Published online Sep 6, 2025. doi: 10.12998/wjcc.v13.i25.105204
Revised: March 31, 2025
Accepted: May 18, 2025
Published online: September 6, 2025
Processing time: 173 Days and 22.6 Hours
Poorly differentiated thyroid carcinoma (PDTC) is a rare and aggressive form of thyroid cancer. Distant metastasis occurs frequently in PDTC.
To determine factors associated with distant metastasis and the effects of metastasis, either diagnosed on initial presentation or developing during follow-up, on mortality in PDTC patients.
Patients with PDTC diagnosed between January 1, 1985 and July 31, 2022 were identified using a thyroid cancer database at a medical center in Taiwan. Factors associated with distant metastasis and cancer-specific survival (CSS) were analyzed using binary logistic analysis and Cox regression, respectively. Survival analysis was conducted using the Kaplan–Meier method.
The study cohort included 39 patients with PDTC, including 16 with distant metastasis on initial presentation, 5 with metastasis during the follow-up period, and 18 with no evidence of metastasis. Older age (≥ 45 years) was significantly associated with a higher risk of distant metastasis (odds ratio: 5.31; 95% confi
Older patients with PDTC have an increased risk of distant metastasis. Patients with metastatic PDTC, both diagnosed at presentation and developing during follow-up, have a dismal prognosis.
Core Tip: Poorly differentiated thyroid carcinoma (PDTC) is a rare but aggressive subtype of thyroid cancer. A significant proportion of patients with PDTC have distant metastasis. Patients with metastatic PDTC diagnosed at presentation or developing during follow-up have dismal 5-year cancer-specific survival rates of only 55.0% and 40.0%, respectively. Older age (≥ 45 years) is a risk factor for metastatic PDTC.
- Citation: Hsu CW, Hsueh C, Lu YL, Hsu CJ, Wong RJ, Lin SF. Risk factors and outcomes of metastatic poorly differentiated thyroid carcinoma. World J Clin Cases 2025; 13(25): 105204
- URL: https://www.wjgnet.com/2307-8960/full/v13/i25/105204.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v13.i25.105204
The incidence of thyroid cancer has increased over the past three decades[1]. The majority of thyroid cancers are differentiated thyroid carcinomas (DTC), including papillary thyroid carcinoma, follicular thyroid carcinoma, and oncocytic cell carcinoma. Most DTC patients have a favorable prognosis, with a 10-year survival rate of approximately 90%. Anaplastic thyroid carcinoma (ATC) is an aggressive and fatal disease, with a 2-year survival rate of only 10%–15%. Poorly differentiated thyroid carcinoma (PDTC) ranks between DTC and ATC with respect to the degree of differentiation. PDTC represents 3–5% of all thyroid cancer cases, with a 5-year survival rate of 47.0%–89.3%[2-4]. Multiple prognostic factors associated with poor survival in PDTC patients have been reported, including older age (≥ 45 years), larger tumor size (> 4 cm), extrathyroidal extension, and distant metastasis at presentation[5,6].
Distant metastases are observed at presentation in 26.0%–65.5% of PDTC patients. The lung and bone are the most common metastatic sites[5-7]. Among these patients, the 5-year cancer-specific survival (CSS) is only 34%[5]. A significant proportion of PDTC patients (22%–50%) develop metastatic disease during follow-up[6,8]. However, few studies have addressed the clinical course of patients who develop metastatic PDTC during follow-up.
This study aimed to identify factors associated with PDTC metastasis and to assess the effect of distant metastasis diagnosed upon initial presentation or during follow-up on CSS.
This study examined data from a thyroid cancer database at Chang Gung Memorial Hospital in Taiwan. The database was specifically developed to support thyroid cancer research[9]. Data were collected prospectively for all patients receiving thyroid cancer treatment at this medical center. Comprehensive records, including clinical characteristics (age, sex), laboratory results (thyroid function tests, thyroglobulin, anti-thyroglobulin antibody), imaging data [chest radiography, computed tomography (CT), magnetic resonance imaging, radioactive iodine (RAI) scan, bone scan], pathological findings, treatment regimens [surgery, RAI therapy, external beam radiation therapy (EBRT), chemotherapy, targeted therapy, immunotherapy], clinical course, and outcomes were collected and maintained.
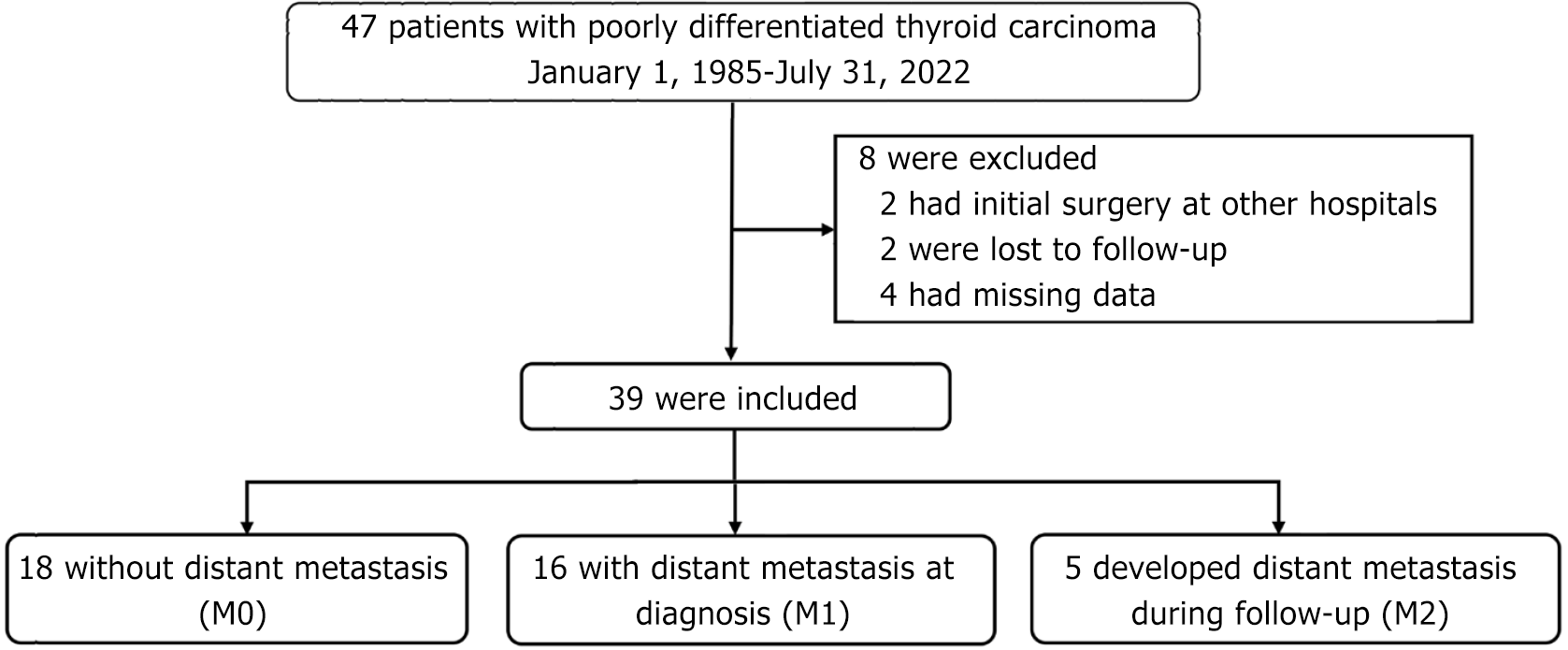
A total of 47 patients with PDTC diagnosed between January 1, 1985 and July 31, 2022 were identified in the database. The exclusion criteria included having primary surgery at another hospital, loss to follow-up, or missing data. The eligible patients were assigned to one of three groups according to the timing of distant metastasis diagnosis, as follows: M0, no evidence of metastasis during the study period; M1, metastasis identified on initial presentation; and M2, appearance of metastasis during the follow-up period.
