Published online Jan 16, 2025. doi: 10.12998/wjcc.v13.i2.96876
Revised: October 15, 2024
Accepted: October 24, 2024
Published online: January 16, 2025
Processing time: 174 Days and 16.1 Hours
The classification of uterine sarcomas is based on distinctive morphological and immunophenotypic characteristics, increasingly supported by molecular genetic diagnostics. Data on neurotrophic tyrosine receptor kinase (NTRK) gene fusion-positive uterine sarcoma, potentially aggressive and morphologically similar to fibrosarcoma, are limited due to its recent recognition. Pan-TRK immunohistochemistry (IHC) analysis serves as an effective screening tool with high sensitivity and specificity for NTRK-fusion malignancies.
We report a case of a malignant mesenchymal tumor originating from the uterine cervix, which was pan-TRK IHC-positive but lacked NTRK gene fusions, accom
The clinical significance of NTRK gene fusion lies in potential treatment with TRK inhibitors for positive sarcomas. Identifying such rare tumors is crucial due to the potential applicability of tropomyosin receptor kinase inhibitor treatment.
Core Tip: This case report highlights a rare malignant mesenchymal tumor originating from the uterine cervix that was pan-TRK immunohistochemistry-positive but lacked neurotrophic tyrosine receptor kinase (NTRK) gene fusions. Although NTRK-fusion sarcomas are aggressive and may benefit from targeted TRK inhibitor therapy, this case underscores the importance of molecular genetic diagnostics to guide accurate treatment decisions. The report contributes to the limited literature on NTRK-positive uterine sarcomas and emphasizes the potential clinical value of TRK inhibitors in selected cases.
- Citation: Lee S, Jeon YR, Shin C, Kwon SY, Shin S. Pan-TRK positive uterine sarcoma in immunohistochemistry without neurotrophic tyrosine receptor kinase gene fusions: A case report. World J Clin Cases 2025; 13(2): 96876
- URL: https://www.wjgnet.com/2307-8960/full/v13/i2/96876.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v13.i2.96876
Sarcoma of uterine cervix is very less common histological type of cervical cancer. This, information regarding the incidence and characteristics of sarcoma of the cervix is based on smaller case series. In a hospital-based tumor registry, 8 cervical sarcomas were identified among 1583 cervical malignancies[1]. Treatment of such rare cervical tumors is not currently included in the National Comprehensive Cancer Network Guidelines for cervical cancer and current treatment strategies may be extrapolated from data regarding uterine sarcomas and soft tissue sarcomas.
The classification of cervical sarcomas is based on distinctive morphological and immunophenotypic characteristics, increasingly supported by molecular genetic diagnostics[2]. Neurotrophic tyrosine receptor kinase (NTRK) fusion-positive uterine sarcoma is a recently recognized mesenchymal tumor that is defined by its morphologic resemblance to soft tissue fibrosarcoma[3]. Pan-TRK immunohistochemistry (IHC) analysis serves as an effective screening tool with high sensitivity and specificity for NTRK-fusion malignancies. The clinical significance of NTRK gene fusion lies in the potential treatment with TRK inhibitors for positive sarcomas. We report a case of a malignant mesenchymal tumor originating from the uterine cervix, which was pan-TRK IHC-positive but lacked NTRK gene fusions.
A 55-year-old woman (gravida 1, para 1) was admitted to the emergency department with the chief complaints of progressive abdominal distension and dyspnea.
Her symptoms, which had developed over the preceding 2 weeks, were accompanied by a body mass index of 19 and an unremarkable medical history.
No significant past history or any illness or surgery.
No relevant family history contributed to the current condition.
