately 500 participants will be recruited. Data—sociodemographic information, medication history, height, weight, vital signs, and assessment questionnaires (Montreal Cognitive Assessment-Korean, short form of Korean-Everyday Cognition, Numeric Rating Scale, Korean version of the Insomnia Severity Index)—will be collected at 3-month intervals over a year. This study will also document the TKM treatment administered and any adverse events. Routine TKM procedures will be followed, with acupuncture and acupotomy administered as per protocol; treatments including herbal medicine, Chuna therapy, and moxibustion may be administered at the practitioner’s discretion.
The registry will capture a wide range of real-world clinical data regarding demographic profiles, treatment processes, and adverse events. This detailed documentation is expected to clarify patient characteristics, evaluate the clinical course, and identify factors that may affect cognitive improvement in patients with MCI and SCD.
This research may provide evidence supporting acupotomy for cognitive impairment in primary care by confirming its efficacy and safety, providing preliminary evidence for TKM-based interventions aimed at improving cognitive function.
Core Tip: This multicenter registry study will investigate real-world clinical data from patients with mild cognitive impairment and subjective cognitive decline treated with traditional Korean medicine (TKM), including acupotomy. It is the first registry-based study to focus on this population in primary care. By collecting information on demographics, cognitive function, treatment procedures, and adverse events, this study aims to examine the safety and effectiveness of comprehensive TKM treatment, including acupotomy. Findings from this registry are expected to serve as a foundation for planning and conducting future clinical studies.
- Citation: Jun H, Ryu M, Chae H, Chu H, Kim K, Lee DE, Jin H, Joo S, Park D, Leem J, Kang HW. Response to Korean medicine with acupotomy in patients with cognitive impairment in primary care: A multicenter registry protocol. World J Clin Cases 2025; 13(25): 107484
- URL: https://www.wjgnet.com/2307-8960/full/v13/i25/107484.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v13.i25.107484
Globally, the population is rapidly aging. In 2022, the global population aged ≥ 65 years was 883.481 million, and is projected to reach 1.007 billion by 2030 and 1.578 billion by 2050[1]. Among countries in the Organization for Economic Cooperation and Development, South Korea’s aging rate is particularly fast. South Korea’s old-age dependency ratio-i.e., the number of people aged ≥ 65 years per 100 working-age individuals-is expected to increase from 19.11 in 2015 to 88.68 in 2060[2]. In response to this global trend, the World Health Organization (WHO) has emphasized the importance of healthy aging. The WHO defines healthy aging as “the process of developing and maintaining the functional ability that enables well-being in older age,” where functional ability includes physical, mental, and cognitive capacities[3].
Although aging is a major risk factor for chronic diseases, the health status of older adults is often considered resilient, and the aging process is viewed as modifiable[4]. Research on healthy aging highlights the importance of assessing both disease markers and intrinsic capacity, with a focus on diverse interventions and integrative treatments. Additionally, there is a need to focus on primary care institutions to ensure continuous care and provide older adults with equitable access to healthcare services[5]. In South Korea, owing to the dual medical system, individuals commonly visit primary care institutions-such as Korean medicine clinics or hospitals-for traditional Korean medicine (TKM) treatments. As of 2022, 94.4% of patients visited Korean medicine clinics, while 12.4% visited Korean medicine hospitals, reflecting a strong preference for clinics[6]; moreover, there were 14549 clinics compared with 546 hospitals, indicating an easier access to clinics[7].
People fear losing their independence, becoming a burden to others, or suffering from physical and mental problems, which makes them reluctant towards experiencing progressive dementia[8]. However, over 55 million people worldwide are currently living with dementia, and approximately 10 million new cases are reported annually[9]. Subjective cognitive decline (SCD) and mild cognitive impairment (MCI) are considered conditions on a continuum leading to dementia, with approximately 5%–10% of patients with MCI progressing to dementia annually[10]. SCD is a state in which individuals perceive a decline in cognitive function and seek medical help, even in the absence of objective cognitive impairment. In 2014, researchers introduced the term 'subjective cognitive decline' to describe this condition[11], which has received increasing attention owing to evidence suggesting that it is associated with a higher risk of future objective cognitive decline[12,13]. Therefore, although SCD and MCI do not necessarily lead to dementia, they are important indicators of increased dementia risk that require ongoing management at the individual, community, national, and global levels.
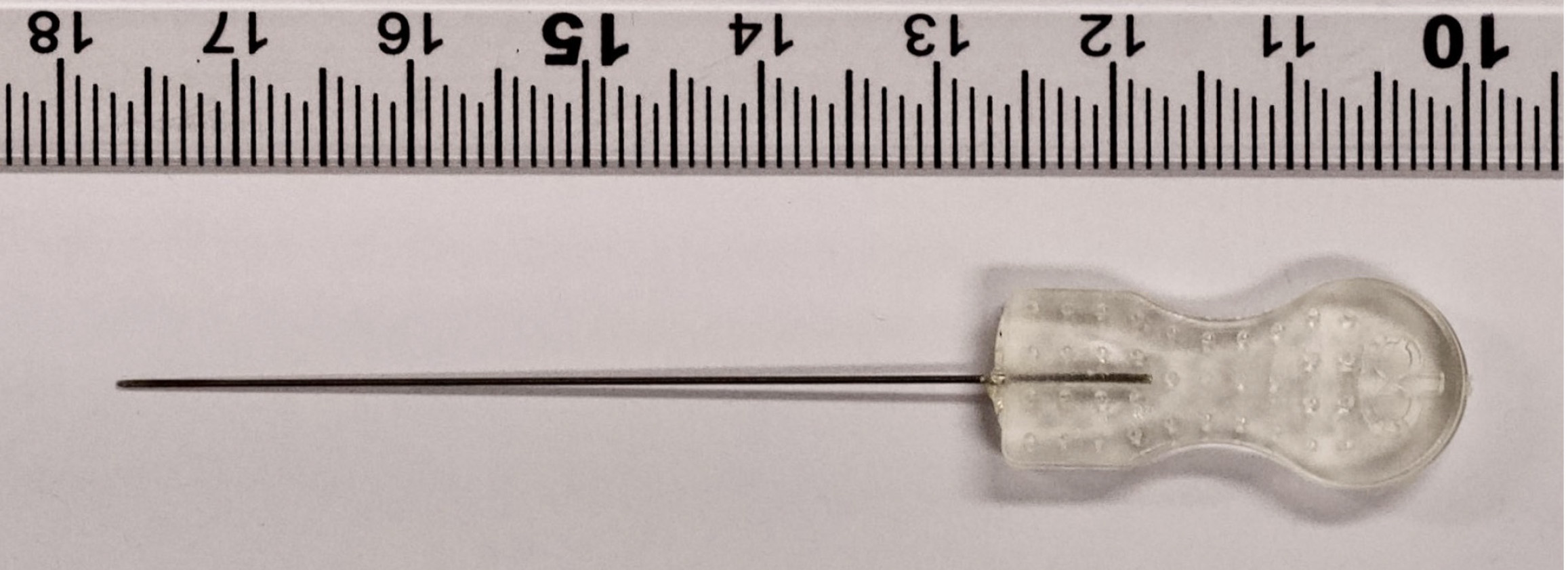
Acupuncture and herbal medicines are primarily used to treat cognitive impairment in TKM and have been reported to be effective for improving cognitive function[14–18]. In patients with MCI, acupuncture significantly improved scores on the Mini-Mental State Examination (MMSE) and Montreal Cognitive Assessment (MoCA)[14]. Additionally, a systematic review found that acupuncture, whether used alone or in combination with other treatments, generally outperformed control interventions such as nimodipine, huperzine, perphenazine, duxil, donepezil, hygiene, sham acupuncture, and cognitive training, with significant improvements in MMSE scores[15]. In patients with SCD, acupuncture led to improvements in composite Z-scores on multidomain neuropsychological tests and increased hippocampal volume and functional connectivity[16]. Additionally, herbal medicine demonstrated efficacy in improving MMSE and MoCA scores in patients with MCI[17,18]. Another intervention used in TKM is acupotomy, which is similar to acupuncture but involves the insertion of a tool with a flat, knife-like tip into the body, making it a minimally invasive surgical approach[19] (Figure 1). Acupotomy needles are thicker and the procedure is more invasive than standard acupuncture, which may produce stronger effects. Acupotomy is primarily used to treat chronic musculoskeletal pain conditions such as frozen shoulder, cervical spondylosis, and trigger finger, and has shown superior outcomes compared with conventional conservative treatments[20]. However, few studies have investigated the effects of acupotomy on cognitive impairment in clinical practice, especially when compared with acupuncture.