Covariates examined in survival analysis included age, sex, tumor size, T stage, N stage, extrathyroidal extension, type of surgery, RAI treatment, and EBRT[3-6]. Tumor staging was performed in accordance with the eighth edition of the AJCC staging system[10].
Thyroidectomy was the first-line treatment for PDTC in this study. Neck lymph nodes were removed by neck dissection if metastasis was suspected. RAI was given if deemed appropriate by the treating physician. Following primary treatment, levothyroxine was prescribed for thyroid hormone supplementation or thyroid-stimulating hormone suppression. Assessments of serum thyroglobulin and antithyroglobulin antibody and cervical ultrasound were typically performed every 3-12 months during the follow-up period. Imaging assessments including chest X-ray, RAI uptake and scan, CT, and 18F-fluorodeoxyglucose positron emission tomography/CT were performed when metastasis was suspected. EBRT was used for locoregional control and palliation of symptomatic metastasis. Four patients with metastatic PDTC were treated with targeted therapy including lenvatinib, sorafenib, and cabozantinib.
Continuous variables are presented as the median [interquartile range (IQR)]. Categorical variables are presented as a number (percentage). The Kruskal-Wallis H test was used to analyze continuous variables not following a normal distribution, and the χ2 test was used to analyze categorical variables. Cox regression analysis was used to determine the hazard ratio (HR) of risk factors associated with CSS. Binary logistic regression was used to calculate the odds ratio (OR) of risk factors for distant metastasis. In multivariable analysis, only the independent variables were included for further evaluation. Survival analysis was conducted using the Kaplan–Meier method. Statistical analyses were performed using SPSS statistical software (version 22.0, SPSS Inc., IBM corp., Armonk, NY, United States). P < 0.05 was considered statistically significant.
This study was approved by the Chang Gung Medical Foundation Institutional Review Board (No. 202401356B0). The Institutional Review Board waived the requirement for obtaining informed consent. The study was performed in accordance with the principles of the Declaration of Helsinki.
A total of 47 patients with PDTC were identified in our thyroid cancer database. Eight patients were excluded, including two who underwent primary cancer surgery at other hospitals, two who were lost to follow-up, and four who had missing data. Thus, 39 PDTC patients were included in the final analysis. These patients were divided into three groups based on the presence of metastasis or not: M0, no evidence of metastasis (n = 18); M1, metastasis on initial presentation
The clinical characteristics of the study cohort are shown in Table 1. The median age at PTDC diagnosis was highest in the M2 group (58 years), followed by the M1 group (52 years) and the M0 group (41 years) (P = 0.042). The highest percentage of female patients was discovered in the M2 group (100%), followed by the M0 group (72.2%), and the M1 group (43.8%) (P = 0.045). Differences among the three cohorts in the proportion of older patients (≥ 45 years), tumor size (≤ 2 cm, > 2–4 cm, and > 4 cm), T stage, N stage, and extrathyroidal invasion did not reach statistical significance (P > 0.05). The most common site of metastasis was the lung in the M1 (56.3%) and M2 (60.0%) groups. The other metastatic organs included bone, the lung and bone, the lung and brain, and bone and the brain. The M0 group and M2 group had the largest percentage of patients who had total thyroidectomy (100%, both groups), compared to the M1 group (75%) (P = 0.041). The proportions of patients who received RAI were similar in the M0 group (61.1%), M1 group (75.0%), and M2 group (100%) (P = 0.217). The median cumulative dose of RAI was highest in the M2 group (580 mCi), followed by the M1 group (100 mCi) and the M0 group (75 mCi) (P = 0.005). The percentage of patients who had EBRT was highest in the M2 group (60.0%), followed by the M1 group (43.8%) and the M0 group (16.7%). However, these differences among the three groups were not statistically significant (P = 0.099). The median dose of EBRT was similar in all the three groups (M0 group, 5600 cGy; M1 group, 3720 cGy; and M2 group, 5000 cGy; P = 0.634). Targeted therapy was used in each group as follows: M1 group, 3 patients; M2 group, 1 patient; and M0 group, no patients. The M2 group had the highest cancer-specific mortality (100%), followed by the M1 group (62.5%) and the M0 group (5.6%) (P = 0.001). The M2 group had the worst overall mortality (100%), followed by the M1 group (75.0%) and the M0 group (11.1%) (P = 0.001). The median follow-up duration in the M0, M1, and M2 groups was 8.0 years, 2.0 years, and 4.2 years, respectively (P = 0.156).