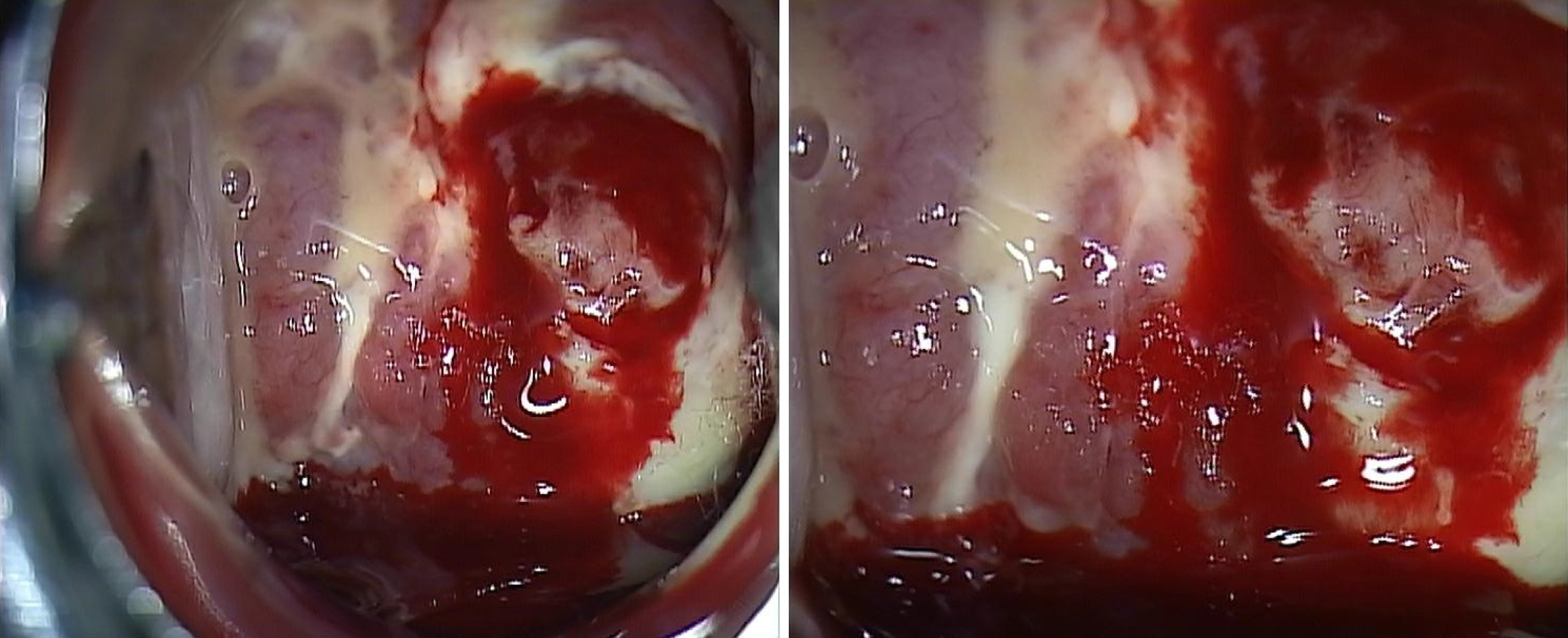
Pelvic examination revealed a tumor engulfing the uterine cervix and extensively invading the vagina and uterine corpus. Colposcopy indicated abnormal vasculature and necrotic change in the cervical mass, with heavy bleeding upon touching. A cervical punch biopsy was performed during the colposcopy (Figure 1).
The patient initially underwent tests for CA125, HE4, and SCC, with results of 30.4 U/mL, 29.4 pmol/mL, and 0.66 ng/mL, respectively, all within normal ranges. For follow-up tests, only SCC was measured, and it remained within the normal range, below 1 ng/mL, during chemotherapy.
