Published online Jul 26, 2025. doi: 10.12998/wjcc.v13.i21.104723
Revised: March 12, 2025
Accepted: March 20, 2025
Published online: July 26, 2025
Processing time: 118 Days and 11.6 Hours
Hereditary alpha tryptasemia was first described in 2016 and is the most common (up to 72%) cause of elevated serum basal tryptase (TPS). The clinical presentation of this condition, which is caused by copy number gains in the TPSAB1 gene encoding serum α TPS, is variable for each patient. Some patients are asymp
Core Tip: Hereditary alpha tryptasemia was first described in 2016 and is the most common cause of elevated serum basal tryptase (TPS). The clinical presentation of this condition, which is caused by copy number gains in the TPSAB1 gene encoding serum α TPS, is variable for each patient.
- Citation: Tüsüz Önata E, Özdemir Ö, Savaşan S. Hereditary alpha tryptasemia and clinical implications. World J Clin Cases 2025; 13(21): 104723
- URL: https://www.wjgnet.com/2307-8960/full/v13/i21/104723.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v13.i21.104723
Tryptase (TPS) is a tetrameric serine protease molecule that is predominantly expressed and stored in tissue mast cells (MCs) and is also expressed in basophils in lesser amounts[1]. MCs are long-lived cells originating from CD34+/CD117+ pluripotent hematopoietic stem cells in the bone marrow and play a role in innate and acquired immunity as well as hypersensitivity reactions.
The majority of mature MCs are found in epithelial and mucosal tissues where the host encounters foreign antigens (such as skin, respiratory system, gastrointestinal system, and ocular tissue)[2]. Mature MCs contain cytoplasmic granules that store many mediators including TPS, histamine, heparin, leukotrienes, growth factors, cytokines, chemokines, serotonin, chymases, and cathepsins[3]. The activation of MCs is mainly initiated by high-affinity immunoglobulin E receptor Fc epsilon RI and a tyrosine kinase protein, c-KIT. Apart from these, there are many receptors such as complement receptors (CRs) (CR2, CR4, CR5), chemokine receptors (CCR) (CCR3, CCR5, and CXCR4), and toll-like receptors on the MC surface[4]. MCs are activated and degranulated through stimulation of these receptors. After degranulating MCs can remain in tissues for a long time by generating granules again[5]. Although basophils have similar granules to MCs, they contain 500 times less TPS than MCs[6]. Therefore, high TPS levels are mostly associated with excessive activation of MCs[7].
In MCs TPS is present in two different forms: (1) Monomeric pro-α TPS and pro-β TPS; and (2) Mature α TPS and β TPS in the tetrameric structure. Pro-TPSs are inactive TPS precursors and form mature active tetrameric TPSs after proteolysis and assembly. Mature TPSs are stabilized in MC secretory granules depending on heparin[6,8]. Serum basal TPS (sBT) is formed by inactive pro-α TPS and pro-β TPSs released continuously from unstimulated tissue MCs and then transported from the interstitial space to the peripheral circulation[9]. Mature forms of TPS are released only when MCs are activated[6]. The most commonly used clinical test measuring total TPS does not differentiate between pro-α TPS and pro-β TPS and cannot differentiate between mature and pro-TPSs; therefore, it is important to evaluate TPS measurement together with the clinical condition[3].
The TPS locus is located on the short arm of chromosome 16 and contains four TPS encoding genes (TPSAB1, TPSB2, TPSG1, and TPSD1). Although these four genes encode TPS, only the TPSAB1 and TPSB2 genes encode TPS isoforms measured as serum TPSs[9]. While the TPSB2 gene encodes β-2 and β-3 TPS isoforms, the TPSAB1 gene encodes α TPS and β-1 TPS. As a result of the competition of these α TPS and β-1 TPS alleles at the same locus, it is possible to observe individuals in which α TPS is completely absent[9-11]. The three defined β TPS isoforms are 99% similar to each other. The β TPS, which is the dominant form in MCs, is found in the blood both as an inactive monomer and as a heparin-bound tetramer[12,13]. The β TPS is the most effective TPS in the extracellular space and is assumed to play a role in physiological and pathological responses[14]. Two isoforms of α TPS, which is the second most important TPS after β TPS, have been defined as α-1 and α-2[15]. Although α TPS and β TPS have 95% similarity, they are not actually the same forms.
Mature β TPSs are neutral serine proteases with trypsin-like activity. This enzymatic activity of mature α TPSs is negligible, and it is a more positively charged molecule than β TPS[16]. In allergic reactions, the β TPS level increases in the blood with MC stimulation, but the α TPS level does not increase. Among other TPS forms, γ TPS is a transmembrane protein bound to the plasma membrane or secretory granule membrane. Delta TPS, on the other hand, is a TPS form that lacks a significant part of its active site and has very little catalytic activity. It is preferentially synthesized in MCs of the large intestine, lung, and heart[15].
In healthy individuals, the sBT level is generally stable and averages 5 ng/mL (< 1.0-30.7 ng/mL) in most individuals[17]. The upper limit (95 percentile value) of sBT level has been suggested as 11.4-15.0 ng/mL so far[17,18].
The sBT total serum TPS concentrations are used as the measure of body MCs burden in the clinic. The serum TPS level is important in the diagnosis of anaphylactic reactions in which MCs degranulate suddenly[19]. Since MCs are found in tissues rather than in the peripheral circulation, serum TPS will start to be highly detected in the blood approximately 30 min after the onset of symptoms and will return to the sBT value in time. For the diagnosis of anaphylaxis, a comparison of the serum acute TPS value taken 30 min after the onset of symptoms and within the first 2 h with sBT value has a high specificity and positive predictive value. The 1.2X sBT + 2 formula has been suggested for this purpose. If the patient does not have a previous sBT level, the TPS value 24 h after the symptoms completely disappear can be used as the sBT level[20-22].