| | M0 (n = 18) | M1 (n = 16) | M2 (n = 5) | P value |
| Age, median (years, IQR) | 41 (26-59) | 52 (49-63) | 58 (51-62) | 0.042 |
| Age | 0.060 | |||
| < 45 years | 10 (55.6) | 3 (18.8) | 1 (20.0) | |
| ≥ 45 years | 8 (44.4) | 13 (81.2) | 4 (80.0) | |
| Sex | 0.045 | |||
| Female | 13 (72.2) | 7 (43.8) | 5 (100) | |
| Male | 5 (27.8) | 9 (56.2) | 0 | |
| Tumor size (cm) | 0.222 | |||
| ≤ 2 | 3 (16.7) | 0 | 0 | |
| > 2-4 | 6 (33.3) | 6 (37.5) | 3 (75.0) | |
| > 4 | 9 (50.0) | 10 (62.5) | 1 (25.0) | |
| T stage | 0.536 | |||
| T1 | 3 (16.7) | 0 | 0 | |
| T2 | 4 (22.2) | 5 (31.2) | 1 (20.0) | |
| T3 | 5 (27.8) | 3 (18.8) | 1 (20.0) | |
| T4 | 6 (33.3) | 8 (50.0) | 3 (60.0) | |
| N stage | 0.323 | |||
| N0 | 17 (94.4) | 13 (81.2) | 5 (100) | |
| N1 | 4 (10.3) | 3 (18.8) | 0 | |
| Extrathyroidal invasion | 6 (33.3) | 8 (50.0) | 4 (80.0) | 0.166 |
| Initial metastatic sites | ||||
| Lung | 0 | 9 (56.3) | 3 (60.0) | |
| Bone | 0 | 3 (18.8) | 0 | |
| Lung, bone | 0 | 2 (12.5) | 2 (40.0) | |
| Lung, brain | 0 | 1 (6.3) | 0 | |
| Bone, brain | 0 | 1 (6.3) | 0 | |
| Type of thyroid surgery | 0.041 | |||
| Lobectomy or subtotal thyroidectomy | 0 | 4 (25.0) | 0 | |
| Total thyroidectomy | 18 (100) | 12 (75.0) | 5 (100) | |
| 131I therapy | 11 (61.1) | 12 (75.0) | 5 (100) | 0.217 |
| 131I cumulative dose, median (mCi, IQR) | 75 (15-100) | 100 (97-162) | 580 (100-835) | 0.005 |
| External beam radiation therapy | 3 (16.7) | 7 (43.8) | 3 (60.0) | 0.099 |
| External beam radiation therapy, median (cGy, IQR) | 5600 (3950-6200) | 3720 (1725-5310) | 5000 (4750-5500) | 0.634 |
| Targeted therapy | 0 | 3 (18.8) | 1 (20.0) | - |
| Lenvatinib | 0 | 1 (6.25) | 0 | |
| Lenvatinib, sorafenib | 0 | 2 (12.5) | 0 | |
| Lenvatinib, sorafenib, cabozantinib | 0 | 0 | 1 (20.0) | |
| Cancer-specific mortality | 1 (5.6) | 10 (62.5) | 5 (100) | 0.001 |
| Overall mortality | 2 (11.1) | 12 (75.0) | 5 (100) | 0.001 |
| Follow-up, median (years, IQR) | 8.0 (2.4-14.3) | 2.0 (1.4-5.2) | 4.2 (2.8-8.1) | 0.156 |
We sought to identify factors associated with cancer-specific mortality in PDTC patients (Table 2). Univariate Cox regression analysis showed that cancer-specific mortality was associated with N stage [HR: 6.86; 95% confidence interval (CI): 1.82–25.8; P = 0.004], M stage (HR: 2.96; 95%CI: 1.53–5.74; P = 0.001), and EBRT (HR: 4.42; 95%CI: 1.50–13.0; P = 0.007). Using multivariate Cox regression analysis adjusted for N stage, M stage, and EBRT, we found that N1 stage (adjusted HR: 10.1; 95%CI: 2.34–43.3; P = 0.002) and distant metastasis (adjusted HR: 2.41; 95%CI: 1.07–5.39; P = 0.033) were significantly associated with cancer-specific mortality.
| | Survival (n = 23) | Mortality (n = 16) | Univariate HR (95%CI) | P value | Multivariate HR (95%CI)1 | P value |
| Age | ||||||
| < 45 years | 11 (47.8) | 3 (18.8) | Reference | |||
| ≥ 45 years | 12 (52.2) | 13 (81.2) | 3.47 (0.95–12.6) | 0.060 | ||
| Sex | ||||||
| Female | 14 (60.9) | 11 (68.8) | Reference | |||
| Male | 9 (39.1) | 5 (31.2) | 1.58 (0.52–4.81) | 0.416 | ||
| Tumor size | ||||||
| ≤ 2 cm | 3 (13.0) | 0 | Reference | |||
| > 2-4 cm | 7 (30.4) | 8 (53.3) | ||||
| > 4 cm | 13 (56.5) | 7 (46.7) | 1.39 (0.62–3.12) | 0.423 | ||
| T stage | ||||||
| T1/T2 | 8 (34.8) | 5 (31.2) | Reference | |||
| T3/T4 | 15 (65.2) | 11 (68.8) | 1.31 (0.44–3.85) | 0.627 | ||
| N stage | ||||||
| N0 | 23 (100) | 12 (75.0) | Reference | Reference | ||
| N1 | 0 | 4 (25.0) | 6.86 (1.82–25.8) | 0.004 | 10.1 (2.34–43.3) | 0.002 |
| M stage | ||||||
| M0 | 17 (73.9) | 1 (37.5) | Reference | Reference | ||
| M1 | 6 (26.1) | 10 (62.5) | ||||
| M2 | 0 | 5 (31.2) | 2.96 (1.53–5.74) | 0.001 | 2.41 (1.07–5.39) | 0.033 |
| Extrathyroidal invasion | ||||||
| No | 14 (60.9) | 7 (43.8) | Reference | |||
| Yes | 9 (39.1) | 9 (56.2) | 1.64 (0.61–4.43) | 0.329 | ||