To date, the only known study to report cognitive improvement after acupotomy in patients with MCI is a case study by Koo et al[21]. The study suggests that impaired cerebrospinal fluid circulation may contribute to cognitive impairment[22] and that acupotomy may alleviate neck muscle tension, promote intracranial circulation, and enhance brain function[23]. Therefore, prospective studies with larger sample sizes are needed to confirm whether TKM treatment, including acupotomy, effectively improves cognitive function in patients with SCD or MCI.
Previous studies on TKM treatment for cognitive impairment were primarily conducted in university hospitals[24,25]; however, > 90% of TKM treatments in actual clinical practice are performed in primary care Korean medicine clinics, where patient severity may differ. Therefore, this registry aimed to achieve the following objectives: (1) To evaluate the characteristics and clinical course of cognitive function in patients visiting primary care Korean medicine clinics for the treatment of cognitive impairment; (2) To analyze the characteristics of TKM interventions, including acupotomy, performed in real-world clinical settings to improve cognitive function; and (3) To assess the efficacy and safety of TKM treatment.
This study will contribute to a more accurate evaluation of the efficacy and safety of TKM treatments for cognitive impairment in real-world clinical environments, while also improving our understanding of the treatment process and patient characteristics in primary care settings.
This study adhered to the Standard Protocol Items: Recommendations for Interventional Trials (SPIRIT) guidelines (see Supplementary material 1). This study was registered with the Clinical Research Information Service (https://cris.nih.go.kr, KCT0009928) and is currently following protocol version 1.1 (see Supplementary material 2 and 3).
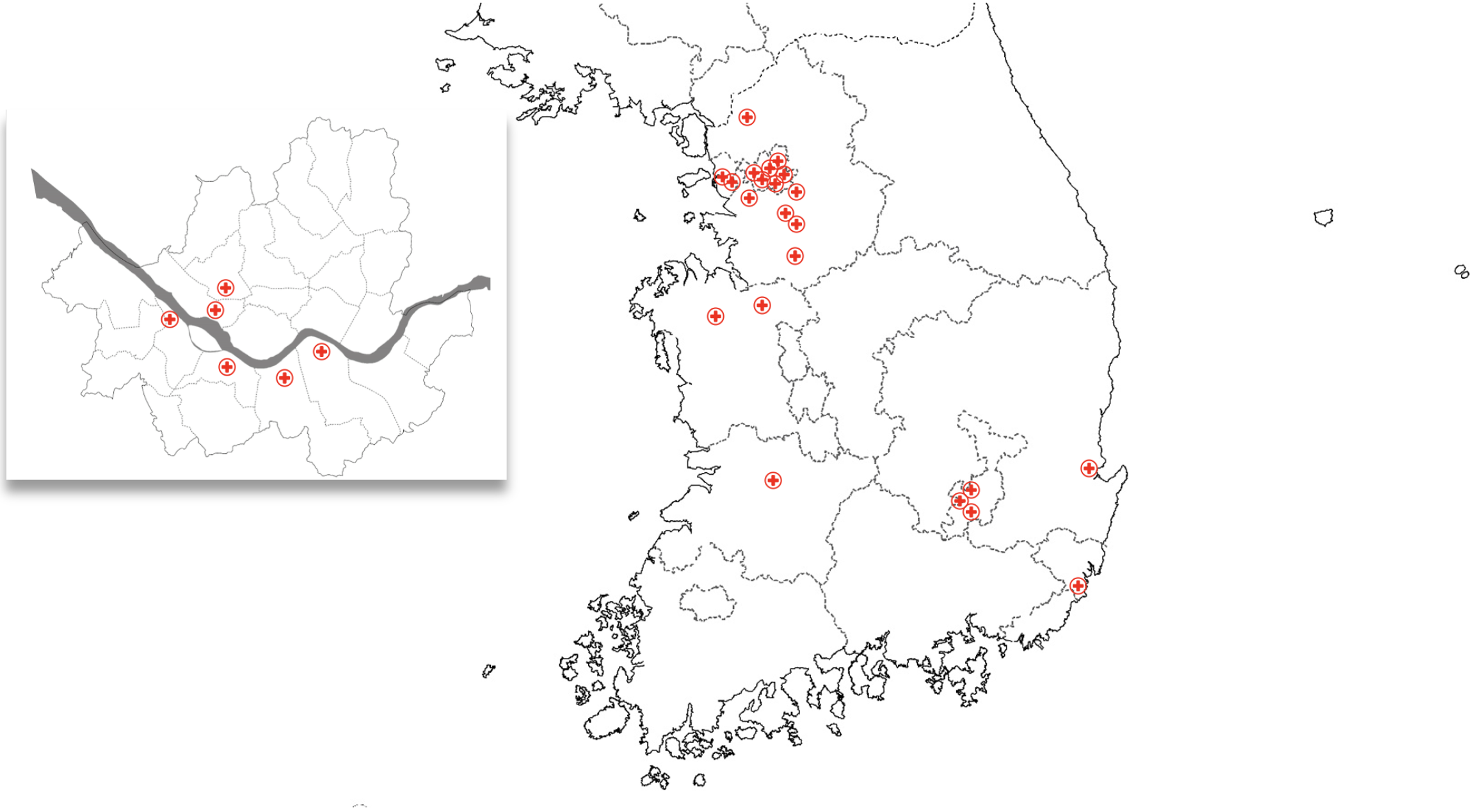
This prospective, multicenter, observational registry targeted patients with SCD and MCI who visited medical institutions in South Korea for TKM treatment. The patient recruitment will take place at the following 22 medical institutions: Daemyung Korean medicine clinic (Yeongdeungpo-gu, Seoul), Seonyujae Korean medicine clinic (Yongin-si, Gyeonggi-do), Mapo Hongik Korean medicine clinic (Mapo-gu, Seoul), Chamjouen Korean medicine clinic (Yongin-si, Gyeonggi-do), Jeongwoori Korean medicine clinic (Dongjak-gu, Seoul), Jayang Korean medicine hospital (Yeonsu-gu, Incheon), Unjeong Seum Korean medicine clinic (Paju-si, Gyeonggi-do), Bareun Kyung Hee Korean medicine clinic (Seocho-gu, Seoul), Bonecure Korean medicine clinic (Gangnam-gu, Seoul), Jaemin Korean medicine clinic (Dong-gu, Busan), Daejung Korean medicine clinic (Pyeongtaek-si, Gyeonggi-do), Cheongidam Korean medicine clinic (Seongnam-si, Gyeonggi-do), Daon Korean medicine clinic (Cheonan-si, Chungcheongnam-do), Yesan Kyung Hee Korean medicine clinic (Yesan-gun, Chungcheongnam-do), Korea-Su Medical Clinic (Bucheon-si, Gyeonggi-do), Haenamu Korean medicine clinic (Seodaemun-gu, Seoul), 365 Okgil Korean medicine clinic (Bucheon-si, Gyeonggi-do), Kimhakdong Korean medicine clinic (Pohang-si, Gyeongsangbuk-do), Ona Korean medicine clinic - Wolbae Wolseong branch (Dalseo-gu, Daegu), Ona Korean medicine clinic - Kumho branch (Buk-gu, Daegu), Ona Korean medicine clinic - Seojae Secheon branch (Dalseong-gun, Daegu), and Bonsuho Korean medicine clinic (Jeonju-si, Jeonbuk-do; Figure 2). The study will be conducted from October 14, 2024, the date of Institutional Review Board (IRB) approval, until December 31, 2026.