More recently, Mateja et al[23] presented a new formula with higher specificity and sensitivity to confirm anaphylaxis in all patients [including patients with hereditary alpha tryptasemia (HAT) and systemic mastocytosis (SM)]. Accordingly, a serum acute TPS/sBT ratio of 1.685 or higher indicates anaphylaxis[23]. In addition to this information, it should be kept in mind that serum TPS levels may not always increase in anaphylaxis, especially in food-induced anaphylaxis, and a normal TPS value does not exclude anaphylaxis[24]. The sBT level may also be increased in various clinical conditions and diseases other than these conditions. TPS levels may be increased in patients with severe renal dysfunction[25,26] and parasitic infections[27,28]. TPS values are elevated in myeloid neoplasms (acute myeloid leukemia, chronic myeloid leukemia, myeloproliferative neoplasms, myelodysplastic syndrome, chronic myelomonocytic leukemia, chronic eosinophilic leukemia)[29,30] and MC diseases (SM, cutaneous mastocytosis), and the TPS value is used as a biomarker in the follow-up of these diseases (Table 1)[31]. In lymphoid neoplasms, TPS values generally remain stable[30].
| Etiology | Diagnostic methods | Serum basal tryptase range | Incidence |
| Hereditary alpha tryptasemia | ddPCR | 8-50 ng/mL | ≤ 67% |
| No disease is detected | Exclusion diagnosis | Variable | ≤ 23% |
| Renal failure | Glomerular filtration rate < 60 mL/minute, elevated creatinine | 10-50 ng/mL | ≤ 16% |
| Systemic mastocytosis | Bone marrow aspiration, c-KIT D816V mutation by ddPCR, skin biopsy | 20-200 ng/mL | ≤ 5% |
| Other myeloid neoplasms, acute myeloid leukemia, myeloproliferative neoplasms, myeloid neoplasms with eosinophilia, myelodysplastic neoplasms | Complete blood count, bone marrow aspiration, molecular genetics, cytogenetics | 15-50 ng/mL | Rare |
| Hypereosinophilic syndrome | Bone marrow aspiration, molecular genetics, cytogenetics | 10-50 ng/mL | Rare |
| Chronic inflammatory diseases, rheumatoid arthritis, eosinophilic esophagitis | Rheumatological examinations, endoscopy, biopsy | 5-25 ng/mL | Rare |
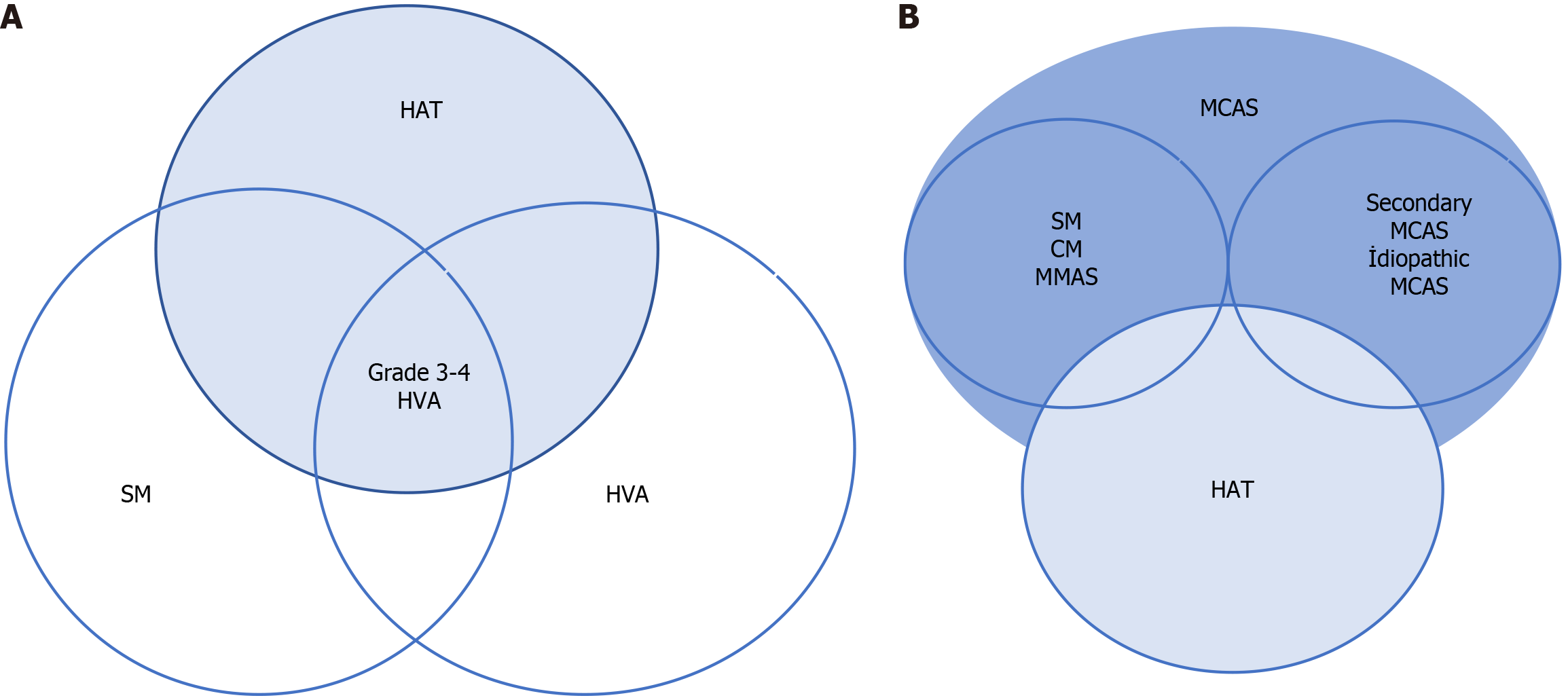
In 2014, Lyons et al[32] identified severe allergic phenotypes and elevated sBT levels in several families without evidence of any other underlying disease. These individuals had chronic and episodic symptoms such as cramping abdominal pain, flushing, diarrhea, urticaria, and a history of anaphylaxis (food anaphylaxis, venom anaphylaxis, idiopathic anaphylaxis) (Figure 1 and Figure 2A). The authors reported that these symptoms were induced by specific triggers such as stress, food, heat, vibration, exercise, trauma, and sometimes without any trigger. They initially suspected MC disease due to the similarity of symptoms and high levels of sBT; however, they failed to detect clonal MC disease and MC activation syndrome in most of the affected patients[32]. Two years later in 2016, Lyons et al[33] described HAT with high sBT levels resulting from congenital copy number gains in a single allele in the TPSAB1 gene encoding α TPS. Having identified a single aberration at the human TPS locus on chromosome 16p13.3 by exome and genome sequencing and linkage segregation analysis, Lyons et al[33] performed additional analysis of the TPSAB1 gene with a digital droplet PCR assay that validated the accurate detection of α TPS and β TPS sequences and direct measurement of copy numbers. Monoallelic extra alpha copies of the TPSAB1 gene were detected in the majority of patients, thus demonstrating an autosomal dominant inheritance pattern[34]. The prevalence of HAT has been reported in 4%-6% of the whole population in various studies[33,34]. On the other hand, HAT is the most common cause of high sBT, accounting for 64%-72% of cases in the general population with pathologically elevated sBT levels[35-37].