| Type of thyroid surgery | ||||||
| Total thyroidectomy | 21 (91.3) | 14 (87.5) | Reference | |||
| Lobectomy or subtotal thyroidectomy | 2 (4.3) | 2 (6.2) | 3.48 (0.71–17.0) | 0.124 | ||
| 131I cumulative dose | ||||||
| < 100 mCi | 14 (60.9) | 7 (43.8) | Reference | |||
| ≥ 100 mCi | 9 (39.1) | 9 (56.2) | 1.42 (0.52–3.83) | 0.493 | ||
| External beam radiation therapy | ||||||
| No | 20 (87.0) | 6 (37.5) | Reference | Reference | ||
| Yes | 3 (13.0) | 10 (62.5) | 4.42 (1.50–13.0) | 0.007 | 3.07 (0.86–11.0) | 0.084 |
Five patients developed distant metastasis during follow-up. The clinical features of these patients are shown in Table 3. All of these patients were female, aged 44–77 years. Most patients (60.0%) had T4 stage, followed by T3 stage (20.0%) and T2 stage (20.0%). No patients had metastatic neck lymph nodes at presentation. All patients (100%) received total thyroidectomy, and four patients (80%) underwent RAI treatment with a cumulative dose of 30–160 mCi before metastasis was discovered. The time interval between the discovery of distant metastasis and the diagnosis of PDTC ranged from 0.7 to 11.9 years. More distant metastases were identified by chest X-ray (40%), followed by RAI scan (20%), CT (20%), and positron emission tomography/CT (20%). The sites of metastasis included the lung (60%) and both the lung and bone (40%). Three patients (60%) received palliative EBRT (range, 4500–6000 cGy). One patient (20%) received targeted therapy (sequential lenvatinib, sorafenib, and cabozantinib treatment). All patients had RAI treatment for PDTC, including three patients (60%) with a cumulative RAI dose ≥ 580 mCi. All patients died of PDTC, with the time from the diagnosis of metastasis to death within 5.9 years (range, 0.6–5.9 years).
| Patient number | Age/Sex | TNM at diagnosis | Type of thyroid surgery | Time from PDTC diagnosis to metastasis (years) | Cumulative dose of RAI before metastasis (mCi) | Positive finding at detection | Sites of metastasis | Time from metastasis to death (years) | Cumulative dose of RAI (mCi) | External beam radiation therapy, dose (cGy) | Targeted therapy |
| 1 | 44/F | T4N0M0 | Total thyroidectomy | 11.9 | 0 | CT | Lung, bone | 5.9 | 980 | 6000 | |
| 2 | 51/F | T4N0M0 | Total thyroidectomy | 1.7 | 100 | PET/CT | Lung | 2.5 | 100 | 0 | Lenvatinib, sorafenib, cabozantinib |
| 3 | 58/F | T4N0M0 | Total thyroidectomy | 0.7 | 30 | RAI scan | Lung | 2.1 | 580 | 5000 | |
| 4 | 62/F | T2N0M0 | Total thyroidectomy | 4.9 | 160 | Chest X-ray | Lung, bone | 3.2 | 835 | 4500 | |
| 5 | 77/F | T3N0M0 | Total thyroidectomy | 0.9 | 30 | Chest X-ray | Lung | 0.6 | 60 | 0 |
In these 39 PDTC patients, 16 (41.0%) had metastasis on initial presentation and 5 (12.8%) had metastasis during follow-up. Factors associated with metastasis were assessed (Table 4). Our data demonstrate that distant metastasis was significantly associated with older age (≥ 45 years) (OR: 5.31; 95%CI: 1.27-22.2; P = 0.018), while sex, tumor size, T stage, and N stage did not correlate with distant metastasis. The lung was the most common metastatic site for PDTC, a finding consistent with a previous study[5].
| M0 (n = 18) | M1 + M2 (n = 21) | Univariate OR (95%CI) | P value | |
| Age | ||||
| < 45 years | 10 (55.6) | 4 ((19.0) | Reference | |
| ≥ 45 years | 8 (44.4) | 17 (81.0) | 5.31 (1.27–22.2) | 0.018 |
| Sex | ||||
| Female | 13 (72.2) | 12 (57.1) | Reference | |
| Male | 5 (27.8) | 9 (42.9) | 1.95 (0.51–7.49) | 0.328 |
| Tumor size | ||||
| ≤ 2 cm | 3 (16.7) | 0 | Reference | |
| > 2-4 cm | 6 (33.3) | 9 (45.0) | ||
| > 4 cm | 9 (50.0) | 11 (55.0) | 1.72 (0.62–4.83) | 0.301 |
| T stage | ||||
| T1/T2 | 7 (38.9) | 6 (28.6) | Reference | |
| T3/T4 | 11 (61.1) | 15 (71.4) | 1.59 (0.42–6.07) | 0.496 |