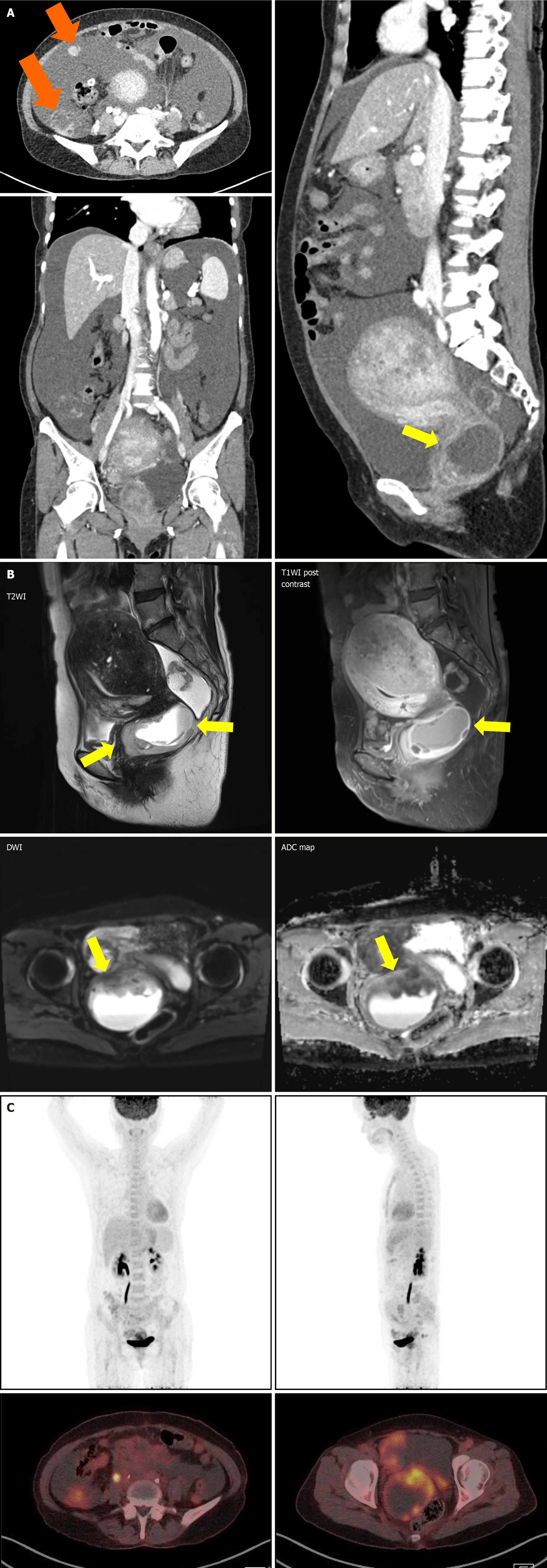
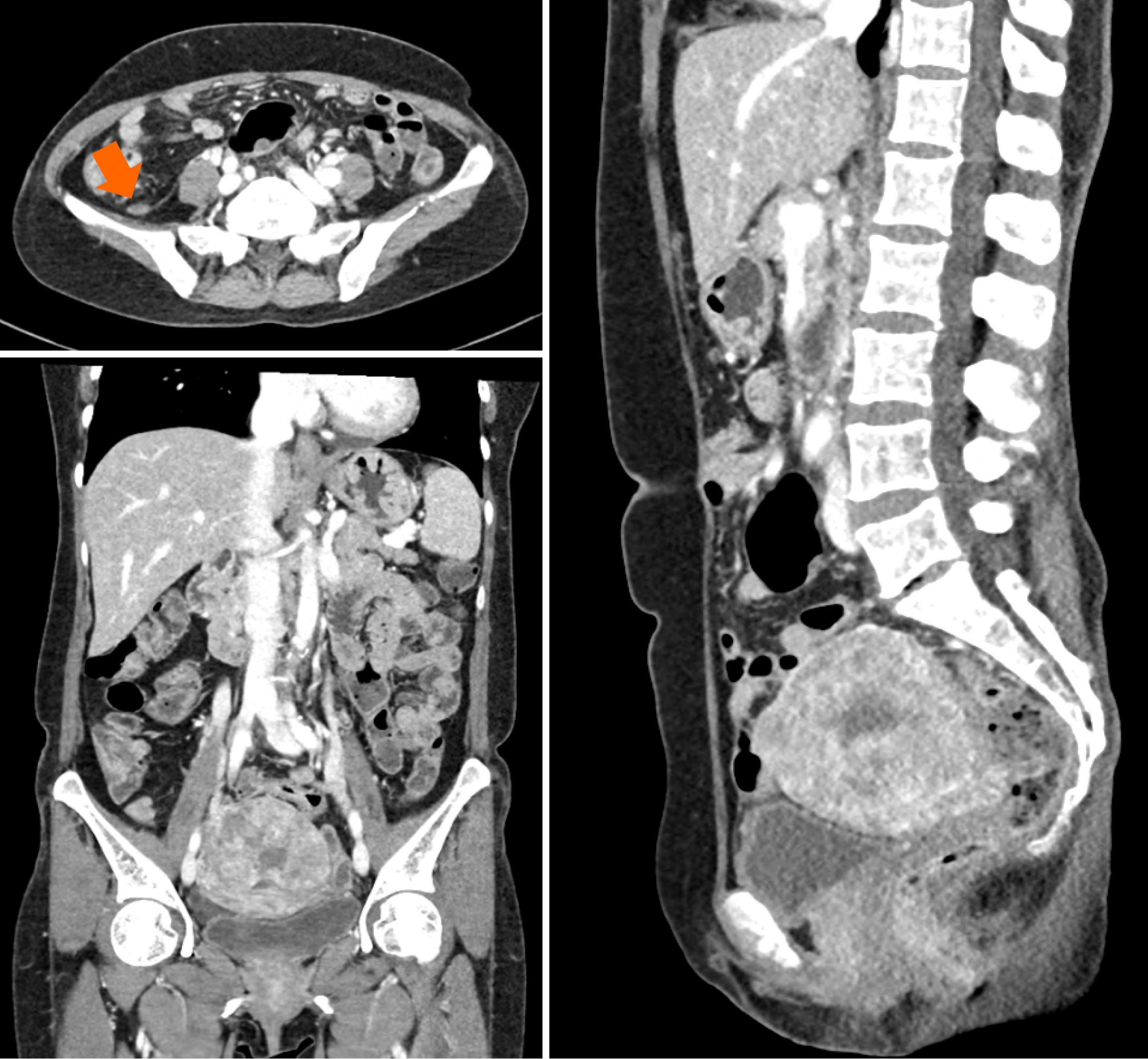
Computed tomography (CT) of the abdomen and pelvis showed significant ascites and multiple ill-defined lesions along the pelvic peritoneum, including a 6 cm mass in the pelvic cavity, initially suspected to be ovarian in origin. However, laboratory tests did not reveal any specific findings to confirm this. Consequently, ovarian cancer with peritoneal carcinomatosis was considered as a preliminary diagnosis, necessitating further imaging for clarification.
Magnetic resonance imaging (MRI) of the pelvis revealed a 4.5 cm infiltrative mass in the cervix, significant hematocolpos, and parametrial invasion. Pronounced adenomyosis was observed in the posterior uterine wall. Diffuse hemoperitoneum and peritoneal thickening, suggestive of peritoneal seeding, were also noted (Figure 2A). A Subsequent positron emission tomography-CT (PET-CT) scan showed a large hypermetabolic cervical mass (SUVmax: 9.8) invading the upper-mid-vagina and