Not a single or uniform clinical phenotype is observed in patients with HAT. Some individuals may have no symptoms[38]. In one study, symptoms were reported in one-third of individuals with HAT with the same frequency as healthy individuals, while mild-to-moderate symptoms were reported in one-third, and significant symptoms were reported in the remaining individuals[33]. Functional gastrointestinal complaints are observed most frequently. Approximately half of the affected individuals reported irritable bowel syndrome (IBS)-like symptoms[39,40]. When samples taken from the duodenal and terminal ileum of patients with HAT were analyzed, it was observed that these individuals had more MCs than healthy individuals[41].
Recurrent skin symptoms including flushing and itching are observed in half of the symptomatic HAT cases. It has been observed that these symptoms usually occur spontaneously, but vibration and mild trauma are the most common triggers[37]. Mood changes (Table 2), chronic fatigue/pain, joint hypermobility, and autonomic dysfunction (orthostatic hypotension, tachycardia, presyncope, and syncope) are other reported clinical symptoms[40,42]. Multiple food intolerances, failure to thrive, and eosinophilic gastrointestinal disease have also been described in a few very symptomatic families with increased copies of TPSAB1[40].
| Clinical findings | |
| Gastrointestinal symptoms | Nausea |
| Abdominal pain | |
| Diarrhea | |
| Vomiting | |
| Dyspepsia | |
| Neuropsychiatric findings | Cognitive disorders (such as memory disorders) |
| Fatigue | |
| Sleep disorders | |
| Depressive disorders | |
| Nervousness | |
| Cutaneous and allergic symptoms | Sudden hot flushes |
| Pruritus | |
| Rash, urticaria | |
| Pain | Muscle pain |
| Headache | |
| Joint pain | |
| Other findings | Systemic sudden hypersensitivity reaction |
| Joint hypermobility | |
The prevalence of HAT in patients with mastocytosis (systemic or cutaneous) is 2-3 times higher than in the general population (12%-21%) in Europe[34,43,44]. The prevalence of venom allergy in cases with SM and HAT is 3-fold higher (Figure 2) than in patients with SM alone[34,45].
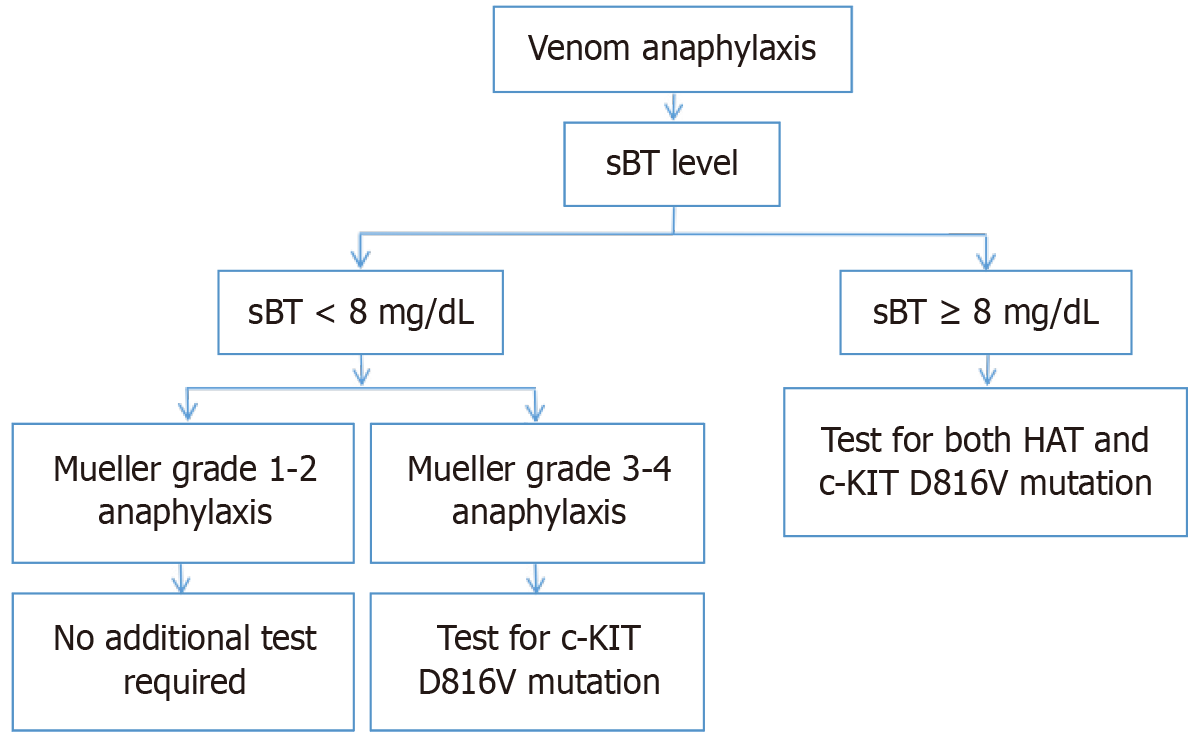
The rate of venom anaphylaxis in patients with HAT is not higher than in the general population[46,47]. However, more severe reactions are observed in patients with HAT as a result of insect bites[46]. Figure 1 shows the recommended approach algorithm according to the level of sBT and severity of anaphylaxis in patients who develop anaphylaxis as a result of insect bites (Figure 1 and Figure 2A).
Excluding individuals with HAT, the range of TPS in normal individuals was found to be mostly between 1.0 ng/mL and 11.4 ng/mL[37,48]. However, since lower basal TPS levels ranging from 8.0 ng/mL to 11.3 ng/mL (and rarely even lower) have been observed in some HAT-positive cases[18,38,40], a threshold value of 8.0 ng/mL has been recommended[18,38,40]. It was observed that higher sBT levels were observed in individuals with additional copy numbers of the TPSAB1 alpha gene. This resulted in sBT levels of 15.0 ng/mL ± 5.0 ng/mL in individuals with one extra gene copy number, 24.0 ng/mL ± 6.0 ng/mL in individuals with two extra gene copies, and 37.0 ng/mL ± 14.0 ng/mL in individuals with four extra copies[39].