| N stage | ||||
| N0 | 17 (94.4) | 18 (85.7) | Reference | |
| N1 | 1 (5.6) | 3 (14.3) | 2.83 (0.27–29.9) | 0.370 |
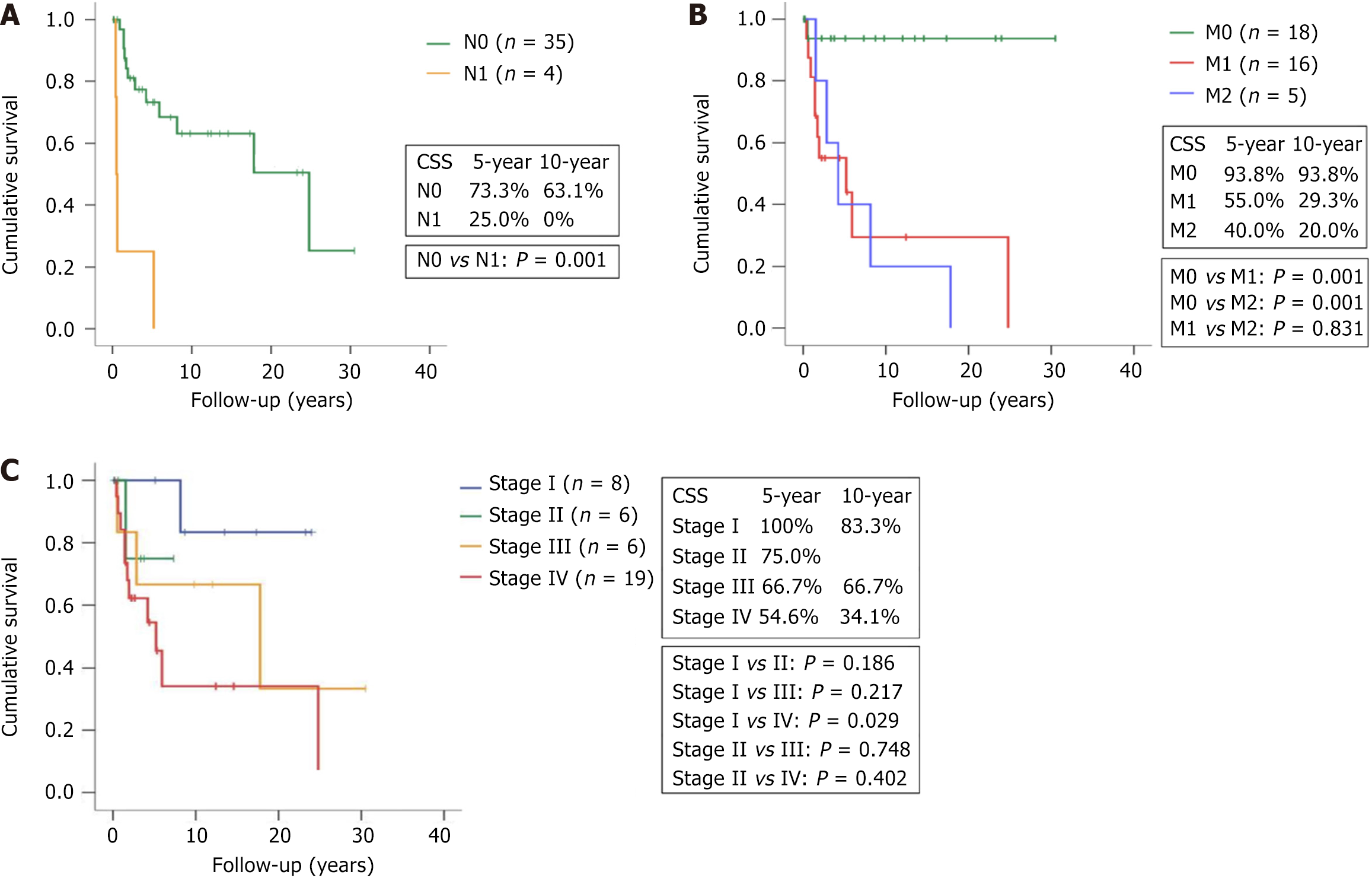
Our results show that patients who died of PDTC were more likely to have lymph node metastasis and distant metastasis. Kaplan–Meier survival analysis stratified by N stage and M stage showed that the N1 group had poorer 5-year and 10-year CSS (25.0% and 0%) than the N0 group (73.3% and 63.1%) (P = 0.001) (Figure 2A). Patients with M1 and M2 disease were more likely to die of the disease than those with M0 disease (Figure 2B). Poorer 5-year and 10-year CSS rates were seen in the M1 group (55.0% and 29.3%) and M2 group (40.0 and 20.0%), compared with the M0 group (93.8% and 93.8%) (P = 0.001 for both comparisons). CSS was similar between the M1 group and M2 group (P = 0.831).
The 8th edition of the AJCC provides the same definition of TNM for PDTC and DTC[10]. This staging system includes a stage classification to stratify the risk of mortality for patients with DTC, but that classification is not indicated for patients with PDTC. We sought to analyze the CSS of these 39 PDTC patients based on the 8th edition of the stage classification in DTC patients aged ≥ 55 years. Our data reveal that PDTC patients with stage IV disease had worse 5-year and 10-year CSS (54.6% and 34.1%) than those with stage I disease (100% and 83.3%) (P = 0.029). However, the differences in survival between the other cohorts did not reach statistical significance (P > 0.05 for 4 comparisons) (Figure 2C).
We found that PDTC patients without distant metastasis had a promising outcome, with a 5-year CSS rate of 93.8%. In contrast, the prognosis for patients with metastatic PDTC, whether diagnosed upon presentation or during follow-up, was dismal, with 5-year CSS rates of 55% and 40%, respectively. Our data are consistent with a previous report showing a 5-year CSS rate of 34% for patients with metastatic PDTC diagnosed at presentation[5]. We additionally showed that PDTC patients who developed metastasis during follow-up had similarly dismal outcomes to those who had metastasis at diagnosis.
Our study revealed that a substantial percentage of PDTC patients with M0 disease (21.7%) initially eventually developed distant metastasis. The time interval between the diagnosis of PDTC and the identification of metastasis ranged from 0.7 to 11.9 years. Most metastases (80%) occurred within 5 years after the diagnosis of PDTC in older patients (≥ 51 years), suggesting that older patients should be monitored more intensively within 5 years. The majority of patients (80%) had advanced T stage (≥ T3). However, one patient (20%) who had T2N0M0 disease initially developed lung and bone metastases 4.9 years after PDTC diagnosis and died subsequently. These data are consistent with a previous report showing that a small proportion of patients with T1/T2 disease (6%) would succumb to PDTC[5]. The most common metastatic sites in the M2 group were the lung (100.0%) and bone (40.0%), which was similar to the M1 group (lung, 75.0%; bone, 31.2%).
Previous reports have demonstrated that older age (> 45 years), T4a stage, extrathyroidal extension, tumor mitosis and necrosis, and distant metastasis at presentation were predictors of cancer-specific mortality in PDTC patients[3,5,6,7,11]. In this study, we found that metastatic PDTC, either cervical lymph node metastasis or distant metastasis, was an independent risk factor correlating with cancer-specific mortality, while age, sex, tumor size, T stage, extrathyroidal invasion, type of thyroid surgery, RAI cumulative dose, and EBRT were not. Our data indicate that metastasis, whether to lymph nodes or solid organs, is the most important prognostic factor for PDTC mortality.