The study will include patients with SCD or MCI who express a willingness to participate after reviewing the recruitment notices posted on the websites or bulletin boards of each participating institution. Participants will be informed that there will be no additional financial burden related to the study, treatment, or assessments, and that all safety matters will be thoroughly explained. Written informed consent will be obtained from participants who voluntarily agree to participate in the study; only those who provide this written consent will be registered, and a screening evaluation will be conducted to assess whether they satisfy or violate the inclusion and exclusion criteria, respectively.
This study is part of a research project funded by the National Institute for Korean Medicine Development. The data collected may be linked and integrated with national public institution databases for future research; therefore, the participants will be asked to provide separate consent for third-party data access and secondary use of the data. However, patients can still participate in the registry study even if they decline consent to these additional data uses.
Inclusion criteria: Aged 55–85 years (both men and women). A diagnosis of MCI according to the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5) criteria (or a subjective report of cognitive decline for those who do not meet the criteria for MCI). MCI is evidenced by a mild decline in one or more cognitive domains compared with previous levels. This decline may be based on concerns raised by the patient, knowledgeable informants, or clinicians. Additionally, it must be quantitatively supported by a score of < 23 on the Korean version of the MoCA. Cognitive decline should not interfere with an individual’s ability to perform independent daily activities, nor should it occur solely in the context of delirium, or be explained by another mental disorder. Clearly understanding the study and voluntarily expressing intent to participate by signing an informed consent form. Seeking TKM treatments (acupuncture, pharmacopuncture, herbal medicine, etc.), including acupotomy, for cognitive decline.
Exclusion criteria: A diagnosis of any major neurocognitive disorder according to the DSM-5 criteria. Being illiterate, blind, deaf, or having severe speech disorders that make testing more difficult. Cannot understand Korean. Being deemed unsuitable for participation in this clinical study at the discretion of the researcher.
Because this registry study is an epidemiological investigation conducted without a predefined statistical hypothesis, the sample size was determined by considering the number of patients who can realistically be enrolled at each participating institution, and the covariates needed for the regression analysis. Across 22 institutions, 1670 patients with cognitive impairment receive TKM treatment annually; it is estimated that up to 60% of these patients will agree to participate in the study. Based on this, approximately 500 patient data points are expected to be collected over 6 months. Additionally, the linear regression analysis planned for this study will include 25 covariates: Sex, age, geographic region, smoking history, alcohol consumption, physical activity status, family history, education level, past occupation, current occupation, marital status, presence of depressive symptoms, insurance coverage type, hypertension, diabetes, dyslipidemia, stroke, cardiovascular disease, depression, insomnia, cognitive impairment treatment status, and baseline scores on the MoCA-Korean (MoCA-K), short form of Korean-Every Day Cognition (K-ECog-12), cognitive decline Numeric Rating Scale (NRS), Korean version of the Insomnia Severity Index (ISI-K). Following the commonly cited empirical guideline of requiring about 20 subjects per covariate[26,27], a total of around 500 participants are needed to include all 25 covariates. Therefore, based on this calculation, the study plans to recruit a total of 500 participants across the 22 institutions for 6 months following IRB approval.
Data collection: Only patients who consent to participate in the study will be registered, and their health status will be assessed at 3-month intervals for 1 year from the time of registration. The baseline and comprehensive data collection variables include the following: Personal information, sociodemographic information, medication history, height and weight, vital signs, MoCA-K, K-ECog-12, NRS, ISI-K, details of the TKM treatments administered, and adverse events. Data will initially be collected using Moaform (https://www.moaform.com/), and later transferred into an electronic case report form (eCRF) built into the myTrial system (https://www.mytrial.co.kr), which is structured to match the same items collected via Moaform. The online database allows for information modification, updates, and real-time export when necessary for quality control and statistical analysis. Once permission has been granted for public use, the data can be accessed through an online database. The data collection schedule is shown in Table 1.
| Study period | ||||||
| Screening | Post-screening | |||||
| Timepoint (month ± day) | To (0) | T1 (0 + 14)6 | T2 (3 ± 30) | T3 (6 ± 30) | T4 (9 ± 30) | T5 (12 ± 30) |
| Screening | ||||||
| Informed consent | X | |||||
| Inclusion/exclusion criteria | X | |||||
| Assign participant number | X | |||||
| Personal information1 | X | |||||
| Sociodemographic information2 | X | |||||
| Medication history3 | X | |||||
| Height and weight | X | |||||
| Vital signs4 | X | |||||
| Assessments | ||||||
| MoCA-K | X | X | X | X | X | |
| K-ECog-12 | X | X | X | X | X | |
| NRS | X | X | X | X | X | |
| ISI-K | X | X | X | X | X | |
| Korean medicine treatment5 | X | X | X | X | ||
| Changes in medical and medication history | X | X | X | X | ||
| Adverse events | X | X | X | X | ||
Screening and enrollment phase: During the screening phase, the following information and test items will be collected: Personal information, primary reason for visiting the Korean medicine clinic, vital signs, height and weight, sociodemographic information [smoking history, alcohol consumption, physical activity, family medical history, education, past and current occupations, marital status, presence of depressive symptoms (Patient Health Questionnaire-2), and type of insurance], and medication history. Patients who meet the inclusion criteria will be enrolled in the study. Once enrolled, the participants will complete clinical assessments using the MoCA-K, K-ECog-12, NRS, and ISI-K questionnaires.
