Published online Sep 6, 2025. doi: 10.12998/wjcc.v13.i25.107471
Revised: April 22, 2025
Accepted: May 24, 2025
Published online: September 6, 2025
Processing time: 105 Days and 1.7 Hours
Thyroid cartilage noncontiguous metastatic involvement is an extremely rare entity due to the absence of vessels within the cartilaginous tissue. A literature review revealed that metastasis from lung cancer is even rarer.
A 51-year-old male smoker presented with progressive, painful left upper neck lumps for 8 months, accompanied by 10 kg weight loss, poor appetite, and mentation. He had a 30-year history of chronic alcohol use. Following comprehensive imaging studies and pathological examinations, we have identified metastatic lung adenocarcinoma with widespread dissemination to multiple sites including the thyroid cartilage. The final diagnosis is: Lung adenocarcinoma (pT3N3M1c, Stage IVb), epidermal growth factor receptor-mutated (p.E746_S752
Monitor for uncommon thyroid cartilage noncontiguous metastases; early detection improves outcomes.
Core Tip: This rare case documents thyroid cartilage metastasis from an epidermal growth factor receptor (EGFR)-mutated lung adenocarcinoma (p.E746_S752delinsV) in a 51-year-old smoker. Despite the avascular nature of cartilaginous tissue, the patient presented with neck masses, weight loss, and widespread metastases (pT3N3M1c, stage IVB). First-line gefitinib achieved remarkable regression of cervical, abdominal subcutaneous, and oropharyngeal lesions at 6 months. Key highlights: (1) Thyroid cartilage metastasis, though exceptionally rare, warrants vigilance in lung cancer; (2) EGFR targeting remains effective even in unusual metastatic patterns; and (3) Multidisciplinary management optimizes outcomes. Early molecular testing is critical for tailored therapy.
- Citation: Ai MM, Lin T, Guo RY, Zhang YY, Yu F. Unexpected metastasis of thyroid cartilage involvement from lung adenocarcinoma: A case report. World J Clin Cases 2025; 13(25): 107471
- URL: https://www.wjgnet.com/2307-8960/full/v13/i25/107471.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v13.i25.107471
The thyroid cartilage is seldom involved in metastatic processes because its cartilaginous structure lacks vascular supply, preventing hematogenous dissemination. Previous reports have described cases of cartilaginous involvement in a melanoma[1], advanced stage carcinoma prostate[2,3], breast[4], but only a few reports of thyroid cartilage metastatic lesions from the lungs have been published[5]. Lung adenocarcinoma (LUAD), which accounts for the majority of non-small cell lung cancer (NSCLC) cases, often presents with metastatic tumors at diagnosis, contributing to its unfavorable 5-year survival outcomes. Although LUAD can metastasize to any part of the body, the nervous and respiratory systems, bone, liver, and adrenal glands are the most common sites for LC metastasis[6]; however, metastasis to the head and neck region is less prevalent as compared to the other sites. Laryngeal metastases usually remain unnoticed and may or may not present with clinical symptoms. Herein, we report a rare case of thyroid cartilage metastasis from a LUAD. We hope that our report will add to the existing literature on this subject.
A 51-year-old Chinese male presented with neck lumps, accompanied by mild pain, that gradually increased in size over the left side of the upper neck region for 8 months.
The patient presented with neck lumps, and multiple subcutaneous masses on his abdominal wall and back. He began experiencing hemoptysis and fatigue for the last month, which was not relieved with conservative treatment. He had no other symptoms, such as choking while drinking, aspiration, difficulty breathing, dysphagia, or hoarseness; however, he had poor mentation and appetite, with a weight loss of 10 kg over the past 6 months.
The patient’s medical history was unremarkable, with no recorded chronic conditions, surgical procedures, or significant traumatic injuries.
The patient has a 30-year history of smoking and alcohol use. No familial predisposition to neoplastic or heritable conditions potentially associated with the patient's clinical manifestations was identified.