In this study, a total of 53.8% patients had metastatic PDTC. The risk factor correlated with metastasis was older age. Understanding the risk factors for distant metastasis might allow clinicians to plan more intensive surveillance of patients who are at risk of metastasis. A recent study revealed that patient age was significantly associated with distant metastasis in papillary thyroid microcarcinoma patients[12].
A previous study has shown that aggressive surgery including total thyroidectomy and compartment neck dissection in PDTC patients with gross extrathyroidal extension can yield promising locoregional control, with a 5-year local recurrence–free survival and regional recurrence–free survival of 70% and 62%, respectively[6]. In this study, patients in the M2 group had undergone total thyroidectomy as the primary surgery, but all of them developed metastasis and died of PDTC eventually. These results reflect the aggressive and fatal nature of PDTC.
The majority of our patients (71.8%) received RAI therapy. We found that a higher dose of RAI (≥ 100 mCi) was not associated with better survival. A recent study showed that RAI did not improve CSS in PDTC patients[13]. The reason for the ineffectiveness of RAI in this and previous studies is unclear. One study reports that most patients with metastatic PDTC (70%) were RAI-avid[6]. The RAI uptake in metastatic PDTC may not exceed the therapeutic threshold, likely due to varying mixtures of well- and less-well–differentiated tumor components, which may account for the limited efficacy of RAI in PDTC[6,7]. In addition, resistance to RAI might be another cause for the limited efficacy of RAI for treating PDTC. Currently, RAI is recommended as an adjuvant therapy for PDTC patients with distant metastasis that is RAI-avid[6,14,15].
One-third of our patients received EBRT. We found that EBRT did not improve CSS, which is consistent with previous studies reporting that EBRT did not improve survival in PDTC patients[16,17]. EBRT is mainly used for local tumor control and palliative treatment of metastatic disease[16].
Lenvatinib, sorafenib, and cabozantinib are tyrosine kinase inhibitors that have been approved for the treatment of progressive radioiodine-refractory DTC based on the results of the SELECT, DECISION, and COSMIC-311 studies, respectively[18-20]. Although the SELECT study aimed to evaluate the effects of lenvatinib in patients with DTC, that study included 47 patients with PDTC, including 28 patients in the lenvatinib group and 19 patients in the control group. The results show that lenvatinib improved progression-free survival over that of control treatment in PDTC patients (HR: 0.21; 95%CI: 0.08–0.56)[18]. In our study, four PDTC patients received a tyrosine kinase inhibitor: Lenvatinib, n = 1; lenvatinib initially followed by sorafenib, n = 1; sorafenib initially followed by lenvatinib, n = 1; and sequential lenvatinib, sorafenib, and cabozantinib, n = 1. Disease progression was observed in the majority of the patients who underwent tyrosine kinase inhibitor treatment (Supplementary Figure 1). Novel therapies are needed to increase outcomes for patients with advanced PDTC.
Some limitations of this study deserve mentioning. First, the utility of the 8th edition of TNM staging system to stratify the risk of mortality for PDTC patients is unclear and warrants further studies with a larger sample size. Second, we sought to identify factors associated with lymph node metastasis. The results show that age, sex, tumor size, T stage, M stage, and extrathyroidal invasion did not correlate with lymph node metastasis (P > 0.05) (Supplementary Table 1). Third, we did not assess genetic alterations in these PDTC patients. High frequencies of genetic mutations have been found in PDTC, including RAS, BRAF, TERT promoter, TP53, and ATM[21-27]. A previous study showed that RAS-mutated PDTC had an elevated rate of distant metastasis, and BRAF-mutated PDTC had an elevated rate of lymph node metastasis[28]. TERT promoter mutations are usually associated with an aggressive phenotype and poor outcomes[24]. Previous studies have revealed that patients with fatal PDTC had a higher frequency of mutations in HRAS, BRAF, TERT promoter, TP53 and ATM than those with nonfatal PDTC[24,29]. Genetic testing should be performed for patients with refractory PDTC to identify potential cancer-related molecular targets, particularly since the treatment of thyroid cancer has undergone major advancements in recent years[30-34]. Selpercatinib and pralsetinib have been approved for advanced thyroid cancer with RET alterations[30,31]. Larotrectinib and entrectinib were approved for advanced thyroid cancer with NTRK fusions[32,33]. Combination treatment with dabrafenib and trametinib is indicated for solid tumors with the BRAFV600E mutation[34]. Fourth, this study included only 39 PDTC patients. The small sample size may limit the statistical power of this study. Additional studies with larger cohorts will be needed to confirm our results. Fifth, the heterogeneity in treatment approaches, including variations in surgery, RAI therapy, EBRT, and targeted therapy, may influence survival outcomes in this study.
The incidence of PDTC was 0.69% of all thyroid cancer patients in our dataset between 1985 and 2022. In Taiwan, the incidence of PDTC was < 0.48% of all thyroid cancer cases from 2001 to 2022[35]. These data indicate that PDTC is a rare disease in Taiwan. The risk factors associated with PDTC in Taiwan are unclear and need further investigation.
We observed that up to 41.0% of patients with PDTC presented with distant metastasis. In addition, 12.8% developed distant metastasis during follow-up. The survival of patients with distant metastasis, either diagnosed at presentation or during follow-up, is poor. Older age is associated with a higher risk of metastatic PDTC.
