Published online Sep 6, 2025. doi: 10.12998/wjcc.v13.i25.104742
Revised: April 27, 2025
Accepted: May 21, 2025
Published online: September 6, 2025
Processing time: 188 Days and 21.9 Hours
Tramadol is a synthetic opioid analgesic commonly employed in postoperative pain control due to its moderate efficacy and comparatively favorable safety profile. Nonetheless, overdose can result in significant adverse effects, notably central nervous system depression. This risk is amplified in individuals with chro
A 77-year-old male with diabetes mellitus and CKD stage 3 underwent elective right total knee arthroplasty for grade 4 osteoarthritis under spinal anesthesia. Preoperative evaluation revealed deranged renal function tests but no other significant abnormalities. Postoperative pain was managed with multimodal analgesics, including intravenous tramadol and a buprenorphine skin patch. On postoperative third day, the patient was found unconscious (Glasgow Coma Scale 8/15) with mild respiratory depression. Investigations ruled out stroke, pulmo
This case underscores the necessity for judicious tramadol administration in elderly patients with CKD, as diminished renal function markedly impairs drug clearance, predisposing to toxicity. Vigilant assessment of renal function and individualized dose adjustments are essential to mitigate the risk of adverse events in this demographic. Clinicians should maintain a heightened awareness of potential opioid toxicity in postoperative patients presenting with unexplained neurological manifestations. Timely identification and initiation of appropriate supportive measures are pivotal in achieving favorable clinical outcomes.
Core Tip: Clinicians should be aware of opioid toxicity in total knee arthroplasty patients. Cautions should be taken in the presence of kidney disorders. Tramadol and buprenorphine patches were commonly utilized in postoperative analgesia of knee arthroplasty. The combination of these drugs was not recommended and should be monitored for any adverse effects. It is better to use pre-emptive analgesia with minimum use of opioids.
- Citation: Harna B, Arya S. Tramadol overdose in total knee arthroplasty: A case report. World J Clin Cases 2025; 13(25): 104742
- URL: https://www.wjgnet.com/2307-8960/full/v13/i25/104742.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v13.i25.104742
Tramadol is a commonly used opioid analgesic for postoperative pain management due to its moderate potency and safety profile. However, overdose can lead to severe complications, including central nervous system (CNS) depression, particularly in patients with predisposing factors such as chronic kidney disease (CKD), where impaired drug clearance can exacerbate toxicity[1]. The elderly population is especially vulnerable to such adverse effects due to age-related physiological changes. This article presents a case of tramadol overdose in a 77-year-old male with CKD stage 3, who underwent total knee replacement.
A 77-year-old gentleman presented to the outpatient department with moderate-severe right knee pain for the past four years.
He was planned for elective right total knee arthroplasty (TKA) considering the grade 4 osteoarthritis knee on radiographs.
The patient already had a significant history of type 2 diabetes mellitus and CKD stage 3 (probably due to long-standing type 2 diabetes mellitus), managed with oral vildagliptin 50 mg and metformin 1000 mg twice daily dosing for the past eighteen years. The chronic knee pain was managed with pregabalin 75 mg daily dose, tab paracetamol 650 mg daily dose and tramadol 50 mg as and when required for the past two years.
None significant.
The patient underwent left-side TKA under spinal anaesthesia.
Preoperative laboratory investigations revealed a deranged renal function test (blood urea: 72 mg/dL, serum creatinine: 3.8 mg/dL, estimated glomerular filtration rate: 36 mL/minute/1.73 m2) with glycated hemoglobin value of 7.2. The complete blood count, liver function test, blood sugar level, coagulation profile, urine microscopy and chemical examination (protein and sugar), echocardiography, echocardiogram and venous doppler ultrasound for lower limbs were within normal limits.