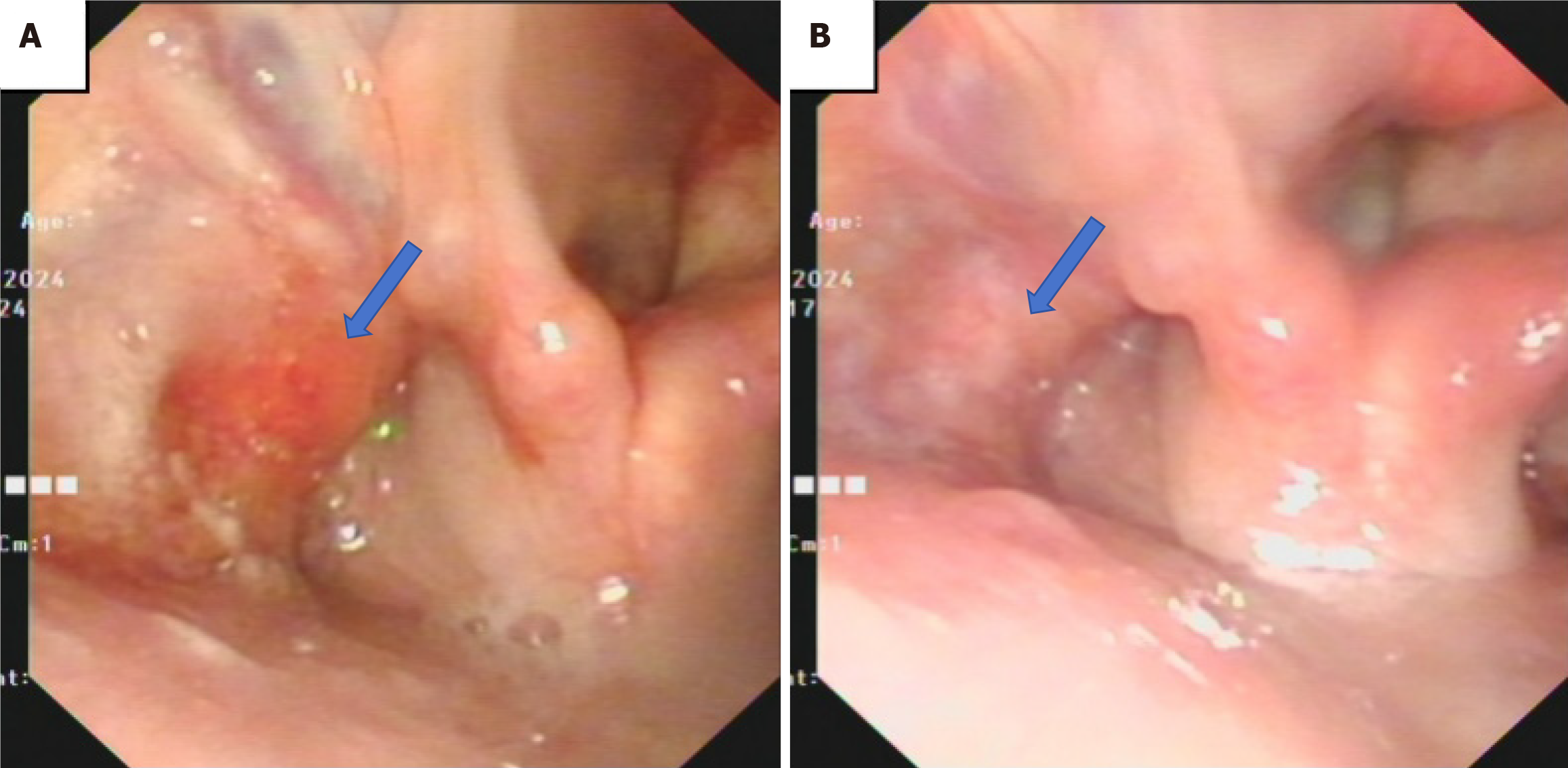
Physical examination revealed multiple enlarged lymph nodes in the left neck regions II and IV, which were approximately 1 cm in diameter, firm in texture, and had limited mobility. Multiple firm, immobile masses were palpable in the chest, abdomen, back, and head, with the largest mass measuring approximately 4 cm in diameter. Laryngoscopy revealed blood-stained secretions in the left piriform sinus, with narrowing of the sinus and disrupted vascular patterns on the lateral wall under the NBI mode. The right piriform sinus and vocal cords showed no abnormalities (Figure 1A). A biopsy was performed concurrently, revealing a squamous epithelial covering with scattered lymphocytes in the stroma, but no definitive tumor was observed. Further imaging studies performed: Enhanced computed tomography (CT), magnetic resonance imaging, and positron emission tomography-CT (PET-CT), along with ultrasound-guided fine needle aspiration biopsy of the oropharyngeal and abdominal masses.