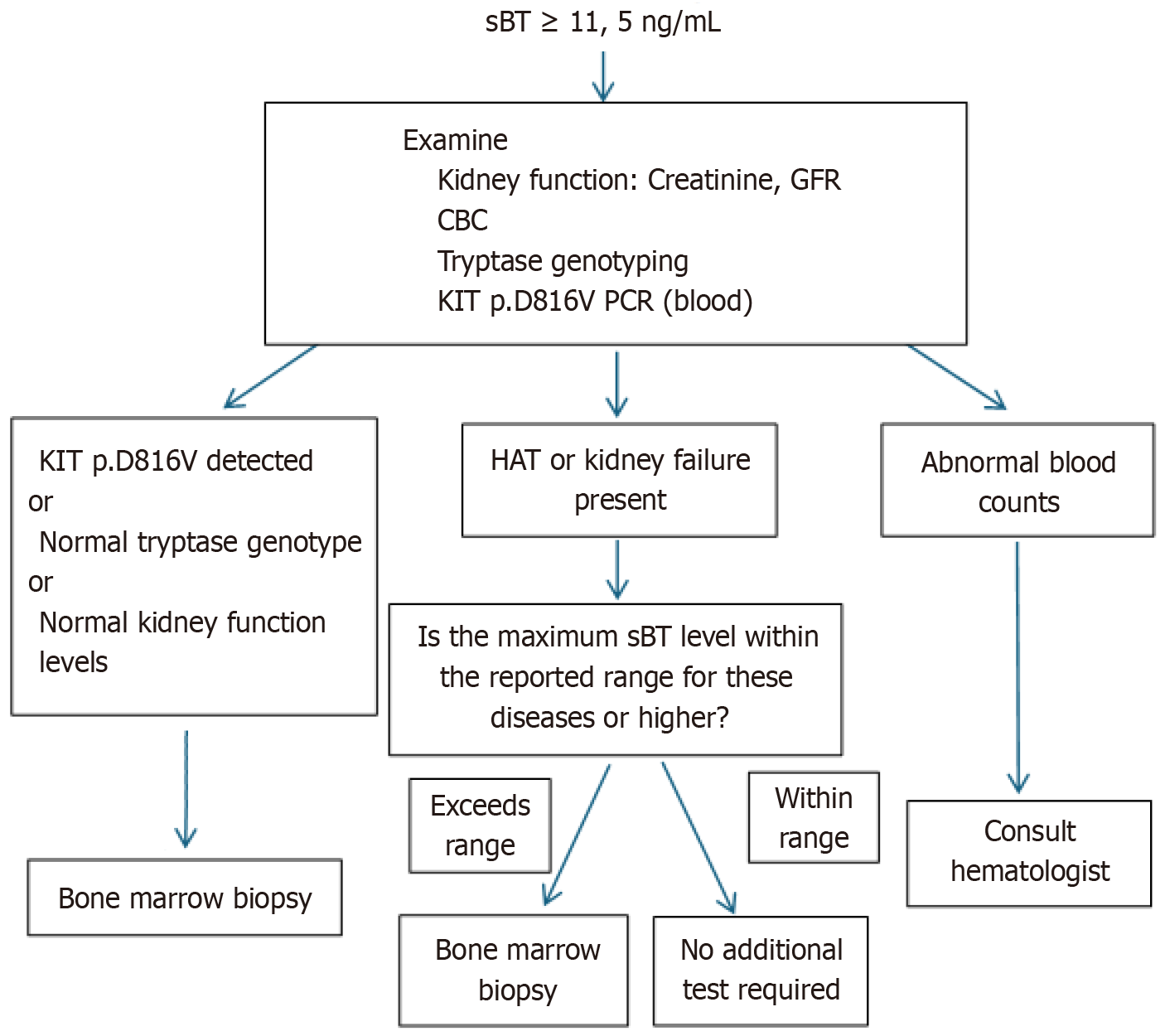
Currently, the most reliable method (with 100% sensitivity and 90% specificity) for the detection of copy number and variations in the TPSAB1 gene is digital droplet PCR[3,40]. Commonly used genetic test indications for HAT are summarized in Table 3. If the HAT test is negative, the patient should be tested for the c-KIT D816V mutation in terms of SM, and bone marrow biopsy should be considered to exclude other bone marrow neoplasms. In asymptomatic individuals, there is no indication for basal TPS level measurement or genetic testing in most patients presenting with certain connective tissue abnormalities or symptoms of IBS[18]. In symptomatic patients with TPS levels higher than 15.0 ng/mL (Figure 2B and Figure 3), testing for the presence of the c-KIT D816V mutation in addition to the presence of HAT is important because of the high risk of developing MC activation syndrome in patients with SM, HAT, and immunoglobulin E-related allergy[49-51].
| sBT level ≥ 8 ng/mL |
| Recurrent signs of MC activation with unclear etiology (such as idiopathic anaphylaxis) |
| Suspected SM with negative c-KIT D816V, but very high TPS level |
| SM or MCAS with severe mediator-related symptoms |
| Serum TPS value > 15.0 ng/mL without underlying mastocytosis or myeloid neoplasm |
| Familial clustering of systemic MC activation symptoms |
| Higher levels of sBT according to the degree of bone marrow mast cell infiltration |
| Diagnosed or suspected idiopathic or secondary MCAS |
Since HAT is a newly defined disease, it is not well known by physicians, and it is difficult to differentiate it from other diseases without genetic testing. Genetic tests are not always available everywhere and can be confused with other allergic diseases with similar findings, making diagnosis difficult. Commonly used indications for genetic testing for HAT have been established as outlined in Table 3. Genetic testing for SM may be required if the HAT test is negative, and genetic testing may not be required in asymptomatic patients. In symptomatic patients with sBT levels higher than 15.0 ng/mL, it is important to test for the presence of the c-KIT D816V mutation in addition to the presence of HAT[49-51].