uterine corpus, with extensive central necrosis. This scan also revealed right sided hydronephroureterosis and multiple hypermetabolic lesions in the right pelvis and paracolic gutter (Figure 2B), leading to a diagnosis of advanced cervical cancer.
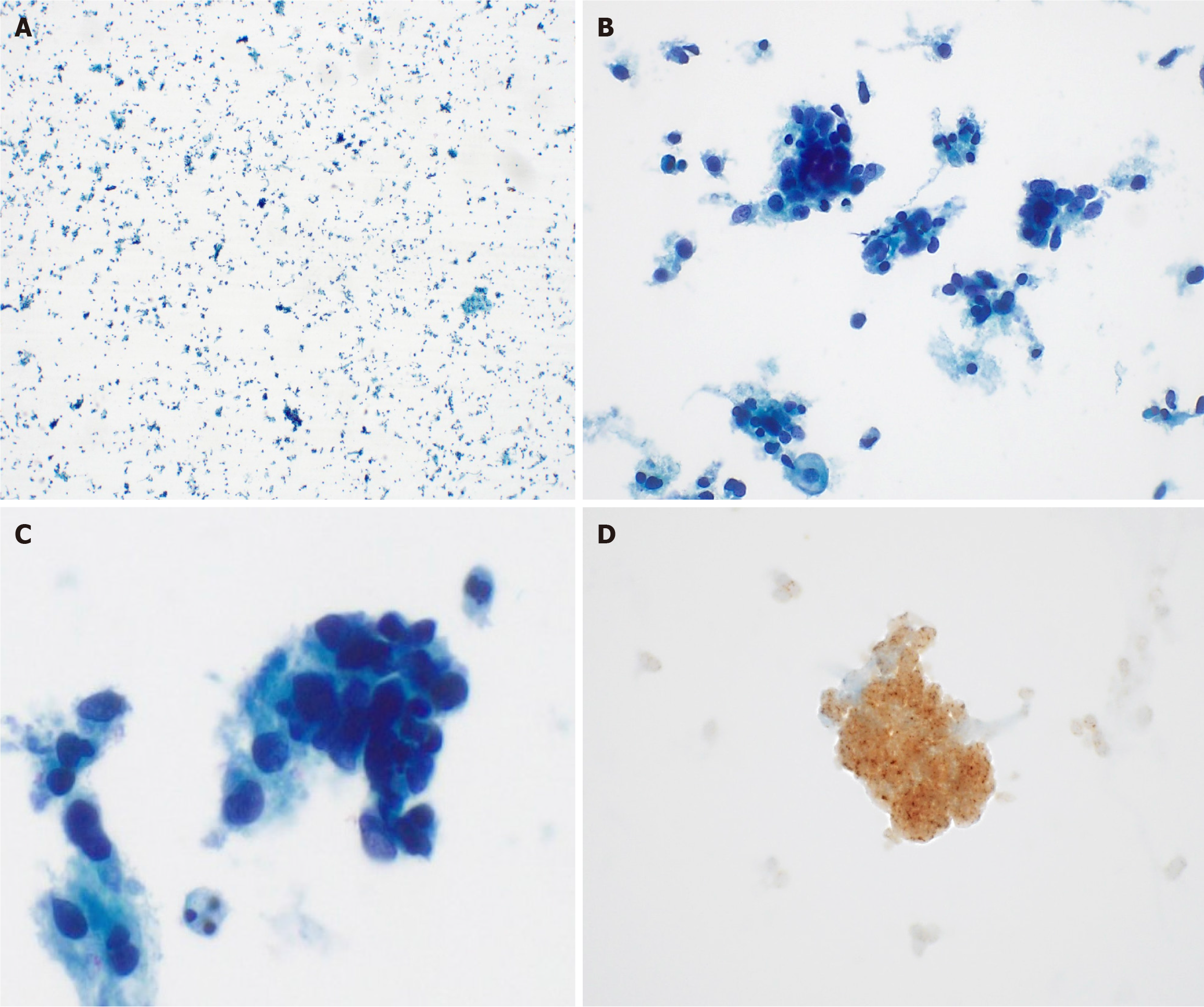
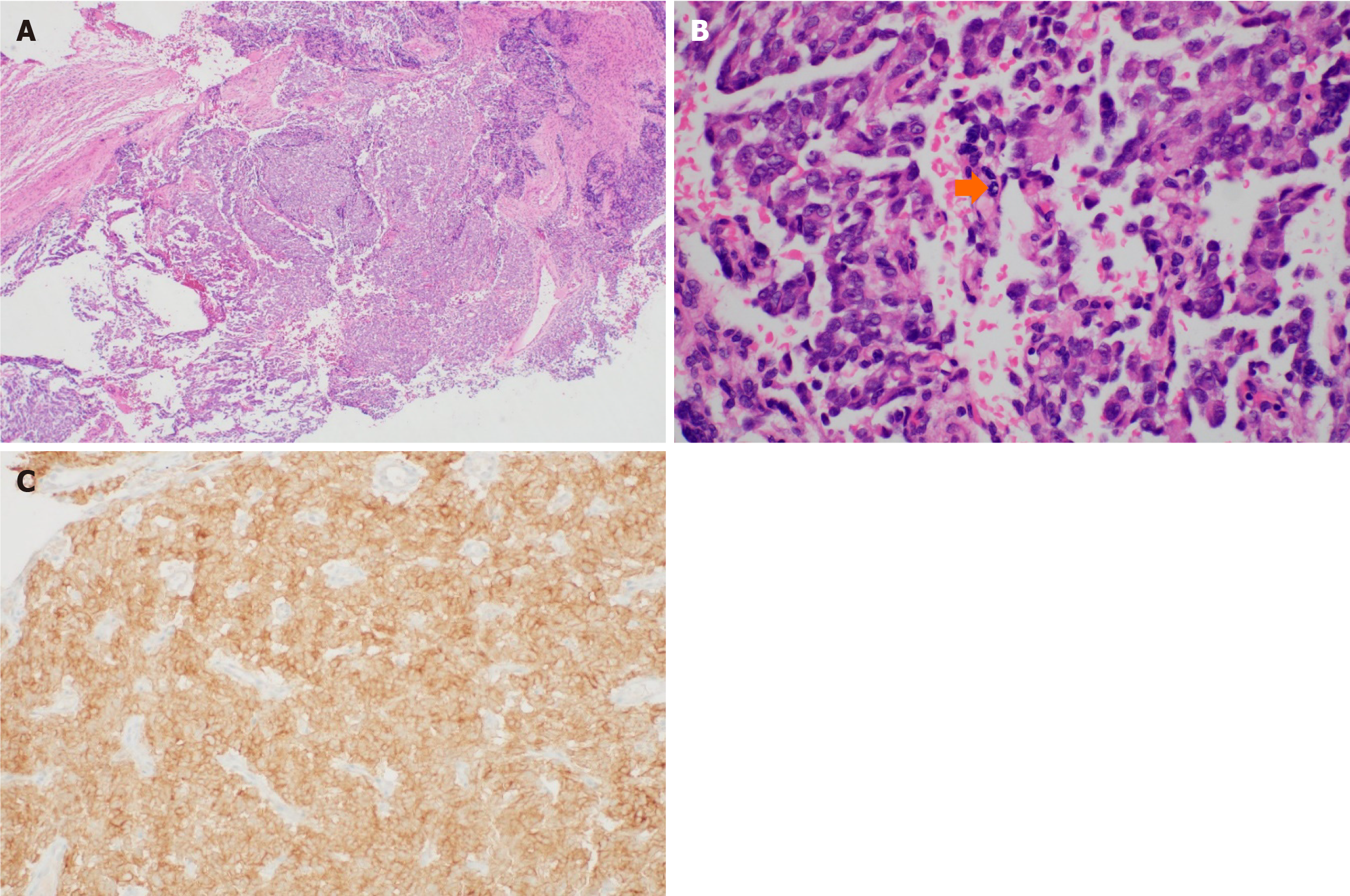
Liquid-based cytology detected ovoid to round cells with hyperchromatic nuclei. IHC exhibited diffuse strong positivity for pan-TRK (Figure 3). The cervical punch biopsy yielded infiltrating tumor cells arranged in a sheet pattern, displaying vesicular nuclei and distinct nucleoli. Mitotic activity was commonly observed as well as some necrotic areas. As in the cytology specimen, pan-TRK staining was positive (Figure 4). Subsequent next-generation sequencing (NGS), the sole method available at our institution to detect NTRK gene fusion, did not identify any relevant variants, including NTRK gene fusion.