The Laboratory tests reveal hypoalbuminemia (26.8 g/L), severely elevated D-dimer (39640 µg/L), mild hypertriglyceridemia (1.99 mmol/L), and low high-density lipoprotein (0.66 mmol/L). Multiple tumor markers are significantly elevated, including carcinoembryonic antigen (11.30 µg/L), CA 125 (57.0 U/mL), CA 15-3 (915.0 U/mL), CA 72-4 (178.00 U/mL), and CYFRA 21-1 (13.7 ng/mL) (Table 1). No significant abnormalities were detected in other tests.
| Test name | Value (Unit) | Reference range |
| albumin | 26.8 g/L (↓) | 34.0–53.0 |
| D-dimer | 39640 µg/L (↑) | 0–550 |
| triglyceride | 1.99 mmol/L (↑) | 0.50–1.90 |
| high-density lipoprotein | 0.66 mmol/L (↓) | 1.10–2.00 |
| carcinoembryonic antigen | 11.30 µg/L (↑) | < 10 |
| carbohydrate antigen 125 | 57.0 U/mL (↑) | < 35 |
| carbohydrate antigen 15-3 | 915.0 U/mL (↑) | < 30 |
| carbohydrate antigen 72-4 | 178.00 U/mL (↑) | < 6.9 |
| CYFRA 21-1 | 13.7 ng/mL (↑) | < 3.3 |
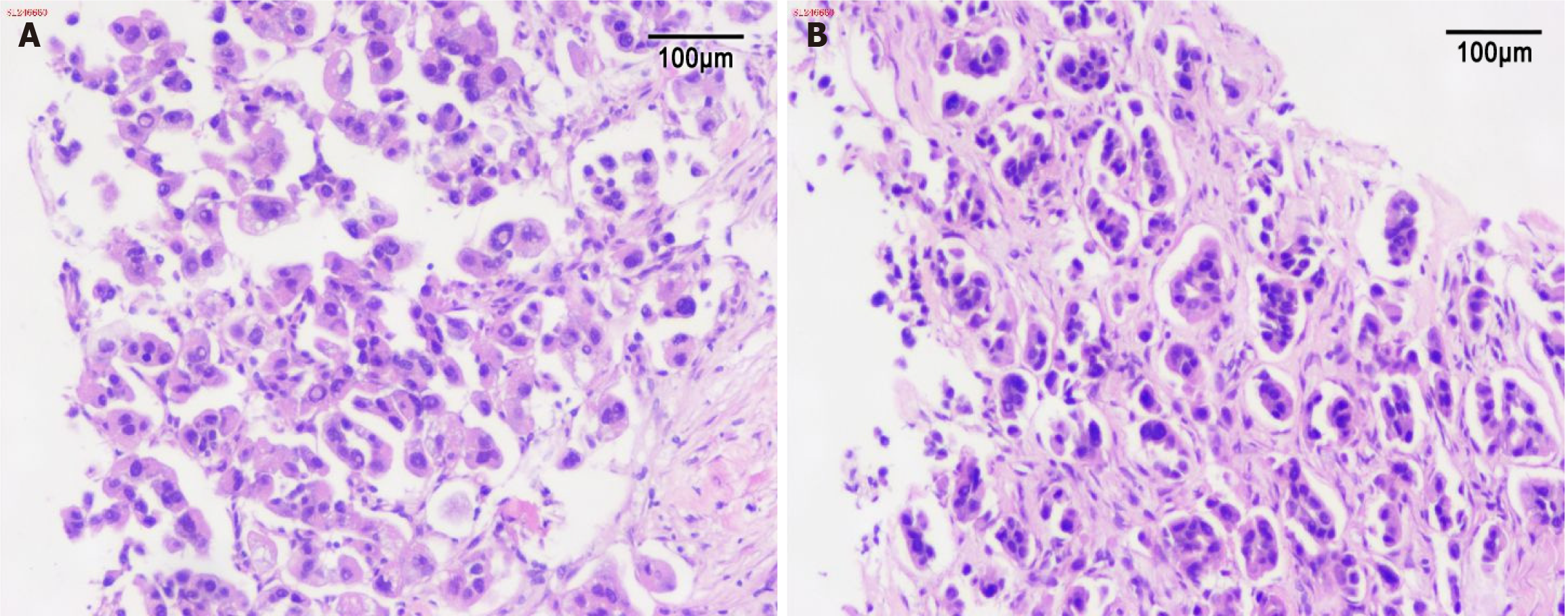
The pathology report showed the following results (Figure 2): Oropharyngeal biopsy revealed fibrous tissues with scattered atypical cells in a nest-like arrangement, with irregularly enlarged nuclei and abundant cytoplasm, consistent with metastatic LUAD; abdominal wall biopsy showed proliferative fibrous tissues with infiltrative growth of atypical cells arranged in glandular or nest-like patterns, with irregularly enlarged nuclei and abundant cytoplasm, which was also consistent with metastatic LUAD.