We would like to thank the Center for Big Data Analytics and Statistics at Chang Gung Memorial Hospital at Linkou, Taiwan for their assistance.
| 1. | Rossi ED, Pantanowitz L, Hornick JL. A worldwide journey of thyroid cancer incidence centred on tumour histology. Lancet Diabetes Endocrinol. 2021;9:193-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 72] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 2. | Sassolas G, Hafdi-Nejjari Z, Remontet L, Bossard N, Belot A, Berger-Dutrieux N, Decaussin-Petrucci M, Bournaud C, Peix JL, Orgiazzi J, Borson-Chazot F. Thyroid cancer: is the incidence rise abating? Eur J Endocrinol. 2009;160:71-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 37] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 3. | de la Fouchardière C, Decaussin-Petrucci M, Berthiller J, Descotes F, Lopez J, Lifante JC, Peix JL, Giraudet AL, Delahaye A, Masson S, Bournaud-Salinas C, Borson Chazot F. Predictive factors of outcome in poorly differentiated thyroid carcinomas. Eur J Cancer. 2018;92:40-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 57] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 4. | Tong J, Ruan M, Jin Y, Fu H, Cheng L, Luo Q, Liu Z, Lv Z, Chen L. Poorly differentiated thyroid carcinoma: a clinician's perspective. Eur Thyroid J. 2022;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 33] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 5. | Ibrahimpasic T, Ghossein R, Carlson DL, Nixon I, Palmer FL, Shaha AR, Patel SG, Tuttle RM, Shah JP, Ganly I. Outcomes in patients with poorly differentiated thyroid carcinoma. J Clin Endocrinol Metab. 2014;99:1245-1252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 103] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 6. | Ibrahimpasic T, Ghossein R, Carlson DL, Chernichenko N, Nixon I, Palmer FL, Lee NY, Shaha AR, Patel SG, Tuttle RM, Balm AJ, Shah JP, Ganly I. Poorly differentiated thyroid carcinoma presenting with gross extrathyroidal extension: 1986-2009 Memorial Sloan-Kettering Cancer Center experience. Thyroid. 2013;23:997-1002. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 55] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 7. | Hiltzik D, Carlson DL, Tuttle RM, Chuai S, Ishill N, Shaha A, Shah JP, Singh B, Ghossein RA. Poorly differentiated thyroid carcinomas defined on the basis of mitosis and necrosis: a clinicopathologic study of 58 patients. Cancer. 2006;106:1286-1295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 209] [Cited by in RCA: 241] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 8. | Siironen P, Hagström J, Mäenpää HO, Louhimo J, Heikkilä A, Heiskanen I, Arola J, Haglund C. Anaplastic and poorly differentiated thyroid carcinoma: therapeutic strategies and treatment outcome of 52 consecutive patients. Oncology. 2010;79:400-408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 50] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 9. | Wu MH, Lee YY, Lu YL, Lin SF. Risk Factors and Prognosis for Metastatic Follicular Thyroid Cancer. Front Endocrinol (Lausanne). 2022;13:791826. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 10. | Amin MB, Greene FL, Edge SB, Compton CC, Gershenwald JE, Brookland RK, Meyer L, Gress DM, Byrd DR, Winchester DP. The Eighth Edition AJCC Cancer Staging Manual: Continuing to build a bridge from a population-based to a more "personalized" approach to cancer staging. CA Cancer J Clin. 2017;67:93-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2341] [Cited by in RCA: 4675] [Article Influence: 519.4] [Reference Citation Analysis (4)] |
| 11. | Volante M, Landolfi S, Chiusa L, Palestini N, Motta M, Codegone A, Torchio B, Papotti MG. Poorly differentiated carcinomas of the thyroid with trabecular, insular, and solid patterns: a clinicopathologic study of 183 patients. Cancer. 2004;100:950-957. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 138] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 12. | Huang H, Xu S, Wang X, Liu S, Liu J. Patient Age Is Significantly Related to Distant Metastasis of Papillary Thyroid Microcarcinoma. Front Endocrinol (Lausanne). 2021;12:748238. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 13. | Xu L, Zou Q, Jiao J, Zhang Y. Postoperative radioiodine therapy impact on survival in poorly differentiated thyroid carcinoma: a population-based study. Nucl Med Commun. 2022;43:145-151. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 14. | Fagin JA, Wells SA Jr. Biologic and Clinical Perspectives on Thyroid Cancer. N Engl J Med. 2016;375:1054-1067. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 461] [Cited by in RCA: 679] [Article Influence: 67.9] [Reference Citation Analysis (0)] |
| 15. | Kunte S, Sharett J, Wei W, Nasr C, Prendes B, Lamarre E, Ku J, Lorenz RR, Scharpf J, Burkey BB, Shah A, Joshi N, Geiger JL. Poorly Differentiated Thyroid Carcinoma: Single Institution Series of Outcomes. Anticancer Res. 2022;42:2531-2539. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 16. | Sanders EM Jr, LiVolsi VA, Brierley J, Shin J, Randolph GW. An evidence-based review of poorly differentiated thyroid cancer. World J Surg. 2007;31:934-945. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 126] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 17. | Chao TC, Lin JD, Chen MF. Insular carcinoma: infrequent subtype of thyroid cancer with aggressive clinical course. World J Surg. 2004;28:393-396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 37] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 18. | Schlumberger M, Tahara M, Wirth LJ, Robinson B, Brose MS, Elisei R, Habra MA, Newbold K, Shah MH, Hoff AO, Gianoukakis AG, Kiyota N, Taylor MH, Kim SB, Krzyzanowska MK, Dutcus CE, de las Heras B, Zhu J, Sherman SI. Lenvatinib versus placebo in radioiodine-refractory thyroid cancer. N Engl J Med. 2015;372:621-630. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1207] [Cited by in RCA: 1418] [Article Influence: 128.9] [Reference Citation Analysis (0)] |
| 19. | Brose MS, Nutting CM, Jarzab B, Elisei R, Siena S, Bastholt L, de la Fouchardiere C, Pacini F, Paschke R, Shong YK, Sherman SI, Smit JW, Chung J, Kappeler C, Peña C, Molnár I, Schlumberger MJ; DECISION investigators. Sorafenib in radioactive iodine-refractory, locally advanced or metastatic differentiated thyroid cancer: a randomised, double-blind, phase 3 trial. Lancet. 2014;384:319-328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1260] [Cited by in RCA: 1169] [Article Influence: 97.4] [Reference Citation Analysis (1)] |