Treatment phase: Patients will visit the clinic at their convenience and receive routine care and treatment at the discretion of the attending physician. Three months after screening, assessments will be conducted to evaluate changes in vital signs, medical history, medication use, and the patient's clinical condition. This will be performed using the MoCA-K, K-ECog-12, NRS, and ISI-K questionnaires. Additionally, details of TKM treatments administered for cognitive improvement over the past 3 months will be recorded, and any adverse events will be monitored. Adverse events will be assessed during regular evaluations, as well as at each routine visit, with immediate reporting if they occur. These assessments will be repeated every 3 months until the patient’s treatment is concluded.
End of treatment: Given the nature of MCI and SCD, in which symptoms may fluctuate and persist over long periods, the final treatment endpoint varies among patients. The study will follow patients for up to 1 year; however, treatment may be concluded before the 1-year mark based on the patient’s condition and physician’s judgment.
Data quality control: To enhance the quality of data collection, real-time online meetings and video training sessions were provided to the researchers, offering guidance on data collection methods and standard operating procedures for completing the eCRFs. Continuous communication will be maintained using a real-time online chat platform, and all documents related to clinical trial participants will be stored on encrypted computers and will be accessible only to authorized researchers. Korean medical doctors at each site will collect patient data, initially entering them into Moaform. Once the clinical research coordinator reviews and verifies the data, they will be finalized and entered into the eCRF system for storage.
Since this study is a prospective observational registry without a placebo control group and only involves routine medical care and survey assessments without additional interventions or tests, it is considered to pose minimal risk to the participants. Consequently, establishing a monitoring committee is unnecessary; however, if a serious adverse event is suspected to be related to the treatment, the principal investigator will report it to the public IRB within 24 hours and suspend the study. If the study is deemed to pose a risk to the safety and well-being of the participants, it will be terminated early.
Descriptive analyses will be conducted to assess demographic information and baseline characteristics. Categorical variables will be presented as frequencies and percentages, while continuous variables will be expressed as mean ± SD. To evaluate efficacy, the primary measures will include the MoCA-K, K-ECog-12, NRS, and ISI-K scores. Depending on normality, paired t-tests (or Wilcoxon signed rank tests) will be conducted for within-group (pre-post) comparisons. Subgroup comparisons-such as high- vs low-dose acupotomy, SCD vs MCI, and whether a patient is on medication for cognitive impairment-will be made using independent t-tests (or Wilcoxon rank-sum tests). Categorical variables will be analyzed using McNemar’s test for within-group comparisons, and χ2 tests (or Fisher’s exact tests) for comparisons between two or more groups. In this study, all patients who undergo screening and receive at least one post-screening procedure will be included in the analysis. Accordingly, any missing values will be handled using the Last Observation Carried Forward method. For safety assessments, adverse and serious adverse events will be presented as frequencies and percentages, and differences in incidence rates between treatment interventions will be analyzed using χ2 tests (or Fisher’s exact tests). Factors influencing treatment efficacy will be explored using linear regression for continuous dependent variables, and logistic regression for binary dependent variables.
This study aimed to establish a registry of patients with MCI or SCD who visit primary care institutions for TKM treatment. The registry will collect data on patient characteristics and TKM treatment processes, enabling the evaluation of clinical response rates and identification of treatment responders. In this study, patients will receive routine TKM treatments-including acupotomy, acupuncture, herbal medicine, moxibustion, cupping, Chuna therapy, and pharmacopuncture-as deemed appropriate by the attending physicians. Treatments may be modified or discontinued based on clinical judgment, and no additional interventions will be introduced specifically for the study. TKM treatments and assessment tools will follow the cognitive impairment protocol established by the Korean Medical Society of Acupotomology, and will be conducted in institutions with certified TKM practitioners who are society members, ensuring alignment with routine clinical practice. The participants are not restricted from receiving standard medical treatments or medications; however, changes in medical history and medication use will be checked and recorded at each 3-month follow-up. Additionally, if a sudden decline in cognitive function is detected during regular cognitive assessments conducted every 3 months, participants will be advised to schedule an additional hospital visit.
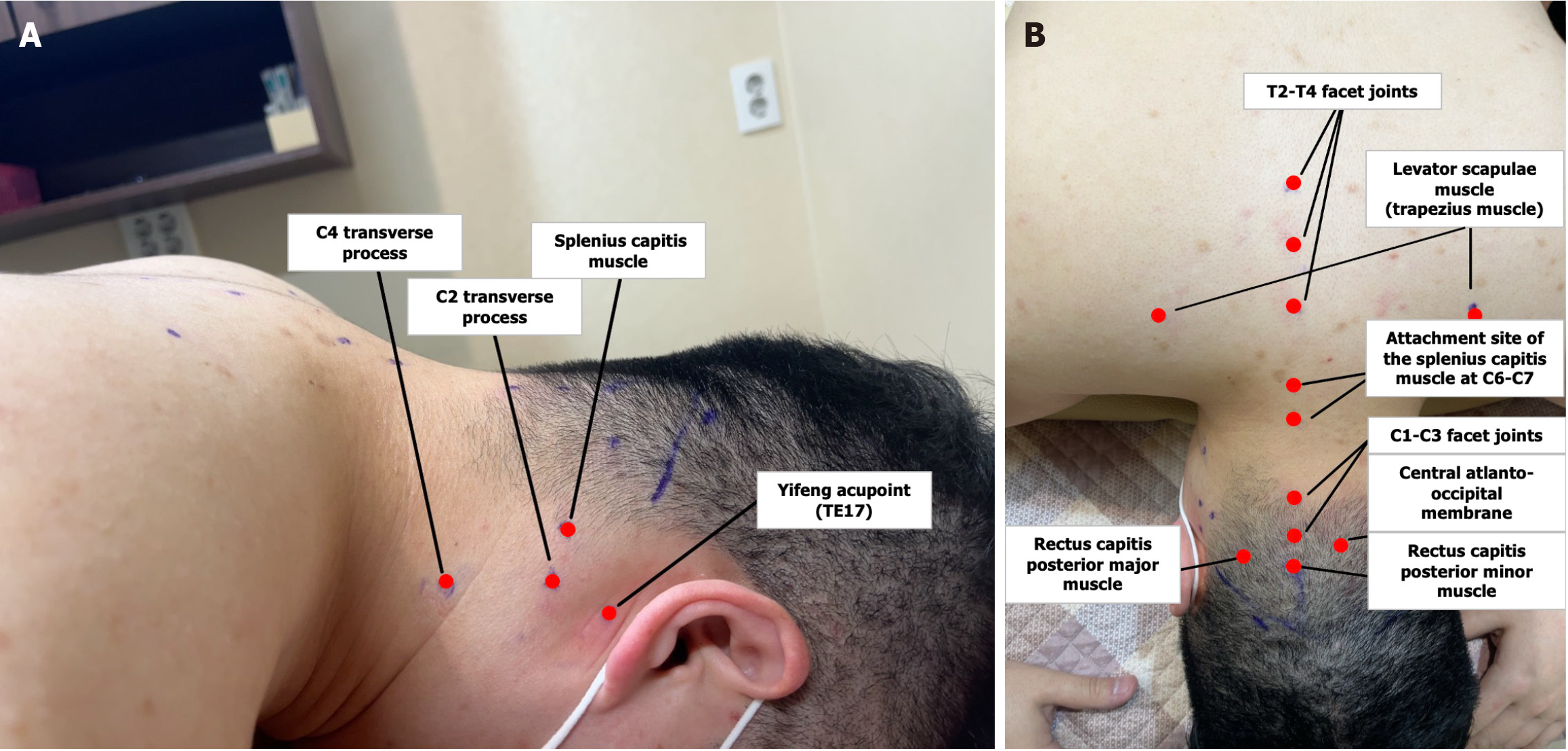
The treatment processes of acupuncture and acupotomy, which are typically applied to patients with cognitive decline, are as follows. Additional treatments-such as herbal medicine, Chuna therapy, and moxibustion-will be freely administered at the discretion of the attending physician. At each visit, patients will receive either acupotomy or acupuncture at the following points: (1) The C2 and C4 transverse process; (2) Yifeng (TE17); (3) The splenius capitis; (4) The rectus capitis posterior minor; (5) The rectus capitis posterior major; (6) Three myofascial points around the atlantoaxial region (the central myofascial area and C1–C3 facet joints); (7) The attachment of the splenius capitis at C6–C7; (8) The T2–T4 facet joints; (9) The levator scapulae (trapezius); and (10) The exit points of the emissary veins (Figure 3). These treatments are generally administered at least once per week; however, because acupotomy exerts a stronger stimulus than acupuncture, its frequency will be restricted to no more than once per week.