Programmed death-ligand 1 (PD-L1) expression was negative (tumor proportion score, TPS < 1%). LC next-generation sequencing panel testing identified one somatic mutation, including one with clear or potential clinical significance (EGFR p. E746-S752 delinsV). No hereditary risk-related mutations were detected, and no level II targeted drug mutations were found.
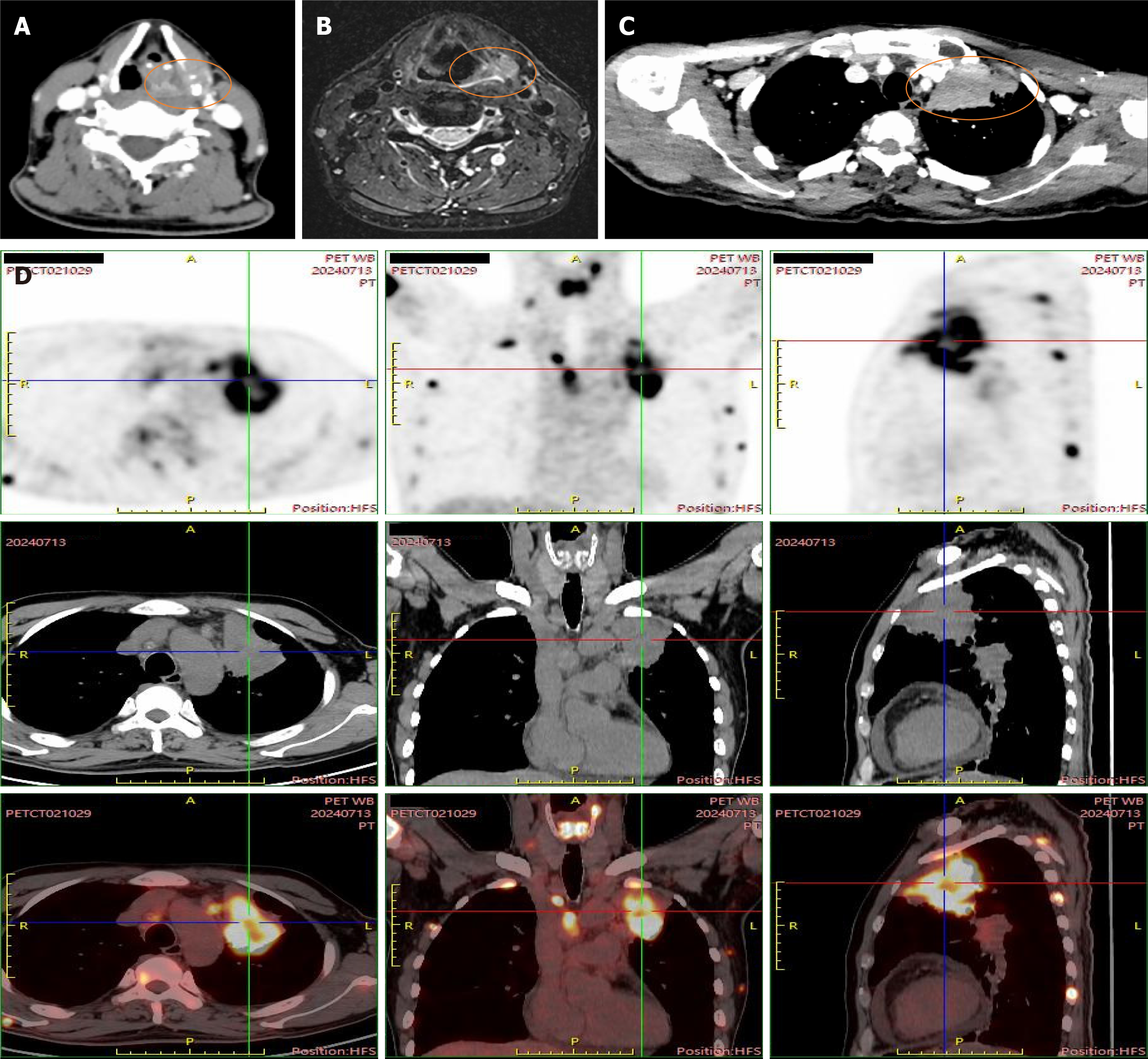
The imaging studies showed the following findings (Figure 3): Peripheral-type LC in the left upper lobe with adjacent pleural invasion; multiple metastatic tumors in both lungs; multiple lymph node metastases in the bilateral supraclavicular, mediastinal, left hilum, and neck regions II and IV; bilateral adrenal metastases; multiple bone metastases; dispersed soft tissue nodules in the chest wall; slightly hyperdense masses and nodules in the left temporal and right parietal lobes, with increased metabolism, all suggesting metastasis; and nodules in the left piriform sinus with bone destruction of the thyroid cartilage.
After multidisciplinary discussion involving thoracic surgery, oncology, radiation therapy, pulmonology, and neurosurgery, the patient was diagnosed with widely metastatic advanced LUAD. Targeted therapy was initiated as the primary treatment.
The final diagnosis was LUAD (pT3N3M1c, IVb), with characteristic EGFR exon 19 p.E746_S752delinsV mutation.
The patient was treated with oral gefitinib (0.25 g, once a day) for targeted therapy.