Intraoperatively, the patient was given periarticular analgesia with a cocktail of 0.2% ropivacaine, tranexamic acid and adrenaline. The operative and postoperative periods were uneventful. The patient was mobilized with a walker on 1st postoperative day (POD). The visual analog scale (VAS) on POD 1 was 7, requiring multimodal analgesic intervention. The patient was given an intravenous 1 g injection of paracetamol every six hours, a tablet of pregabalin 75 mg at night time and an intravenous injection of tramadol 75 mg every twelve hours for pain management on POD 1. The postoperative investigations revealed the haemoglobin: 11.2 g/dL, renal function test (blood urea: 88 mg/dL, serum creatinine: 3.9 mg/dL, estimated glomerular filtration rate: 34 mL/minute/1.73 m2) with normal coagulation profile, blood sugar levels. VAS score was increased to 8 on POD 2, which required further pain management. Considering the CKD stage 3, advanced age, and increasing pain, a buprenorphine skin patch (10 mcg/hour) was applied on the left thigh with the continuation of injection paracetamol 1 g every six hours and 100 mg tramadol every twelve hours. On POD 3, the patient was found unconscious in the early morning with a Glasgow Coma Scale (GCS) of 8/15. In the last 24 hours, 200 mg of intravenous tramadol was given along with the buprenorphine patch. The vital parameters showed blood pressure: 122/68 mmHg, heart rate: 72 beats per minute, respiratory rate: 15 breaths per minute, and oxygen saturation: 92% on room air. The patient was shifted to the intensive care unit, considering poor consciousness (Table 1). The patient was investigated considering the expected complications like stroke, pulmonary embolism or any cardiopulmonary event. The investigation revealed normal serum electrolytes, blood glucose and ammonia levels, with mild respiratory acidosis (pH = 7.25) on arterial blood gas analysis, probably due to hypoventilation (Table 2).
| Test | Result |
| Blood urea | 72 mg/dL |
| Serum creatinine | 3.8 mg/dL |
| eGFR | 36 mL/minute/1.73 m2 |
| HbA1c | 7.2 |
| CBC, LFT, coagulation, echo, doppler | Within normal limits |
| Urinalysis (protein, sugar) | Normal |
| Vital signs | BP: 122/68 mmHg, HR: 72 bpm, RR: 15, SpO2: 92% |
| ABG | pH: 7.25 (mild respiratory acidosis) |
| CT & MRI brain | Cortical atrophy, lacunar infarcts |
| CT pulmonary angiography | No embolism or pneumonitis |
| Echocardiogram | Normal |
| Doppler (lower limbs) | No DVT |
| Tramadol level | 1.8 mg/L (elevated; normal: 0.1-0.8 mg/L) |
| Intervention | Details |
| Intubation & ventilation | SIMV mode, PEEP 6, FiO2 60%, VT: 550 |
| Opioid reversal | Buprenorphine patch removed, naloxone on standby |
| Diuresis | IV frusemide 40 mg BID |
| Recovery | GCS improved to 15/15 within 48 hours |
| Extubation & mobilization | POD 6 |
| Pain management post-recovery | Paracetamol 1 g Q8H, pregabalin 75 mg QHS |
| Discharge | POD 10, stable vitals, no respiratory distress |
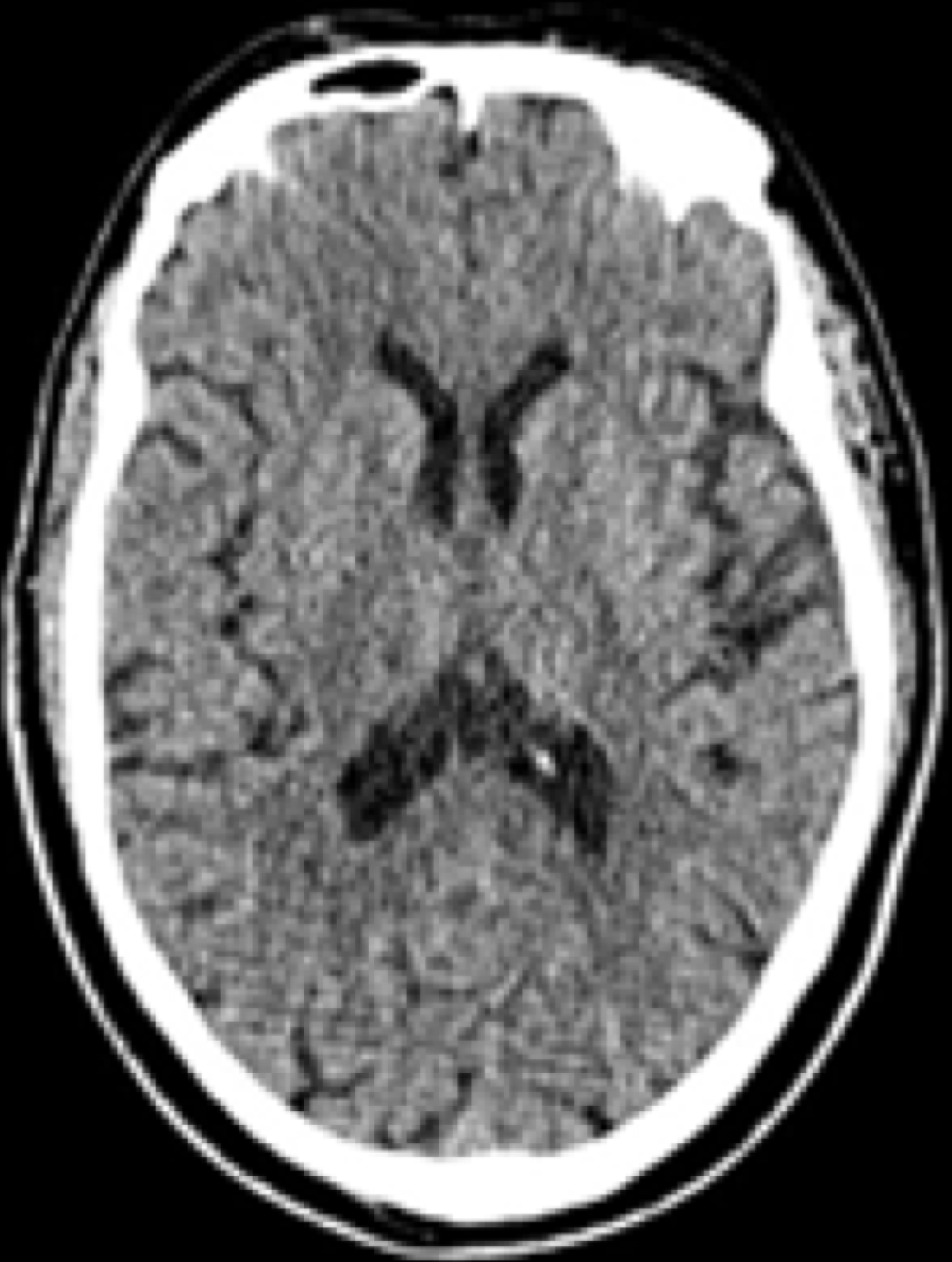