Consequently, combination chemotherapy was chosen as the first-line treatment. The patient was started on a regimen of mesna, doxorubicin, ifosfamide, and dacarbazine (MAID).
After six cycles of MAID chemotherapy, a follow-up pelvic CT was performed to assess the treatment response. The 5 cm cervical tumor observed in the pre-treatment images had completely disappeared after chemotherapy, and the ascites had also resolved. Most of the multiple peritoneal seeding observed in the right paracolic gutter, which measured up to 4 cm, had improved, leaving only small peritoneal seeding less than 1 cm (Figure 5). The patient is currently undergoing concurrent chemoradiation with cisplatin as second-line treatment.
NTRK gene fusions, first identified in colon carcinoma in 1982[4], have gain significant attention in oncology, particularly in sarcoma research. Notably, infantile fibrosarcoma demonstrates NTRK gene fusion in over 90% of the cases[5]. These fusions are characterized as ‘druggable’ alterations, responsive to TRK inhibitors regardless of the tumor’s tissue of origin.
Recent advancements in tumor-agnostic therapies, particularly TRK inhibitors, have shown promising efficacy and safety. The oncologic outcomes of TRK inhibitors in previous studies are presented in Table 1. Most studies have not focused solely on the uterine cervix but have utilized TRK inhibitors across various organs with NTRK gene fusion, evaluating the efficacy based on the NTRK status. Larotrectinib, a pioneering ATP-competitive small-molecule TRK inhibitor, demonstrated significant effectiveness in TRK fusion cancers across various age groups and tumor types in a combined analysis of three phase I/II trials[6]. An integrated analysis involving 159 patients revealed an objective response rate (ORR) of 79% [95% confidence interval (CI): 72% to 85%], median response duration of 35.2 months (median follow-up 12.9 months), and a median time to response of 1.8 months[7]. Larotrectinib’s favorable safety profile, combined with its clinical efficacy, has translated into substantial, sustained improvements in the quality of life[8].