At the 6-month of follow-up, the patient reported a reduction in the oropharyngeal mass (Figure 1B), with considerable shrinkage of the abdominal, back, and scalp masses, along with notable improvement in mental status and appetite.
The thyroid cartilage's avascular structure makes metastatic infiltration a rare phenomenon. We describe an uncommon case of thyroid cartilage metastasis in a 51-year-old man with LUAD. The patient's significantly elevated tumor markers
Potential explanations may lie within the hematogenous or lymphatic routes of distant cancer metastases. Hyaline cartilage is known to undergo bony metaplasia and ossification during the aging process and, as the bone has a better vascular supply than the cartilage, Ehrlich[10] hypothesized that this phenomenon could explain the development of a distant metastasis through the hematogenous route. Laryngeal cartilage metastasis starts by micro-metastases within the hematopoietic tissue of the ossified laryngeal parts. Then, they merge to form visible focal tumor nodules, and further growth causes destruction of the laminas. Subsequently, tumor extension is seen into the perilaryngeal soft tissue. Although he is a relatively young man, the tumor progression may have led to increased blood supply and subsequent exacerbation of ossification. Additionally, this patient has a markedly elevated D-dimer level, it primarily result from tumor-associated hypercoagulability (via tissue factor overexpression and fibrinolysis activation) and may serve as a prognostic biomarker for venous thromboembolism risk and disease progression[11].