Computer tomography (Figure 1) with angiography and magnetic resonance imaging brain revealed degenerative cortex atrophy in the parietal and temporal region with lacunar infarcts in the gangliothalamic region. The echocardiography and echocardiogram depicted no abnormality. The computer tomography pulmonary angiography revealed no embolism or thrombus or any aspiration pneumonitis. The venous doppler of lower limbs revealed no evidence of deep venous thrombosis. The patient was hemodynamically stable with low consciousness. The low GCS score and unconscious state warranted the prophylactic intubation to prevent aspiration and further deterioration of the patient’s condition. The patient was intubated and maintained on synchronized intermittent mandatory ventilation mode with positive end-expiratory pressure of 6, fraction of inspired oxygen 60% and tidal volume point setting of 550. The patient’s pupil was constricted and reacting timidly to the light stimulus.
Ruling out the obvious causes for the decreased consciousness, opioid toxicity was considered. The tramadol levels were investigated and found to be 1.8 mg/L (normal: 0.1 mg/L to 0.8 mg/L).
The buprenorphine patch was removed. Injection naloxone was kept as a backup antagonist drug for the management. Diuresis was performed with an intravenous injection of frusemide 40 mg twice daily. The patient’s condition improved over the next 48 hours.
The patient regained full consciousness with a GCS 15/15. The patient was extubated and was mobilized again on POD 6. The VAS score of the patient decreased to 4 and was managed with oral tablet paracetamol 1 g every 8 hours and tablet pregabalin 75 mg at night time. The patient was discharged on POD 10. At the time of discharge, the vital parameters of the patient were within normal limits with normal consciousness and no respiratory distress. The CKD stage 3 was managed with the nephrologists in the follow-up.
Effective pain management after TKA remains critical to postoperative care. While opioids have traditionally been employed, their use is increasingly questioned due to significant risks, particularly in elderly populations, including dependency, respiratory depression, and severe adverse effects. Consequently, there has been a shift toward alternative pain management strategies, including the use of weaker opioids such as tramadol. Concurrently, buprenorphine, a partial opioid agonist with a ceiling effect on respiratory depression, has gained attention in patients undergoing chronic opioid therapy[2]. However, the combination of tramadol and buprenorphine presents significant pharmacological challenges that necessitate careful consideration. Studies have demonstrated the potential of tramadol to reduce overall opioid consumption without compromising pain relief or early functional recovery in TKA patients[3]. A low-opioid regimen incorporating tramadol led to reduced morphine milligram equivalent consumption compared to high-opioid regimens while maintaining comparable VAS pain scores[4].