Acupotomy treatment process: (1) Mark the treatment points on the patient’s body using a medical marker while the patient is in the prone position; (2) Apply povidone to the treatment points, followed by disinfection with alcohol; (3) Perform acupotomy by inserting and withdrawing a 0.40 mm × 40 mm acupotomy needle at the treatment points. The specific techniques for each point are as follows: The splenius capitis, rectus capitis posterior minor, and rectus capitis posterior major are needled at their origin, ensuring that the acupotomy tip contacts the cranial bone. The levator scapulae is targeted at both its origin and insertion, allowing the acupotomy tip to contact the C2 transverse process and the superior angle of the scapula. The Yifeng (TE17) point is needled vertically from the skin surface until the acupotomy tip reaches the styloid process. For the central region of the atlantoaxial membrane, insert the acupotomy tip between the C1 and C2 spinous processes to a depth of approximately 3–4 cm, incising the membrane while taking care not to penetrate deeper layers. To access the C1–C3 and T2–T4 facet joints, insert the acupotomy tip horizontally 1–1.5 cm lateral to the spinous process tip, and incise the joint capsule when the acupotomy tip contacts the facet joint. The emissary vein exit point is treated by identifying a tender spot near the superior nuchal line or in the vicinity of Sishencong (EX-HN1), then performing an oblique incision beneath the scalp; (4) Apply cupping to all treatment points to facilitate blood drainage and bleeding monitoring, then remove the cups once the process is complete; (5) After treatment, reapply povidone to the treated areas and disinfect again with alcohol; and (6) To prevent infection, apply circular bandages to all treatment points.
Acupuncture treatment process: (1) While the patient is in the prone position, apply a hot pack wrapped in a towel heated to 80 °C to the cervical region for approximately 10 min as part of transdermal myofascial thermotherapy; (2) For acupuncture treatment of the three myofascial points in the atlantoaxial region, cervical and thoracic facet joints, and C4 transverse process, insert 0.30 mm × 40 mm needles; for other points, use 0.25 mm × 30 mm needles; (3) Attach one cupping cup to each side of the C1–C3 facet joints and perform wet cupping therapy for approximately 1 minutes; (4) Administer electroacupuncture by applying a 100 Hz electric stimulus to the needles inserted into the atlantoaxial joints for 10–15 minutes; and (5) After needle removal, apply interferential current therapy to the cervical region for 15 minutes to complete the treatment.
Questionnaires: (1) MoCA-K. The MoCA-K was developed to detect MCI in individuals with a normal MMSE score[28]. The Korean version, developed by Lee et al[29], will be used. The MoCA-K assesses short-term memory, visuospatial abilities, executive functions, attention-working memory, language, and orientation, with a total possible score of 30. The test takes approximately 10 min to administer, and a score of ≥ 23 is considered normal[30]. The MoCA-K is not recommended for individuals with reading or writing difficulties; (2) K-ECog-12. The ECog questionnaire was originally developed by Farias et al[31] to assess cognitive-related daily functioning; Song et al[32] developed a Korean version and validated its reliability. Recently, a 12-item short form tailored to the older Korean population was created[33]. The K-ECog-12 evaluates everyday abilities in areas such as memory, language, visuospatial and perceptual abilities, and executive function (planning, organizing, and multitasking). Responses were rated on a 5-point scale: (a) No change or an improvement from 10 years ago; (b) Sometimes worse than 10 years ago; (c) Slightly worse than 10 years ago in almost all cases; (d) Much worse than 10 years ago in almost all cases; and (e) Unknown (or are not applicable). In distinguishing individuals with MCI from cognitively normal controls, the optimal cut-off score was reported as 1.33, with a sensitivity of 72.7% and specificity of 67.4%; (3) NRS. The severity of cognitive decline symptoms, as subjectively perceived by patients, will be assessed; and (4) ISI-K. There is a significant correlation between sleep disorders and cognitive impairment[34–36]. In this study, the sleep status of patients reporting cognitive decline will be assessed to explore the relationship between cognitive impairment and sleep, and to evaluate their response to treatment. The ISI-K, adapted and validated by Cho et al[37] from the original tool developed by Bastien and Morin[38], assesses the severity of insomnia symptoms (difficulty falling asleep, maintaining sleep, and early waking), satisfaction with sleep, the impact of insomnia on daily functioning, and the level of concern caused by sleep difficulties. It consists of seven items scored on a 0–4 Likert scale (0 = none, 4 = very severe), with a total score ranging from 0 to 28. Higher scores indicate more severe insomnia. A score of ≥ 8 indicated insomnia, and scores of ≥ 15 reflected clinical levels of insomnia.
Korean medicine treatment: As part of the routine clinical process, TKM treatments will be administered, and treatment details will be recorded in the electronic medical record system within the clinic. Information on TKM treatments will be reported at 3-month intervals through the Moaform and eCRF. These reports will include details about the TKM interventions provided to the patient, number of treatments, herbal prescriptions, and duration of herbal medicine intake over the past 3 months.