Secondary laryngeal tumors account for 0.09%–0.4% of all laryngeal tumors[8]. Distal tumors rarely metastasize to the larynx because they are terminally located in the lympho-vascular circulation. The difference in the symptoms between secondary laryngeal and primary tumors is the presence of hemoptysis. Rossini et al[12] conceivable that it is a standard indicator of laryngeal metastasis because these tumors have a highly vascular stroma. As seen in the present case, despite the fact that hemoptysis may be associated with the primary LC, it explains why blood-stained secretions are seen attached to the lateral wall of the piriform sinus, while the other areas lack such secretions.
Evidence from Prescher et al[13] suggests that the thyroid cartilage involvement is suggestive of a poor prognosis as it indicates the presence of extensive metastases. Laryngeal metastases usually remain unnoticed and may or may not present with clinical symptoms. Consequently, these metastases are rarely detected at early stages, typically manifesting only when advanced disease is established, thereby portending an unfavorable prognosis. The same was seen in our case; our patient had no thyroid cartilage involvement symptoms even with the presence of a relatively large mass in the piriform sinus. Additionally, tumors metastasizing to the laryngeal regions are often highly aggressive.
The patient was diagnosed with LUAD exhibiting metastases to the brain, adrenal gland, neck, and widespread bone, classified as unresectable advanced metastatic NSCLC. Epidemiological data indicate that median overall survival (OS) of 18-24 months for patients with unresectable locally advanced or metastatic NSCLC, reflecting the aggressive nature of advanced disease. However, Shi et al[14] proposed that patients with NSCLC harboring sensitizing epidermal growth factor receptor (EGFR) gene mutations demonstrate significantly prolonged median OS compared to wild-type patients (34.7 vs 18.1 months, P < 0.001), largely explained by conferred by EGFR tyrosine kinase inhibitors (EGFR-TKIs) therapy[15].
Shi et al[16] demonstrated that EGFR mutations associated with NSCLC are predominantly located in exons 18–21, with the most common classic sensitizing mutations being exon 19 deletions (EGFR 19del) and the exon 21 L858R point mutation (EGFR L858R). In this case, the patient carried a p.E746_S752delinsV non-frameshift deletion mutation in exon 19. Previous studies have shown that, in treatment-naïve advanced NSCLC patients with sensitizing EGFR mutations, compared to platinum-doublet chemotherapy, first-/second-generation EGFR-TKIs show significantly higher objective response rate and longer median progression-free survival in first-line setting[17-19]. Therefore, this patient was treated with targeted therapy as the first-line approach. As a first-generation EGFR-TKI, gefitinib demonstrates high efficacy against EGFR-sensitive mutations (Ex19del/L858R), offers convenient once-daily oral administration, and exhibits a favorable safety profile with lower incidence of severe adverse events. Its cost-effectiveness and inclusion in national insurance reimbursement, gefitinib was selected as the first-line treatment for this patient.
Immune checkpoint inhibitors, specifically programmed death-1/PD-L1 monoclonal antibodies, have revolutionized the treatment paradigm for advanced NSCLC, advancing from second-line to standard first-line therapy for certain patients. Consequently, PD-L1 testing was performed for this patient. Unfortunately, his TPS was < 1%, rendering immunotherapy unsuitable in this case.
In our case, despite the presence of multiple metastases throughout the body, gene testing revealed an EGFR mutation, which aligns with the NCCN 2024 version 4 guidelines for advanced or metastatic NSCLC[20]. The patient received a targeted therapy with gefitinib. After > 6 months of follow-up, he had significant symptom improvement, with a notable reduction in the size of the abdominal wall, back, neck, and scalp masses, leading to an enhanced quality of life.