In patients with HAT, treatment is recommended only in symptomatic patients with symptoms of MC activation (severe allergy or anaphylaxis)[3]. The World Allergy Organization and the European Mastocytosis Competence Network recommend that triggering factors should be well identified and avoided, recurrence should be prevented by allergen immunotherapy and desensitization if necessary, and a personalized emergency action plan should be established by ensuring the use of epinephrine in emergencies[52-55]. Since severe allergic reactions and anaphylaxis can be observed frequently in patients with HAT and SM together, it is important to establish an anaphylaxis emergency action plan, especially in these patients[33,53].
The combination of H1 and H2 antihistamines has been shown to provide relief, especially in gastrointestinal symptoms[56,57]. In some patients, it has been observed that intestinal symptoms were alleviated with MC stabilizers such as cromoglycate or flavonoid quercetin[40]. In the first study investigating the effect of omalizumab in symptomatic patients with HAT, the response rate to urticaria and anaphylaxis was found to be 100% in 13 patients, and the response rate to itching, rash, fatigue, nausea, and abdominal pain was found to be 50%.
In another study conducted with 18 patients, response to omalizumab was found to be 100% for urticaria and 80% for anaphylaxis[46,55]. Another candidate drug in the treatment of HAT and other MC-related disorders is AK002, an antibody that binds and inactivates the inhibitory receptor Siglec-8 on the surface of MCs, eosinophils, and to a lesser extent basophils. The safety and efficacy of this treatment continues to be investigated[58]. Maun et al[59] produced a non-competitive inhibitor antibody that binds to human α TPS and β TPS and induces dissociation of the active tetramer structure into its monomers. This antibody was shown to potently inhibit active β TPS in vivo. Since this antibody can also inhibit α/β TPS heterotetramers, it is thought to be a treatment option for patients with HAT[59].
The most important limitation is the lack of different drugs and targeted therapies in addition to drugs such as antihistamines and omalizumab, which have been used since the past. Long-term management strategies for patients with mild or moderate symptoms are unclear. The efficacy and potential side effects of current older therapies are well known, but they remain palliative. Future development of targeted biological agent therapies and perhaps genetic therapies will result in more definitive and effective treatment.
HAT is inherited and is common in the population. It is the most common cause of elevated sBT. The recent discovery of HAT has led to a better understanding of the genetics, expression, and secretion mechanism of TPS. The demonstration that HAT increases the risk of severe allergic reactions and anaphylaxis, especially when associated with SM, has been important for the treatment of these patients. Further research is required to clarify how HAT plays a role in the pathogenesis of MC disorders. A comprehensive understanding of HAT may contribute to the development of new specific/targeted therapeutic methodologies for cases with MC disorders along with HAT.
| 1. | Schwartz LB, Irani AM, Roller K, Castells MC, Schechter NM. Quantitation of histamine, tryptase, and chymase in dispersed human T and TC mast cells. J Immunol. 1987;138:2611-2615. [PubMed] |
| 2. | Gilfillan AM, Austin SJ, Metcalfe DD. Mast cell biology: introduction and overview. Adv Exp Med Biol. 2011;716:2-12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 108] [Cited by in RCA: 109] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 3. | Sprinzl B, Greiner G, Uyanik G, Arock M, Haferlach T, Sperr WR, Valent P, Hoermann G. Genetic Regulation of Tryptase Production and Clinical Impact: Hereditary Alpha Tryptasemia, Mastocytosis and Beyond. Int J Mol Sci. 2021;22:2458. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 35] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 4. | Marshall JS. Mast-cell responses to pathogens. Nat Rev Immunol. 2004;4:787-799. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 576] [Cited by in RCA: 621] [Article Influence: 28.2] [Reference Citation Analysis (0)] |
| 5. | Artuc M, Hermes B, Steckelings UM, Grützkau A, Henz BM. Mast cells and their mediators in cutaneous wound healing--active participants or innocent bystanders? Exp Dermatol. 1999;8:1-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 152] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 6. | Krystel-Whittemore M, Dileepan KN, Wood JG. Mast Cell: A Multi-Functional Master Cell. Front Immunol. 2015;6:620. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 283] [Cited by in RCA: 501] [Article Influence: 50.1] [Reference Citation Analysis (0)] |
| 7. | Oštrić Pavlović I, Radović S, Krtinić D, Spirić J, Kusić N, Veličković A, Tomić-Spirić V. Tryptase: The Silent Witness of Past and Ongoing Systemic Events. Medicina (Kaunas). 2024;60:1380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 8. | Sakai K, Ren S, Schwartz LB. A novel heparin-dependent processing pathway for human tryptase. Autocatalysis followed by activation with dipeptidyl peptidase I. J Clin Invest. 1996;97:988-995. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 110] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 9. | Caughey GH. Tryptase genetics and anaphylaxis. J Allergy Clin Immunol. 2006;117:1411-1414. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 122] [Cited by in RCA: 100] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 10. | Soto D, Malmsten C, Blount JL, Muilenburg DJ, Caughey GH. Genetic deficiency of human mast cell alpha-tryptase. Clin Exp Allergy. 2002;32:1000-1006. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 61] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 11. | Atiakshin D, Buchwalow I, Samoilova V, Tiemann M. Tryptase as a polyfunctional component of mast cells. Histochem Cell Biol. 2018;149:461-477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 66] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 12. | Cairns JA. Inhibitors of mast cell tryptase beta as therapeutics for the treatment of asthma and inflammatory disorders. Pulm Pharmacol Ther. 2005;18:55-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 54] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 13. | Caughey GH. Mast cell proteases as pharmacological targets. Eur J Pharmacol. 