| 20. | Brose MS, Robinson B, Sherman SI, Krajewska J, Lin CC, Vaisman F, Hoff AO, Hitre E, Bowles DW, Hernando J, Faoro L, Banerjee K, Oliver JW, Keam B, Capdevila J. Cabozantinib for radioiodine-refractory differentiated thyroid cancer (COSMIC-311): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2021;22:1126-1138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 160] [Article Influence: 32.0] [Reference Citation Analysis (0)] |
| 21. | Cancer Genome Atlas Research Network. Integrated genomic characterization of papillary thyroid carcinoma. Cell. 2014;159:676-690. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1650] [Cited by in RCA: 2273] [Article Influence: 206.6] [Reference Citation Analysis (0)] |
| 22. | Nikiforov YE. Thyroid carcinoma: molecular pathways and therapeutic targets. Mod Pathol. 2008;21 Suppl 2:S37-S43. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 296] [Cited by in RCA: 295] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 23. | Nikiforova MN, Nikiforov YE. Molecular genetics of thyroid cancer: implications for diagnosis, treatment and prognosis. Expert Rev Mol Diagn. 2008;8:83-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 197] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 24. | Landa I, Ibrahimpasic T, Boucai L, Sinha R, Knauf JA, Shah RH, Dogan S, Ricarte-Filho JC, Krishnamoorthy GP, Xu B, Schultz N, Berger MF, Sander C, Taylor BS, Ghossein R, Ganly I, Fagin JA. Genomic and transcriptomic hallmarks of poorly differentiated and anaplastic thyroid cancers. J Clin Invest. 2016;126:1052-1066. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 596] [Cited by in RCA: 921] [Article Influence: 92.1] [Reference Citation Analysis (0)] |
| 25. | Nikiforov YE. Genetic alterations involved in the transition from well-differentiated to poorly differentiated and anaplastic thyroid carcinomas. Endocr Pathol. 2004;15:319-327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 147] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 26. | Hannallah J, Rose J, Guerrero MA. Comprehensive literature review: recent advances in diagnosing and managing patients with poorly differentiated thyroid carcinoma. Int J Endocrinol. 2013;2013:317487. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 34] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 27. | Sobrinho-Simões M, Máximo V, Rocha AS, Trovisco V, Castro P, Preto A, Lima J, Soares P. Intragenic mutations in thyroid cancer. Endocrinol Metab Clin North Am. 2008;37:333-362, viii. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 73] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 28. | Ibrahimpasic T, Ghossein R, Shah JP, Ganly I. Poorly Differentiated Carcinoma of the Thyroid Gland: Current Status and Future Prospects. Thyroid. 2019;29:311-321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 187] [Article Influence: 26.7] [Reference Citation Analysis (0)] |
| 29. | Lee DY, Won JK, Lee SH, Park DJ, Jung KC, Sung MW, Wu HG, Kim KH, Park YJ, Hah JH. Changes of Clinicopathologic Characteristics and Survival Outcomes of Anaplastic and Poorly Differentiated Thyroid Carcinoma. Thyroid. 2016;26:404-413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 58] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 30. | Wirth LJ, Sherman E, Robinson B, Solomon B, Kang H, Lorch J, Worden F, Brose M, Patel J, Leboulleux S, Godbert Y, Barlesi F, Morris JC, Owonikoko TK, Tan DSW, Gautschi O, Weiss J, de la Fouchardière C, Burkard ME, Laskin J, Taylor MH, Kroiss M, Medioni J, Goldman JW, Bauer TM, Levy B, Zhu VW, Lakhani N, Moreno V, Ebata K, Nguyen M, Heirich D, Zhu EY, Huang X, Yang L, Kherani J, Rothenberg SM, Drilon A, Subbiah V, Shah MH, Cabanillas ME. Efficacy of Selpercatinib in RET-Altered Thyroid Cancers. N Engl J Med. 2020;383:825-835. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 404] [Cited by in RCA: 548] [Article Influence: 91.3] [Reference Citation Analysis (0)] |
| 31. | Subbiah V, Hu MI, Mansfield AS, Taylor MH, Schuler M, Zhu VW, Hadoux J, Curigliano G, Wirth L, Gainor JF, Alonso G, Adkins D, Godbert Y, Ahn MJ, Cassier PA, Cho BC, Lin CC, Zalutskaya A, Barata T, Trask P, Scalori A, Bordogna W, Heinzmann S, Brose MS. Pralsetinib in Patients with Advanced/Metastatic Rearranged During Transfection (RET)-Altered Thyroid Cancer: Updated Efficacy and Safety Data from the ARROW Study. Thyroid. 2024;34:26-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 34] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 32. | Drilon A, Laetsch TW, Kummar S, DuBois SG, Lassen UN, Demetri GD, Nathenson M, Doebele RC, Farago AF, Pappo AS, Turpin B, Dowlati A, Brose MS, Mascarenhas L, Federman N, Berlin J, El-Deiry WS, Baik C, Deeken J, Boni V, Nagasubramanian R, Taylor M, Rudzinski ER, Meric-Bernstam F, Sohal DPS, Ma PC, Raez LE, Hechtman JF, Benayed R, Ladanyi M, Tuch BB, Ebata K, Cruickshank S, Ku NC, Cox MC, Hawkins DS, Hong DS, Hyman DM. Efficacy of Larotrectinib in TRK Fusion-Positive Cancers in Adults and Children. N Engl J Med. 2018;378:731-739. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1577] [Cited by in RCA: 2067] [Article Influence: 258.4] [Reference Citation Analysis (0)] |
| 33. | Demetri GD, De Braud F, Drilon A, Siena S, Patel MR, Cho BC, Liu SV, Ahn MJ, Chiu CH, Lin JJ, Goto K, Lee J, Bazhenova L, John T, Fakih M, Chawla SP, Dziadziuszko R, Seto T, Heinzmann S, Pitcher B, Chen D, Wilson TR, Rolfo C. Updated Integrated Analysis of the Efficacy and Safety of Entrectinib in Patients With NTRK Fusion-Positive Solid Tumors. Clin Cancer Res. 2022;28:1302-1312. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 148] [Article Influence: 37.0] [Reference Citation Analysis (0)] |
| 34. | Gouda MA, Subbiah V. Expanding the Benefit: Dabrafenib/Trametinib as Tissue-Agnostic Therapy for BRAF V600E-Positive Adult and Pediatric Solid Tumors. Am Soc Clin Oncol Educ Book. 2023;43:e404770. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 67] [Reference Citation Analysis (0)] |
| 35. | Health Promotion Administration. Cancer Registry Report, (accessed 29 March, 2025). Available from: https://www.hpa.gov.tw/Home/Index.aspx. |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/