Although distant metastasis of tumors to the larynx is rare, and even rarer to the thyroid cartilage, it remains possible that micro-metastases could occur through hematogenous routes within hematopoietic tissues during the cartilage ossification process, leading to distant spread, and mainly suggestive of poor prognosis. In the diagnosis and treatment of malignant tumors, it is essential to conduct a thorough history-taking, comprehensive physical examination, and supportive investigations to avoid any potential oversights.
| 1. | Martín-Jiménez DI, Palacios-García J, Sánchez-Gómez S. Metastatic malignant melanoma of the larynx. Acta Otorrinolaringol Esp (Engl Ed). 2023;74:198-199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 2. | Tohfe M, Baki SA, Saliba W, Ghandour F, Ashou R, Ghazal G, Bahous J, Chamseddine N. Metastatic prostate adenocarcinoma presenting with pulmonary symptoms: a case report and review of the literature. Cases J. 2008;1:316. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 3. | Tupalli A, Damle NA, Thankarajan AS, Mangu BS, Kumar A, Khan D, Sagar S, Bal C. An Unusual Case of Simultaneous Cricoid and Thyroid Cartilage Metastases from Prostatic Adenocarcinoma on (68)Ga-PSMA PET/CT. Nucl Med Mol Imaging. 2020;54:61-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 4. | Usmani S, Ahmed N, Ilyas MW, Murad S, Al Kandari F. Rare Thyroid Cartilage Metastasis From Breast Cancer Visualized on 18F-NaF PET/CT. Clin Nucl Med. 2021;46:43-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 5. | Ogata H, Ebihara S, Mukai K, Mashima K, Saikawa M, Asai M, Noguchi M, Matsuno Y. Laryngeal Metastasis from a Pulmonary Papillary Adenocarcinoma: A Case Report. Japanese J Clin Oncol. 1993;23: 199-203. [RCA] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 6. | Riihimäki M, Hemminki A, Fallah M, Thomsen H, Sundquist K, Sundquist J, Hemminki K. Metastatic sites and survival in lung cancer. Lung Cancer. 2014;86:78-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 623] [Cited by in RCA: 643] [Article Influence: 53.6] [Reference Citation Analysis (0)] |
| 7. | Timon CI, Gullane PJ, Brown D, Van Nostrand AW. Hyoid bone involvement by squamous cell carcinoma: clinical and pathological features. Laryngoscope. 1992;102:515-520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 22] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 8. | Haddad G, Sataloff RT, Hamdan AL. Laryngeal Metastatic Lesions: A Literature Review. J Voice. 2024;38:1458-1464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 9. | Verma S, Singh MM, Deswal S, Kakkar L. A Rare Case of Thyroid Cartilage Metastases Detected on 18F-Fluorodeoxyglucose Positron Emission Tomography/Computed Tomography. Indian J Nucl Med. 2023;38:172-173. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 10. | EHRLICH A. Tumor involving the laryngeal cartilages. AMA Arch Otolaryngol. 1954;59:178-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 17] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 11. | Khorana AA, Mackman N, Falanga A, Pabinger I, Noble S, Ageno W, Moik F, Lee AYY. Cancer-associated venous thromboembolism. Nat Rev Dis Primers. 2022;8:11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 270] [Article Influence: 67.5] [Reference Citation Analysis (0)] |