2016;778:44-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 116] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 14. | Caughey GH. Mast cell proteases as protective and inflammatory mediators. Adv Exp Med Biol. 2011;716:212-234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 130] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 15. | Hallgren J, Pejler G. Biology of mast cell tryptase. An inflammatory mediator. FEBS J. 2006;273:1871-1895. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 148] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 16. | Huang C, Li L, Krilis SA, Chanasyk K, Tang Y, Li Z, Hunt JE, Stevens RL. Human tryptases alpha and beta/II are functionally distinct due, in part, to a single amino acid difference in one of the surface loops that forms the substrate-binding cleft. J Biol Chem. 1999;274:19670-19676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 67] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 17. | Gonzalez-Quintela A, Vizcaino L, Gude F, Rey J, Meijide L, Fernandez-Merino C, Linneberg A, Vidal C. Factors influencing serum total tryptase concentrations in a general adult population. Clin Chem Lab Med. 2010;48:701-706. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 56] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 18. | Valent P, Hoermann G, Bonadonna P, Hartmann K, Sperr WR, Broesby-Olsen S, Brockow K, Niedoszytko M, Hermine O, Chantran Y, Butterfield JH, Greiner G, Carter MC, Sabato V, Radia DH, Siebenhaar F, Triggiani M, Gülen T, Alvarez-Twose I, Staudinger T, Traby L, Sotlar K, Reiter A, Horny HP, Orfao A, Galli SJ, Schwartz LB, Lyons JJ, Gotlib J, Metcalfe DD, Arock M, Akin C. The Normal Range of Baseline Tryptase Should Be 1 to 15 ng/mL and Covers Healthy Individuals With HαT. J Allergy Clin Immunol Pract. 2023;11:3010-3020. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 33] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 19. | Michel M, Klingebiel C, Vitte J. Tryptase in type I hypersensitivity. Ann Allergy Asthma Immunol. 2023;130:169-177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 19] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 20. | Vitte J, Amadei L, Gouitaa M, Mezouar S, Zieleskiewicz L, Albanese J, Bruder N, Lagier D, Mertès PM, Mège JL, Schwartz LB, Leone M. Paired acute-baseline serum tryptase levels in perioperative anaphylaxis: An observational study. Allergy. 2019;74:1157-1165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 34] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 21. | Valent P, Akin C, Arock M, Brockow K, Butterfield JH, Carter MC, Castells M, Escribano L, Hartmann K, Lieberman P, Nedoszytko B, Orfao A, Schwartz LB, Sotlar K, Sperr WR, Triggiani M, Valenta R, Horny HP, Metcalfe DD. Definitions, criteria and global classification of mast cell disorders with special reference to mast cell activation syndromes: a consensus proposal. Int Arch Allergy Immunol. 2012;157:215-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 396] [Cited by in RCA: 480] [Article Influence: 32.0] [Reference Citation Analysis (0)] |
| 22. | Baretto RL, Beck S, Heslegrave J, Melchior C, Mohamed O, Ekbote A, Huissoon AP, Krishna MT. Validation of international consensus equation for acute serum total tryptase in mast cell activation: A perioperative perspective. Allergy. 2017;72:2031-2034. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 73] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 23. | Mateja A, Wang Q, Chovanec J, Kim J, Wilson KJ, Schwartz LB, Glover SC, Carter MC, Metcalfe DD, Brittain E, Lyons JJ. Defining baseline variability of serum tryptase levels improves accuracy in identifying anaphylaxis. J Allergy Clin Immunol. 2022;149:1010-1017.e10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 59] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 24. | Muraro A, Worm M, Alviani C, Cardona V, DunnGalvin A, Garvey LH, Riggioni C, de Silva D, Angier E, Arasi S, Bellou A, Beyer K, Bijlhout D, Bilò MB, Bindslev-Jensen C, Brockow K, Fernandez-Rivas M, Halken S, Jensen B, Khaleva E, Michaelis LJ, Oude Elberink HNG, Regent L, Sanchez A, Vlieg-Boerstra BJ, Roberts G; European Academy of Allergy and Clinical Immunology, Food Allergy, Anaphylaxis Guidelines Group. EAACI guidelines: Anaphylaxis (2021 update). Allergy. 2022;77:357-377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 224] [Cited by in RCA: 314] [Article Influence: 78.5] [Reference Citation Analysis (0)] |
| 25. | Sirvent AE, González C, Enríquez R, Fernández J, Millán I, Barber X, Amorós F. Serum tryptase levels and markers of renal dysfunction in a population with chronic kidney disease. J Nephrol. 2010;23:282-290. [PubMed] |
| 26. | Jesky MD, Stringer SJ, Fenton A, Ng KP, Yadav P, Ndumbo M, McCann K, Plant T, Dasgupta I, Harding SJ, Drayson MT, Redegeld F, Ferro CJ, Cockwell P. Serum tryptase concentration and progression to end-stage renal disease. Eur J Clin Invest. 2016;46:460-474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 27. | Lu F, Huang S. The Roles of Mast Cells in Parasitic Protozoan Infections. Front Immunol. 2017;8:363. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 41] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 28. | Cooper PJ, Schwartz LB, Irani AM, Awadzi K, Guderian RH, Nutman TB. Association of transient dermal mastocytosis and elevated plasma tryptase levels with development of adverse reactions after treatment of onchocerciasis with ivermectin. J Infect Dis. 2002;186:1307-1313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 29. | Valent P, Sperr WR, Sotlar K, Reiter A, Akin C, Gotlib J, Horny HP, Arock M. The serum tryptase test: an emerging robust biomarker in clinical hematology. Expert Rev Hematol. 2014;7:683-690. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 65] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 30. | Sperr WR, El-Samahi A, Kundi M, Girschikofsky M, Winkler S, Lutz D, Endler G, Rumpold H, Agis H, Sillaber C, Jäger U, Valent P. Elevated tryptase levels selectively cluster in myeloid neoplasms: a novel diagnostic approach and screen marker in clinical haematology. Eur J Clin Invest. 2009;39:914-923. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 76] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 31. | Valent P, Akin C, Escribano L, Födinger M, Hartmann K, Brockow K, Castells M, Sperr WR, Kluin-Nelemans HC, Hamdy NA, Lortholary O, Robyn J, van Doormaal J, Sotlar K, Hauswirth AW, Arock M, Hermine O, Hellmann A, Triggiani M, Niedoszytko M, Schwartz LB, Orfao A, Horny HP, Metcalfe DD. Standards and standardization in mastocytosis: consensus statements on diagnostics, treatment recommendations and response criteria. Eur J Clin Invest. 2007;37:435-453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 617] [Cited by in RCA: 548] [Article Influence: 28.8] [Reference Citation Analysis (0)] |