Adverse events: During each clinical visit, adverse events will be monitored using patient reports and clinician inquiries. If the researcher determines that the patient has experienced clinically significant symptoms or changes, or if the patient voluntarily reports them, the occurrence, evaluation, and actions taken for the adverse event will be documented using standardized medical terminology in Moaform and eCRF. If no adverse events are observed, the phrase "no adverse effects since the last assessment" will be recorded in the electronic medical record system. In the event of adverse events following acupotomy, the acupotomy-related adverse event CHECKlist (ACUPOCHECK) will be used for reporting. ACUPOCHECK was developed by the research team in 2018 through a Delphi study[39], and was later confirmed for practical use by 73 Korean medicine practitioners who regularly perform acupotomies. The checklist collects data on the date of the adverse event and treatment session, occurrence of serious adverse events, time of onset, outcome after the event, severity and causality assessment, and the type of local or systemic adverse events. In this multicenter study, the use of a standardized checklist, such as ACUPOCHECK, allowed for the systematic collection of adverse event data following acupotomy. Clinical trial liability insurance was secured to minimize potential harm to the participants, and the study participants were eligible for compensation according to the insurance policy.
Participation in the study is voluntary, and participants may withdraw their consent at any time during the study without any penalty. If a participant withdraws consent or drops out, the researcher will document the reason for dropping out, and the collected data may still be used in the study. However, if the participant does not agree to the use of their data, the data will be excluded from the study.
Reasons for withdrawal or dropout include: The participant withdraws consent during the study. The participant no longer meets the inclusion criteria, or violates the exclusion criteria. An adverse or serious adverse event makes it difficult for the participant to continue in the clinical trial. The researcher determines that it is inappropriate to continue the study for any other reason.
This study was approved by the public IRB designated by the Ministry of Health and Welfare (P01-202410-01-022). Before obtaining consent, the researchers will fully explain the study’s objectives and procedures to potential participants using an information sheet and consent form. After the participants voluntarily provide written consent, a copy of the consent form will be given to them; the original will be securely stored at each recruitment site in a locked location, accessible only to authorized personnel. All research materials containing participants’ personal information will be accessed only by the principal investigator and coinvestigators. These materials will be stored for at least 3 years after the conclusion of the study. After this period, electronic files will be permanently deleted, and paper documents will be shredded for permanent disposal. Information provided to the National Institute for Korean Medicine Development through myTrial will be stored for 10 years after study conclusion. If any modifications are made to the protocol approved by the Public IRB, relevant changes will be reported, and reapproval will be obtained.
The researchers are currently in the data collection phase. Since this paper is a protocol, no results are provided.
This is a prospective multicenter observational registry study aimed at exploring the characteristics and clinical processes of patients with MCI or SCD who visited primary care institutions for TKM treatment. Although 94.4% of patients seeking TKM treatment visit Korean medicine clinics[6], most clinical research has been hospital-centered. Specifically, all studies related to TKM treatment for cognitive impairment have been conducted in Korean medicine hospitals[24,25,40], with no clinical studies conducted in Korean medicine clinics. Therefore, this registry study will identify demographic characteristics of patients visiting primary care clinics and provide insights into the clinical processes, with an aim of improving cognitive function.
According to the Agency for Healthcare Research and Quality, a patient registry is a structured system that uses observational study methods to collect consistent data and evaluate specific outcomes in a population defined by a particular disease, condition, or exposure. A key feature of this approach is that data are naturally collected, and treatment is determined through decisions jointly made by healthcare providers and patients, rather than being dictated by the registry protocol[41].
Registries can serve various purposes, such as disease registries for patients with specific conditions[42], product registries for patients using specific drugs or devices[43], and health service registries for patients receiving medical services, such as hospitalization or outpatient care[41]. Although some registries are designed for a single purpose, others have multiple objectives. Large-scale registries are typically conducted at the national level; however, professional societies may also establish registries to improve treatment quality and support public reporting[44]. This study, led by the Korean Medical Society of Acupotomology (https://www.acupotomy.kr/)-a leading society in Korea that utilizes acupotomy-aimed to establish a registry for patients with cognitive impairment receiving acupotomy and other TKM treatments. Using this registry, this study will evaluate the clinical processes employed to improve cognitive function, and explore their effectiveness and potential improvements in treatment practices.
This study has significance in confirming the safety of acupotomy applied to specific areas, such as the cervical spine, shoulders, and head. Previous studies conducted by the research team, including a literature review[45] and prospective observational study in Korean medicine clinics[46], have shown that while mild-to-moderate adverse events are relatively common, most resolve naturally when treatment is discontinued. However, according to the personal experiences of Korean medicine doctors and survey reports on adverse events following acupotomy[47], adverse events tend to be reported more frequently in the head, neck, and shoulder areas than in other parts of the body. To date, no study has analyzed the frequency or factors influencing acupotomy-related adverse events in specific regions of the body, such as the cervical spine and head. Therefore, this study aimed to gather foundational data regarding the safety of acupotomy in these areas.
This study is also significant because it is the first registry to focus on observing and collecting the clinical data of patients with MCI and SCD undergoing TKM treatments, including acupotomy, for cognitive improvement. Additionally, it involves 22 multicenter sites, enabling the collection of data at a national level. However, this study has several limitations; first, although long-term follow-up is generally recommended given the nature of cognitive impairment, this study was limited to a 1-year observation period. This decision was made through internal discussions, considering that patients with cognitive impairment who seek TKM treatments typically do not continue complementary and alternative therapy for an extended period. Nonetheless, to address the limitations of this shorter follow-up period, the research team collected patient names, date of birth, and sex as linkage keys. In the future, these data will be linked with databases from national public institutions-such as the National Health Insurance Service-allowing for the long-term monitoring of clinical outcomes, including dementia conversion rates, medication use, and mortality. Second, as this is a registry study without a control group or randomization, it is difficult to establish a causal relationship when evaluating the efficacy of TKM treatments. Since this is the first clinical study to examine the effect of TKM care (including acupotomy) on patients with MCI and SCD, it was designed as a registry study without a control group to capture real-world clinical data. In the future, based on the results of this study, the researchers plan to design a clinical trial with a control group to evaluate the treatment effects more rigorously. Third, as the study followed a unique treatment protocol developed by the Korean Medical Society of Acupotomology, the protocol may not represent the practices of Korean medicine doctors who are not affiliated with the society. Furthermore, the characteristics of patients with MCI or SCD visiting the participating clinics may not be representative of all patients visiting Korean medicine clinics nationwide. Fourth, a key characteristic of registry studies is their collection of real-world clinical data without imposing additional restrictions on patients. Similarly, in this study, only information on whether patients with cognitive impairment receive pharmacotherapy-and, if so, which medications are used-will be collected to capture the actual clinical status of patients visiting Korean medicine clinics. No artificial control or restriction will be placed on standard treatments or medications that may serve as potential confounders; instead, they will simply be documented. Accordingly, in the statistical analysis, a subgroup analysis will be conducted based on whether standard treatment is provided, and regression analyses will include potential confounders-such as sex, age, and physical activity-as covariates to explore factors influencing the treatment outcomes.
Basic data on the clinical course, safety, and characteristics of acupotomy treatments for cognitive impairment will be collected through the study period and will serve as a foundation for future prospective controlled clinical trials. The results will also inform modifications to cognitive impairment treatment processes within the acupotomy Society. Consequently, this study can be utilized as a foundation for improving clinical treatment processes and developing subsequent controlled clinical trial protocols.