However, tramadol’s use is not without concern. Its side effect profile, including dizziness, nausea, vomiting, and dry mouth, is well-documented. While these adverse effects are generally minor and non-life-threatening, they occur at higher rates than with stronger opioids. Serious adverse events, such as respiratory depression or unresponsiveness, are notably rare with tramadol, which supports its relative safety. A retrospective review revealed that approximately 11.2% of patients required conversion from tramadol to stronger opioids, indicating its limitations in managing severe postoperative pain[4]. Tramadol undergoes extensive hepatic metabolism and renal excretion, with over 90% of the drug and its metabolites eliminated via the kidneys[5]. The liver metabolizes tramadol by N- and O-demethylation mediated by the cytochrome P450 pathways, particularly cytochrome P450 2D6 (CYP2D6), is mainly excreted through the kidneys. The CYP2D6 genotype affects the plasma levels of tramadol and its metabolites, as well as tramadol efficacy and adverse drug reactions. It has been reported that ultra-rapid metabolizers are more prone to experience the side effects of tramadol[6,7]. Poor metabolizers may contain higher tramadol concentrations and the simultaneous use of CYP2D6 or CYP3A4 inhibitors. Although the genetic polymorphism was assessed but the patient never had any adverse effects from oral intake of tramadol for the past two years, with doses ranging from 50 mg to 200 mg per day. As such, renal impairment significantly affects tramadol’s pharmacokinetics, leading to drug accumulation and heightened toxicity risk[8]. In patients with kidney disease, even standard therapeutic doses of tramadol can result in adverse effects, including seizures, CNS depression, and respiratory compromise. The elimination half-life of tramadol is prolonged in individuals with renal failure, exacerbating its side effects. Therapeutic monitoring, hydration, and supportive care are essential in managing tramadol toxicity in this vulnerable group. Preoperatively, the patient was already taking low-dose pregabalin 75 mg daily for the past two years with no side effect of sedation. Moderate frequency and larger doses of pregabalin were associated with dizziness and somnolence. Dizziness and somnolence were observed in 31% and 22% of patients, respectively[9]. These adverse effects often occurred when pregabalin was initiated and often diminished after weeks of therapy[10].
Buprenorphine is a partial agonist at the μ-opioid receptor and an antagonist at the κ-opioid receptor. Its unique pharmacological profile provides effective analgesia with a lower risk of respiratory depression compared to full opioid agonists. However, its high receptor affinity and long half-life complicate the concurrent use of other opioids, including tramadol. While buprenorphine’s partial agonism may reduce the efficacy of additional opioid agonists, it has been shown to exhibit additive analgesic effects with certain opioids, including tramadol, under specific conditions[11]. This interaction is attributed to tramadol’s secondary mechanism of serotonin and norepinephrine reuptake inhibition, which bypasses traditional opioid pathways to enhance pain relief[12].
Despite the potential for additive analgesia, combining tramadol and buprenorphine is fraught with risks. Tramadol’s weak μ-opioid agonism is often antagonized by buprenorphine’s high receptor affinity, potentially diminishing tramadol’s effectiveness. This interaction can attenuate tramadol’s analgesic effects, rendering it less effective in managing acute postoperative pain. In patients receiving buprenorphine for opioid use disorder, the combination with tramadol may precipitate withdrawal symptoms or paradoxical pain exacerbation. Furthermore, buprenorphine’s interference with tramadol metabolism has been observed, particularly concerning its active metabolite O-desmethyl tramadol, which exhibits higher μ-opioid receptor potency than the parent compound[12]. Many medications can interfere with opioid metabolism through the cytochrome system, but the patient was consuming none of such medications.
The concurrent use of tramadol and buprenorphine is generally discouraged due to the increased risk of adverse outcomes. The product labelling for tramadol includes a black box warning against combining it with other CNS depressants, including buprenorphine, due to the potential for severe respiratory depression and death[13]. Monitoring patients for sedation and respiratory compromise in outpatient settings is impractical, further limiting the feasibility of such combinations. These risks must be carefully weighed against the potential analgesic benefits, particularly in populations with limited treatment alternatives. Even a moderate dose of the opioids in renal impaired patients can cause unusual effects pertaining to deranged or loss of consciousness.