| 32. | Lyons JJ, Sun G, Stone KD, Nelson C, Wisch L, O'Brien M, Jones N, Lindsley A, Komarow HD, Bai Y, Scott LM, Cantave D, Maric I, Abonia JP, Rothenberg ME, Schwartz LB, Milner JD, Wilson TM. Mendelian inheritance of elevated serum tryptase associated with atopy and connective tissue abnormalities. J Allergy Clin Immunol. 2014;133:1471-1474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 112] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 33. | Lyons JJ, Yu X, Hughes JD, Le QT, Jamil A, Bai Y, Ho N, Zhao M, Liu Y, O'Connell MP, Trivedi NN, Nelson C, DiMaggio T, Jones N, Matthews H, Lewis KL, Oler AJ, Carlson RJ, Arkwright PD, Hong C, Agama S, Wilson TM, Tucker S, Zhang Y, McElwee JJ, Pao M, Glover SC, Rothenberg ME, Hohman RJ, Stone KD, Caughey GH, Heller T, Metcalfe DD, Biesecker LG, Schwartz LB, Milner JD. Elevated basal serum tryptase identifies a multisystem disorder associated with increased TPSAB1 copy number. Nat Genet. 2016;48:1564-1569. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 278] [Cited by in RCA: 296] [Article Influence: 29.6] [Reference Citation Analysis (0)] |
| 34. | Greiner G, Sprinzl B, Górska A, Ratzinger F, Gurbisz M, Witzeneder N, Schmetterer KG, Gisslinger B, Uyanik G, Hadzijusufovic E, Esterbauer H, Gleixner KV, Krauth MT, Pfeilstöcker M, Keil F, Gisslinger H, Nedoszytko B, Niedoszytko M, Sperr WR, Valent P, Hoermann G. Hereditary α tryptasemia is a valid genetic biomarker for severe mediator-related symptoms in mastocytosis. Blood. 2021;137:238-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 145] [Article Influence: 29.0] [Reference Citation Analysis (0)] |
| 35. | Waters AM, Park HJ, Weskamp AL, Mateja A, Kachur ME, Lyons JJ, Rosen BJ, Boggs NA. Elevated Basal Serum Tryptase: Disease Distribution and Variability in a Regional Health System. J Allergy Clin Immunol Pract. 2022;10:2424-2435.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 31] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 36. | O'Connell MP, Lyons JJ. Hymenoptera venom-induced anaphylaxis and hereditary alpha-tryptasemia. Curr Opin Allergy Clin Immunol. 2020;20:431-437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 34] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 37. | Chovanec J, Tunc I, Hughes J, Halstead J, Mateja A, Liu Y, O'Connell MP, Kim J, Park YH, Wang Q, Le Q, Pirooznia M, Trivedi NN, Bai Y, Yin Y, Hsu AP, McElwee J, Lassiter S, Nelson C, Bandoh J, DiMaggio T, Šelb J, Rijavec M, Carter MC, Komarow HD, Sabato V, Steinberg J, Hafer KM, Feuille E, Hourigan CS, Lack J, Khoury P, Maric I, Zanotti R, Bonadonna P, Schwartz LB, Milner JD, Glover SC, Ebo DG, Korošec P, Caughey GH, Brittain EH, Busby B, Metcalfe DD, Lyons JJ. Genetically defined individual reference ranges for tryptase limit unnecessary procedures and unmask myeloid neoplasms. Blood Adv. 2023;7:1796-1810. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 44] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 38. | Chollet MB, Akin C. Hereditary alpha tryptasemia is not associated with specific clinical phenotypes. J Allergy Clin Immunol. 2022;149:728-735.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 65] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 39. | Lyons JJ. Hereditary Alpha Tryptasemia: Genotyping and Associated Clinical Features. Immunol Allergy Clin North Am. 2018;38:483-495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 130] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 40. | Luskin KT, White AA, Lyons JJ. The Genetic Basis and Clinical Impact of Hereditary Alpha-Tryptasemia. J Allergy Clin Immunol Pract. 2021;9:2235-2242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 48] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 41. | von Bubnoff D, Koch D, Stocker H, Ludwig RJ, Wortmann F, von Bubnoff N. The Clinical Features of Hereditary Alpha-Tryptasemia—Implications for Interdisciplinary Practice. Dtsch Arztebl Int. 2024;121:258-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 42. | Robey RC, Wilcock A, Bonin H, Beaman G, Myers B, Grattan C, Briggs TA, Arkwright PD. Hereditary Alpha-Tryptasemia: UK Prevalence and Variability in Disease Expression. J Allergy Clin Immunol Pract. 2020;8:3549-3556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 80] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 43. | Wu R, Lyons JJ. Hereditary Alpha-Tryptasemia: a Commonly Inherited Modifier of Anaphylaxis. Curr Allergy Asthma Rep. 2021;21:33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 29] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 44. | Polivka L, Madrange M, Bulai-Livideanu C, Barete S, Ballul T, Neuraz A, Greco C, Agopian J, Brenet F, Dubreuil P, Burdet C, Lemal R, Tournilhac O, Terriou L, Launay D, Bouillet L, Gourguechon C, Damaj G, Frenzel L, Meni C, Bouktit H, Collange AF, Gaudy-Marqueste C, Gousseff M, Le Mouel E, Hamidou M, Neel A, Ranta D, Jaussaud R, Guilpain P, Canioni D, Molina TJ, Bruneau J, Lhermitte L, Garcelon N, Javier RM, Pelletier F, Castelain F, Retornaz F, Cabrera Q, Zunic P, Gourin MP, Wierzbicka-Hainaut E, Viallard JF, Lavigne C, Hoarau C, Durieu I, Heiblig M, Dimicoli-Salazar S, Torregrosa-Diaz JM, Soria A, Arock M, Lortholary O, Bodemer C, Hermine O, Rossignol J; CEREMAST network. Pathophysiologic implications of elevated prevalence of hereditary alpha-tryptasemia in all mastocytosis subtypes. J Allergy Clin Immunol. 2024;153:349-353.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 23] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 45. | Lyons JJ, Chovanec J, O'Connell MP, Liu Y, Šelb J, Zanotti R, Bai Y, Kim J, Le QT, DiMaggio T, Schwartz LB, Komarow HD, Rijavec M, Carter MC, Milner JD, Bonadonna P, Metcalfe DD, Korošec P. Heritable risk for severe anaphylaxis associated with increased α-tryptase-encoding germline copy number at TPSAB1. J Allergy Clin Immunol. 2021;147:622-632. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 175] [Article Influence: 29.2] [Reference Citation Analysis (0)] |