This study is the first clinical investigation to register patients with MCI or SCD seeking TKM treatment that includes acupotomy across 22 medical institutions. It aims to assess patient characteristics, the clinical course of cognitive function, and both the effectiveness and safety of TKM interventions. For efficacy evaluations, the MoCA-K, K-ECog-12, NRS, and ISI-K will be administered every 3 months over the course of 1 year, and adverse events will be monitored for safety. All treatments will follow routine TKM practices, with no additional interventions introduced specifically for the study. This research is crucial for establishing evidence of cognitive improvement achieved through multimodal Korean medicine therapy in patients with MCI and SCD.
The authors would like to thank the Korean Medical Society of Acupotomology for its invaluable support and guidance, as well as all the participating clinicians for their dedicated contributions to this study.
| 1. | United Nations, Department of Economic and Social Affairs. World Population Prospects, 2024. Available from: https://population.un.org/wpp/downloads?folder=Probabilistic%20Projections&group=Population. |
| 2. | Rouzet D, Sánchez AC, Renault T, Roehn O. Fiscal challenges and inclusive growth in ageing societies. OECD Economic Policy Papers. 2019;. [RCA] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 3. | World Health Organization. Healthy ageing and functional ability. 2020. Available from: https://www.who.int/news-room/questions-and-answers/item/healthy-ageing-and-functional-ability. |
| 4. | Cosco TD, Howse K, Brayne C. Healthy ageing, resilience and wellbeing. Epidemiol Psychiatr Sci. 2017;26:579-583. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 125] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 5. | Rudnicka E, Napierała P, Podfigurna A, Męczekalski B, Smolarczyk R, Grymowicz M. The World Health Organization (WHO) approach to healthy ageing. Maturitas. 2020;139:6-11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 118] [Cited by in RCA: 834] [Article Influence: 139.0] [Reference Citation Analysis (0)] |
| 6. | Ministry of Health and Welfare. Announcement of the Results of the 2022 Survey on the Utilization of Traditional Korean Medicine. Available from: https://www.mohw.go.kr/board.es?mid=a10503000000&bid=0027&act=view&list_no=375634. |
| 7. | National Health Insurance Service. Status of Healthcare Facilities by Province, 2022. Available from: https://kosis.kr/statHtml/statHtml.do?orgId=350&tblId=TX_35003_A002&conn_path=I2. |
| 8. | Volicer L. Fear of Dementia. J Am Med Dir Assoc. 2016;17:875-878. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 9. | World Health Organization. Dementia. Available from: https://www.who.int/news-room/fact-sheets/detail/dementia. |
| 10. | Sanford AM. Mild Cognitive Impairment. Clin Geriatr Med. 2017;33:325-337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 274] [Article Influence: 30.4] [Reference Citation Analysis (0)] |
| 11. | Jessen F, Amariglio RE, van Boxtel M, Breteler M, Ceccaldi M, Chételat G, Dubois B, Dufouil C, Ellis KA, van der Flier WM, Glodzik L, van Harten AC, de Leon MJ, McHugh P, Mielke MM, Molinuevo JL, Mosconi L, Osorio RS, Perrotin A, Petersen RC, Rabin LA, Rami L, Reisberg B, Rentz DM, Sachdev PS, de la Sayette V, Saykin AJ, Scheltens P, Shulman MB, Slavin MJ, Sperling RA, Stewart R, Uspenskaya O, Vellas B, Visser PJ, Wagner M; Subjective Cognitive Decline Initiative (SCD-I) Working Group. A conceptual framework for research on subjective cognitive decline in preclinical Alzheimer's disease. Alzheimers Dement. 2014;10:844-852. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1323] [Cited by in RCA: 2228] [Article Influence: 185.7] [Reference Citation Analysis (0)] |
| 12. | Mitchell AJ, Beaumont H, Ferguson D, Yadegarfar M, Stubbs B. Risk of dementia and mild cognitive impairment in older people with subjective memory complaints: meta-analysis. Acta Psychiatr Scand. 2014;130:439-451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 583] [Cited by in RCA: 853] [Article Influence: 71.1] [Reference Citation Analysis (0)] |
| 13. | Slot RER, Sikkes SAM, Berkhof J, Brodaty H, Buckley R, Cavedo E, Dardiotis E, Guillo-Benarous F, Hampel H, Kochan NA, Lista S, Luck T, Maruff P, Molinuevo JL, Kornhuber J, Reisberg B, Riedel-Heller SG, Risacher SL, Roehr S, Sachdev PS, Scarmeas N, Scheltens P, Shulman MB, Saykin AJ, Verfaillie SCJ, Visser PJ, Vos SJB, Wagner M, Wolfsgruber S, Jessen F; Alzheimer's Disease Neuroimaging Initiative; DESCRIPA working group; INSIGHT-preAD study group; SCD-I working group, van der Flier WM. Subjective cognitive decline and rates of incident Alzheimer's disease and non-Alzheimer's disease dementia. Alzheimers Dement. 2019;15:465-476. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 142] [Cited by in RCA: 301] [Article Influence: 37.6] [Reference Citation Analysis (0)] |
| 14. | Yin Z, Li Y, Jiang C, Xia M, Chen Z, Zhang X, Zhao L, Liang F. Acupuncture for mild cognitive impairment: A systematic review with meta-analysis and trial sequential analysis. Front Neurol. 2022;13:1091125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 17] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 15. | He W, Li M, Han X, Zhang W. Acupuncture for Mild Cognitive Impairment and Dementia: An Overview of Systematic Reviews. Front Aging Neurosci. 2021;13:647629. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 25] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 16. | Wang X, Zhou H, Yan CQ, Shi GX, Zhou P, Huo JW, Yang JW, Zhang YN, Wang L, Cao Y, Liu CZ. Cognitive and Hippocampal Changes in Older Adults With Subjective Cognitive Decline After Acupuncture Intervention. Am J Geriatr Psychiatry. 2024;32:1014-1027. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 17. | Wang W, Diwu Y, Liu Q, Zhou Y, Sayed TI, Wang D, Gou Y. Chinese herbal medicine for mild cognitive impairment using mini-mental state examination: A systematic review and meta-analysis. Medicine (Baltimore). 2021;100:e27034. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 18. | Dong L, Hyde AJ, Zhang AL, Xue CC, May BH. Chinese Herbal Medicine for Mild Cognitive Impairment Using Montreal Cognitive Assessment: A Systematic Review. J Altern Complement Med. 2019;25:578-592. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 19. | Jun H, Yoon SH, Ryu M, Chae H, Chu H, Leem J, Kim TH. Acupotomy in Korean Medicine Doctors: A Preliminary Survey on Experiences, Perceptions, and Clinical Usage Status. Healthcare (Basel). 2023;11:2577. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 20. | Kwon CY, Yoon SH, Lee B. Clinical effectiveness and safety of acupotomy: An overview of systematic reviews. Complement Ther Clin Pract. 2019;36:142-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 30] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 21. | Koo W, Lee J, Ryu M. Case Report of Patient with Mild Cognitive Impairment Symptoms Improved with RSNA. J Korean Med Soc Acupotomology. 2023;7:97-102. [DOI] [Full Text] |