Although naloxone is an antidote, but also has some adverse effects, ranging from rapid tachycardia, increase in blood pressure, tremulousness, seizures, and cardiac arrest. In large doses, there can be the reversal of analgesia, ventricular fibrillation, and pulmonary edema[14]. In this patient, naloxone was kept as a side-by treatment in case the patient didn’t respond or condition worsened. Initially, the treatment plan was to flush out the opioids from the patient’s body using diuretics, and it improved the patient’s condition. Thus, initially, not in dire situations, opioid intoxication can be managed by stopping opioids and removing them from the patient’s body system.
This case highlights the importance of cautious tramadol use in elderly patients with CKD, as reduced renal function can significantly impair drug clearance, leading to toxicity. Close monitoring of renal function and appropriate dose adjustments are critical in this population to prevent adverse outcomes. Clinicians must maintain a high index of suspicion for opioid toxicity in patients presenting with unexplained neurological symptoms in the postoperative period. Early recognition and prompt supportive management can lead to favourable outcomes in such cases.
| 1. | Odoma VA, Pitliya A, AlEdani E, Bhangu J, Javed K, Manshahia PK, Nahar S, Kanda S, Chatha U, Mohammed L. Opioid Prescription in Patients With Chronic Kidney Disease: A Systematic Review of Comparing Safety and Efficacy of Opioid Use in Chronic Kidney Disease Patients. Cureus. 2023;15:e45485. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 2. | Infantino R, Mattia C, Locarini P, Pastore AL, Maione S, Luongo L. Buprenorphine: Far Beyond the "Ceiling". Biomolecules. 2021;11:816. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 3. | DeMik DE, Carender CN, Shamrock AG, Callaghan JJ, Bedard NA. Opioid Use After Total Knee Arthroplasty: Does Tramadol Have Lower Risk Than Traditional Opioids? J Arthroplasty. 2020;35:1558-1562. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 4. | Kleeman-Forsthuber L, Pollet A, Johnson RM, Boyle J, Jennings JM, Dennis DA. Evaluation of Low-Dose Versus High-Dose Opioid Pathway in Opioid-Naïve Patients After Total Knee Arthroplasty. Arthroplast Today. 2022;14:81-85. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 5. | Manouchehri A, Nekoukar Z, Malakian A, Zakariaei Z. Tramadol poisoning and its management and complications: a scoping review. Ann Med Surg (Lond). 2023;85:3982-3989. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 11] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 6. | Haufroid V, Hantson P. CYP2D6 genetic polymorphisms and their relevance for poisoning due to amfetamines, opioid analgesics and antidepressants. Clin Toxicol (Phila). 2015;53:501-510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 73] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 7. | Miotto K, Cho AK, Khalil MA, Blanco K, Sasaki JD, Rawson R. Trends in Tramadol: Pharmacology, Metabolism, and Misuse. Anesth Analg. 2017;124:44-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 236] [Article Influence: 26.2] [Reference Citation Analysis (0)] |
| 8. | Paar WD, Frankus P, Dengler HJ. The metabolism of tramadol by human liver microsomes. Clin Investig. 1992;70:708-710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 80] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 9. | Parke-Davis Div of Pfizer Inc. Highlights of prescribing information. [cited 12 February 2025]. Available from: https://labeling.pfizer.com/showlabeling.aspx?id=9678. |
| 10. | Toth C. Pregabalin: latest safety evidence and clinical implications for the management of neuropathic pain. Ther Adv Drug Saf. 2014;5:38-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 129] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 11. | Khanna IK, Pillarisetti S. Buprenorphine - an attractive opioid with underutilized potential in treatment of chronic pain. J Pain Res. 2015;8:859-870. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 77] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 12. | Montalvo C, Genovese N, Renner J. The problem of pain: Additive analgesic effect of tramadol and buprenorphine in a patient with opioid use disorder. Subst Abus. 2019;40:136-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 13. | Highlights of prescribing information. [cited 12 February 2025]. Available from: https://www.janssenlabels.com/package-insert/product-monograph/prescribing-information/ULTRAM-pi.pdf. |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/