| 46. | Šelb J, Rijavec M, Eržen R, Zidarn M, Kopač P, Škerget M, Bajrović N, Luzar AD, Park YH, Liu Y, Šerbec VČ, Zver S, Košnik M, Lyons JJ, Korošec P. Routine KIT p.D816V screening identifies clonal mast cell disease in patients with Hymenoptera allergy regularly missed using baseline tryptase levels alone. J Allergy Clin Immunol. 2021;148:621-626.e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 49] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 47. | Šelb J, Rijavec M, Kopač P, Lyons JJ, Korošec P. HαT is associated with increased risk for severe Hymenoptera venom-triggered anaphylaxis. J Allergy Clin Immunol. 2023;151:804-805. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 8] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 48. | Khoury P, Lyons JJ. Mast cell activation in the context of elevated basal serum tryptase: genetics and presentations. Curr Allergy Asthma Rep. 2019;19:55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 24] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 49. | Valent P, Hartmann K, Schwaab J, Alvarez-Twose I, Brockow K, Bonadonna P, Hermine O, Niedoszytko M, Carter MC, Hoermann G, Sperr WR, Butterfield JH, Ustun C, Zanotti R, Radia DH, Castells M, Triggiani M, Schwartz LB, Orfao A, George TI, Sotlar K, Gotlib J, Reiter A, Horny HP, Arock M, Akin C, Metcalfe DD. Personalized Management Strategies in Mast Cell Disorders: ECNM-AIM User's Guide for Daily Clinical Practice. J Allergy Clin Immunol Pract. 2022;10:1999-2012.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 55] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 50. | Hoermann G, Sotlar K, Jawhar M, Kristensen T, Bachelot G, Nedoszytko B, Carter MC, Horny HP, Bonadonna P, Sperr WR, Hartmann K, Brockow K, Lyons JJ, Kluin-Nelemans HC, Hermine O, Akin C, Broesby-Olsen S, Triggiani M, Butterfield JH, Schwaab J, Reiter A, Gotlib J, Metcalfe DD, George TI, Orfao A, Valent P, Arock M. Standards of Genetic Testing in the Diagnosis and Prognostication of Systemic Mastocytosis in 2022: Recommendations of the EU-US Cooperative Group. J Allergy Clin Immunol Pract. 2022;10:1953-1963. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 37] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 51. | Arock M, Hoermann G, Sotlar K, Hermine O, Sperr WR, Hartmann K, Brockow K, Akin C, Triggiani M, Broesby-Olsen S, Reiter A, Gotlib J, Horny HP, Orfao A, Metcalfe DD, Valent P. Clinical impact and proposed application of molecular markers, genetic variants, and cytogenetic analysis in mast cell neoplasms: Status 2022. J Allergy Clin Immunol. 2022;149:1855-1865. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 28] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 52. | Valent P, Akin C, Hartmann K, Nilsson G, Reiter A, Hermine O, Sotlar K, Sperr WR, Escribano L, George TI, Kluin-Nelemans HC, Ustun C, Triggiani M, Brockow K, Gotlib J, Orfao A, Schwartz LB, Broesby-Olsen S, Bindslev-Jensen C, Kovanen PT, Galli SJ, Austen KF, Arber DA, Horny HP, Arock M, Metcalfe DD. Advances in the Classification and Treatment of Mastocytosis: Current Status and Outlook toward the Future. Cancer Res. 2017;77:1261-1270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 175] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 53. | Valent P, Akin C, Gleixner KV, Sperr WR, Reiter A, Arock M, Triggiani M. Multidisciplinary Challenges in Mastocytosis and How to Address with Personalized Medicine Approaches. Int J Mol Sci. 2019;20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 64] [Article Influence: 9.1] [Reference Citation Analysis (1)] |
| 54. | Cardona V, Ansotegui IJ, Ebisawa M, El-Gamal Y, Fernandez Rivas M, Fineman S, Geller M, Gonzalez-Estrada A, Greenberger PA, Sanchez Borges M, Senna G, Sheikh A, Tanno LK, Thong BY, Turner PJ, Worm M. World allergy organization anaphylaxis guidance 2020. World Allergy Organ J. 2020;13:100472. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 522] [Cited by in RCA: 625] [Article Influence: 104.2] [Reference Citation Analysis (0)] |
| 55. | Mendoza Alvarez LB, Barker R, Nelson C, DiMaggio T, Stone KD, Milner JD, Rosenthal JA, Petroni DH, Glover SC, Lyons JJ. Clinical response to omalizumab in patients with hereditary α-tryptasemia. Ann Allergy Asthma Immunol. 2020;124:99-100.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 56. | Giannetti MP, Weller E, Bormans C, Novak P, Hamilton MJ, Castells M. Hereditary alpha-tryptasemia in 101 patients with mast cell activation-related symptomatology including anaphylaxis. Ann Allergy Asthma Immunol. 2021;126:655-660. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 60] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 57. | Bonadonna P, Nalin F, Olivieri F. Hereditary alpha-tryptasemia. Curr Opin Allergy Clin Immunol. 2022;22:277-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 58. | Youngblood BA, Brock EC, Leung J, Falahati R, Bryce PJ, Bright J, Williams J, Shultz LD, Greiner DL, Brehm MA, Bebbington C, Tomasevic N. AK002, a Humanized Sialic Acid-Binding Immunoglobulin-Like Lectin-8 Antibody that Induces Antibody-Dependent Cell-Mediated Cytotoxicity against Human Eosinophils and Inhibits Mast Cell-Mediated Anaphylaxis in Mice. Int Arch Allergy Immunol. 2019;180:91-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 89] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 59. | Maun HR, Jackman JK, Choy DF, Loyet KM, Staton TL, Jia G, Dressen A, Hackney JA, Bremer M, Walters BT, Vij R, Chen X, Trivedi NN, Morando A, Lipari MT, Franke Y, Wu X, Zhang J, Liu J, Wu P, Chang D, Orozco LD, Christensen E, Wong M, Corpuz R, Hang JQ, Lutman J, Sukumaran S, Wu Y, Ubhayakar S, Liang X, Schwartz LB, Babina M, Woodruff PG, Fahy JV, Ahuja R, Caughey GH, Kusi A, Dennis MS, Eigenbrot C, Kirchhofer D, Austin CD, Wu LC, Koerber JT, Lee WP, Yaspan BL, Alatsis KR, Arron JR, Lazarus RA, Yi T. An Allosteric Anti-tryptase Antibody for the Treatment of Mast Cell-Mediated Severe Asthma. Cell. 2019;179:417-431.e19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 86] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/