| 22. | Simon MJ, Iliff JJ. Regulation of cerebrospinal fluid (CSF) flow in neurodegenerative, neurovascular and neuroinflammatory disease. Biochim Biophys Acta. 2016;1862:442-451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 244] [Article Influence: 22.2] [Reference Citation Analysis (0)] |
| 23. | Chae H, Lee J, Ryu M. Treatment Approaches for Cognitive Disorders Associated with CSF Circulation. J Korean Med Soc Soft Tissue. 2022;6:89-95. [DOI] [Full Text] |
| 24. | Choi Y, Kim YE, Jerng UM, Kim H, Lee SI, Kim GN, Cho SH, Kang HW, Jung IC, Han K, Lee JH. Korean Traditional Medicine in Treating Patients with Mild Cognitive Impairment: A Multicenter Prospective Observational Case Series. Evid Based Complement Alternat Med. 2020;2020:4323989. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 25. | Lee HY, Kang HW, Kim N, Hyun EH, Seo JH, Lyu YS, Jung IC, Kim GW, Park B, Choi SY, Kim HW, Kim HM. Effectiveness of collaborative treatment using Korean and Western medicine for mild cognitive impairment or dementia: A protocol for a prospective observational exploratory study. Medicine (Baltimore). 2018;97:e12098. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 26. | Tabachnick B, Fidell L. Using Multivariate Statistics. 7th ed. Pearson Deutschland, 2019. |
| 27. | Memon MA, Ting H, Cheah J, Thurasamy R, Chuah F, Cham TH. Sample Size for Survey Research: Review and Recommendations. J Appl Struct Equ Model. 2020;4:i-xx. [RCA] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 69] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 28. | Nasreddine ZS, Phillips NA, Bédirian V, Charbonneau S, Whitehead V, Collin I, Cummings JL, Chertkow H. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53:695-699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11622] [Cited by in RCA: 17230] [Article Influence: 820.5] [Reference Citation Analysis (0)] |
| 29. | Lee JY, Dong Woo Lee, Cho SJ, Na DL, Hong Jin Jeon, Kim SK, You Ra Lee, Youn JH, Kwon M, Lee JH, Maeng Je Cho. Brief screening for mild cognitive impairment in elderly outpatient clinic: validation of the Korean version of the Montreal Cognitive Assessment. J Geriatr Psychiatry Neurol. 2008;21:104-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 325] [Cited by in RCA: 367] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 30. | Carson N, Leach L, Murphy KJ. A re-examination of Montreal Cognitive Assessment (MoCA) cutoff scores. Int J Geriatr Psychiatry. 2018;33:379-388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 361] [Cited by in RCA: 642] [Article Influence: 80.3] [Reference Citation Analysis (0)] |
| 31. | Farias ST, Mungas D, Reed BR, Cahn-Weiner D, Jagust W, Baynes K, Decarli C. The measurement of everyday cognition (ECog): scale development and psychometric properties. Neuropsychology. 2008;22:531-544. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 469] [Cited by in RCA: 459] [Article Influence: 25.5] [Reference Citation Analysis (0)] |
| 32. | Song M, Lee SH, Jahng S, Kim SY, Kang Y. Validation of the Korean-Everyday Cognition (K-ECog). J Korean Med Sci. 2019;34:e67. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 33. | Song M, Seo DG, Kim SY, Kang Y. Validation of the Short Form of Korean-Everyday Cognition (K-ECog). J Korean Med Sci. 2023;38:e370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 34. | Fernandez-Mendoza J, He F, Puzino K, Amatrudo G, Calhoun S, Liao D, Vgontzas AN, Bixler E. Insomnia with objective short sleep duration is associated with cognitive impairment: a first look at cardiometabolic contributors to brain health. Sleep. 2021;44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 42] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 35. | Brownlow JA, Miller KE, Gehrman PR. Insomnia and Cognitive Performance. Sleep Med Clin. 2020;15:71-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 135] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 36. | Fortier-Brochu E, Morin CM. Cognitive impairment in individuals with insomnia: clinical significance and correlates. Sleep. 2014;37:1787-1798. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 176] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 37. | Cho YW, Song ML, Morin CM. Validation of a Korean version of the insomnia severity index. J Clin Neurol. 2014;10:210-215. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 230] [Cited by in RCA: 319] [Article Influence: 26.6] [Reference Citation Analysis (0)] |
| 38. | Morin CM, Belleville G, Bélanger L, Ivers H. The Insomnia Severity Index: psychometric indicators to detect insomnia cases and evaluate treatment response. Sleep. 2011;34:601-608. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1955] [Cited by in RCA: 3320] [Article Influence: 221.3] [Reference Citation Analysis (0)] |
| 39. | Jun H, Lee H, Yoon SH, Kwon CY, Jeon D, Lee JH, Leem J. Delphi study for developing a checklist of adverse events associated with acupotomy. J Integr Med. 2024;22:579-587. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 40. | Choi Y, Kim AR, Lee JY, Kim HS, Yang C, Kim JK, Go Y, Jung IC. Herbal Medicine for Patients with Cognitive Impairment: An Observational Study. Neuropsychiatr Dis Treat. 2021;17:3183-3194. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 41. | Registries for Evaluating Patient Outcomes: A User's Guide [Internet]. Rockville (MD): Agency for Healthcare Research and Quality (US), 2014 . [PubMed] |
| 42. | Reynolds GO, Manning L, Kirn D, Klein H, Hampton O, Burke O Jr, Buckley R, Rentz D, Sperling R, Marshall GA, Amariglio RE. Subjective Cognitive Decline in a Registry Sample: Relation to Psychiatric History, Loneliness, and Personality. J Prev Alzheimers Dis. 2022;9:435-440. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 43. | Bisdas T, Bohan P, Lescan M, Zeebregts CJ, Tessarek J, van Herwaarden J, van den Berg JC, Setacci C, Riambau V. Research methodology and practical issues relating to the conduct of a medical device registry. Clin Trials. 2019;16:490-501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 44. | Ormseth CH, Sheth KN, Saver JL, Fonarow GC, Schwamm LH. The American Heart Association's Get With the Guidelines (GWTG)-Stroke development and impact on stroke care. Stroke Vasc Neurol. 2017;2:94-105. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 118] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 45. | Yoon S, Kwon C, Leem J. Adverse events of miniscalpel-needle treatment in Korea: A systematic review. European J Integr Med. 2019;27:7-17. [RCA] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 46. | Yoon SH, Kwon CY, Jo HG, Sul JU, Lee H, Won J, Jeong SJ, Lee JH, Leem J. Safety of acupotomy in a real-world setting: A prospective pilot and feasibility study. J Integr Med. 2022;20:514-523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 47. | Jung S, Woo J, Chae H, Oh K, Choi S, Lee J, Kang K, Chu H, Ryu M. A Survey on the Complications Associated with Acupotomy in a Single Korean Medicine Clinic. Korean J Acupunct. 2020;37:253-261. [DOI] [Full Text] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/