| 12. | Rossini M, Bolzoni A, Piazza C, Peretti G. Renal cell carcinoma metastatic to the larynx. Otolaryngol Head Neck Surg. 2004;131:1029-1030. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 13. | Prescher A, Schick B, Stütz A, Brors D. Laryngeal prostatic cancer metastases: an underestimated route of metastases? Laryngoscope. 2002;112:1467-1473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 15] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 14. | Shi Y, Zhang X, Wu G, Xu J, He Y, Wang D, Huang C, Chen M, Yu P, Yu Y, Li W, Li Q, Hu X, Xia J, Bu L, Yin A, Zhou Y. Treatment strategy, overall survival and associated risk factors among patients with unresectable stage IIIB/IV non-small cell lung cancer in China (2015-2017): A multicentre prospective study. Lancet Reg Health West Pac. 2022;23:100452. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 15. | Zhong Q, Tao Y, Chen H, Zhou Y, Huang L, Han X, Shi Y. The changing landscape of anti-lung cancer drug clinical trials in mainland China from 2005 to 2020. Lancet Reg Health West Pac. 2021;11:100151. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 16. | Shi Y, Li J, Zhang S, Wang M, Yang S, Li N, Wu G, Liu W, Liao G, Cai K, Chen L, Zheng M, Yu P, Wang X, Liu Y, Guo Q, Nie L, Liu J, Han X. Molecular Epidemiology of EGFR Mutations in Asian Patients with Advanced Non-Small-Cell Lung Cancer of Adenocarcinoma Histology - Mainland China Subset Analysis of the PIONEER study. PLoS One. 2015;10:e0143515. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 125] [Cited by in RCA: 163] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 17. | Mok TS, Wu YL, Thongprasert S, Yang CH, Chu DT, Saijo N, Sunpaweravong P, Han B, Margono B, Ichinose Y, Nishiwaki Y, Ohe Y, Yang JJ, Chewaskulyong B, Jiang H, Duffield EL, Watkins CL, Armour AA, Fukuoka M. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009;361:947-957. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5906] [Cited by in RCA: 6589] [Article Influence: 387.6] [Reference Citation Analysis (21)] |
| 18. | Shi YK, Wang L, Han BH, Li W, Yu P, Liu YP, Ding CM, Song X, Ma ZY, Ren XL, Feng JF, Zhang HL, Chen GY, Han XH, Wu N, Yao C, Song Y, Zhang SC, Song W, Liu XQ, Zhao SJ, Lin YC, Ye XQ, Li K, Shu YQ, Ding LM, Tan FL, Sun Y. First-line icotinib versus cisplatin/pemetrexed plus pemetrexed maintenance therapy for patients with advanced EGFR mutation-positive lung adenocarcinoma (CONVINCE): a phase 3, open-label, randomized study. Ann Oncol. 2017;28:2443-2450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 261] [Article Influence: 29.0] [Reference Citation Analysis (0)] |
| 19. | Morgan GJ, Child JA, Gregory WM, Szubert AJ, Cocks K, Bell SE, Navarro-Coy N, Drayson MT, Owen RG, Feyler S, Ashcroft AJ, Ross FM, Byrne J, Roddie H, Rudin C, Cook G, Jackson GH, Wu P, Davies FE; National Cancer Research Institute Haematological Oncology Clinical Studies Group. Effects of zoledronic acid versus clodronic acid on skeletal morbidity in patients with newly diagnosed multiple myeloma (MRC Myeloma IX): secondary outcomes from a randomised controlled trial. Lancet Oncol. 2011;12:743-752. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 119] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 20. | Riely GJ, Wood DE, Ettinger DS, Aisner DL, Akerley W, Bauman JR, Bharat A, Bruno DS, Chang JY, Chirieac LR, DeCamp M, Desai AP, Dilling TJ, Dowell J, Durm GA, Gettinger S, Grotz TE, Gubens MA, Juloori A, Lackner RP, Lanuti M, Lin J, Loo BW, Lovly CM, Maldonado F, Massarelli E, Morgensztern D, Mullikin TC, Ng T, Owen D, Owen DH, Patel SP, Patil T, Polanco PM, Riess J, Shapiro TA, Singh AP, Stevenson J, Tam A, Tanvetyanon T, Yanagawa J, Yang SC, Yau E, Gregory KM, Hang L. Non-Small Cell Lung Cancer, Version 4.2024, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2024;22:249-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 525] [Cited by in RCA: 423] [Article Influence: 211.5] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/