| Ref. | TRKi | Sample size | Tumor type | Objective response rate (%) | Duration of response (months) | Progression free survival (months) | Overall survival (months) |
| Demetri et al[9] | Entrectinib | 121 | Sarcoma, salivary, NSCLC, etc. | 61.2 | 20 (95%CI: 13.0-38.2) | 13.8 (95%CI: 23.4-46.4) | 33.8 (95%CI: 23.4-46.4) |
| Entrectinib | 26 | Sarcoma | 57.7 | 15.0 (95%CI: 4.6-NE) | 10.1 (95%CI: 6.3-13.7) | 18.7 (95%CI: 14.5-NE) | |
| Drilon[10] | Larotrectinib | 122 | Salivary, infantile fibrosarcoma, thyroid cancer, lung cancer, etc. | 81 | Not reached | Not reached | Not reached |
| Entrectinib | 54 | Sarcoma, lung cancer, mammary analogue secretory carcinoma, etc. | 58 | 10.4 | 11.2 | 20.9 | |
| Suh et al[11] | Larotrectinib | 23 | Soft tissue sarcoma | 52.2 | Not reached | Not reached | |
| Entrectinib | 16 | Soft tissue sarcoma | 56.3 | 10.1 | 16.8 | ||
| Demetri et al[12] | Larotrectinib | 71 | Sarcoma, salivary, NSCLC, etc. | 87 (95%CI: 77-94) | Not reached | 28.3 (95%CI: 16.8-NE) | 44.4 (95%CI: 44.4-NE) |
| Entrectinib | 13 | Sarcoma, NSCLC, salivary, breast, thyroid | 46 (95%CI: 19-75) | 10.3 (95%CI: 4.6-15.0) | 11.0 (95%CI: 6.5-15.7) | 16.8 (95%CI: 10.6-20.9) |
Entrectinib, a multi-targeted, inhibitor active against pan-TRK, c-ros oncogene 1 (ROS1), and anaplastic lymphoma kinase (ALK), has similarly shown tumor-agnostic efficacy. An integrated analysis from phase I/II trials reported and independently assessed an ORR of 57% (95%CI: 43% to 71%) and a median response duration of 10.4 months (median follow-up 12.9 months)[13]. Notably, treatment-naïve patients demonstrated higher response rates than those who had received prior systemic therapy. Entrectinib led to a higher response rate in treatment-naïve patients with metastatic disease (n = 30/37; ORR = 81.1%) vs those who had received more than 1 line of prior systemic therapy (n = 44/84; ORR = 52.4%)[9].
The detection of NTRK gene fusion is crucial, as it influences the consideration of TRK inhibitors in management strategies for patients with confirmed NTRK gene rearrangement. However, the rarity of NTRK fusions in sarcomas necessitates reliable diagnostic tools and strategies. Techniques such as IHC, fluorescent in situ hybridization (FISH), reverse transcription polymerase chain reaction, and NGS vary in sensitivity, specificity, and turnaround time (Table 2). Pan-TRK IHC is advantageous due to its high sensitivity, specificity, and cost-effectiveness, making it a practical screening tool. However, it is not definitive for determining treatment plans[14]. FISH, while sensitive and specific, can yield false negatives in cases of noncanonical breakpoints or novel genes[15,16].
| Sensitivity | Specificity | Detection of all fusions | Detection of fusion partner | Detection of protein expression | Turnaround time | Material required | |
| IHC | Relatively high (NTRK1 and 2: 75%-96%, NTRK3: 50%-70%) | Relatively high | + | - | + | 1 day | 1 unstained slide |
| FISH | High | High | One per probe | One per probe | - | 1-3 days | 3 unstained slide |
| RNA NGS | High | High | + | + | + | 2-4 weeks | 15 unstained slide |
| DNA NGS | Moderate | High | + | + | - | 2-4 weeks | 10 unstained slide |
NGS, particularly RNA-based, offers high specificity and sensitivity. DNA-based NGS can assess multiple genomic alterations simultaneously, including mutations, amplifications, deletions, microsatellite instability status, and tumor mutation burden and fusions[16]. In contrast, RNA-based NGS offers a more accurate characterization of transcription evidence and specific gene involvement. However, the quality of RNA remains a limiting factor in RNA-based se
Despite the reliability of these methods, strong pan-TRK IHC expression without NTRK gene fusion is seen in less than 2% of uterine leiomyosarcomas[18]. Not all positive IHC or FISH findings indicate actual genes, highlighting the need for confirmatory gene analysis. Thus, accurate diagnosis, pivotal for targeted therapy, requires careful the interpretation of diagnostic tool.
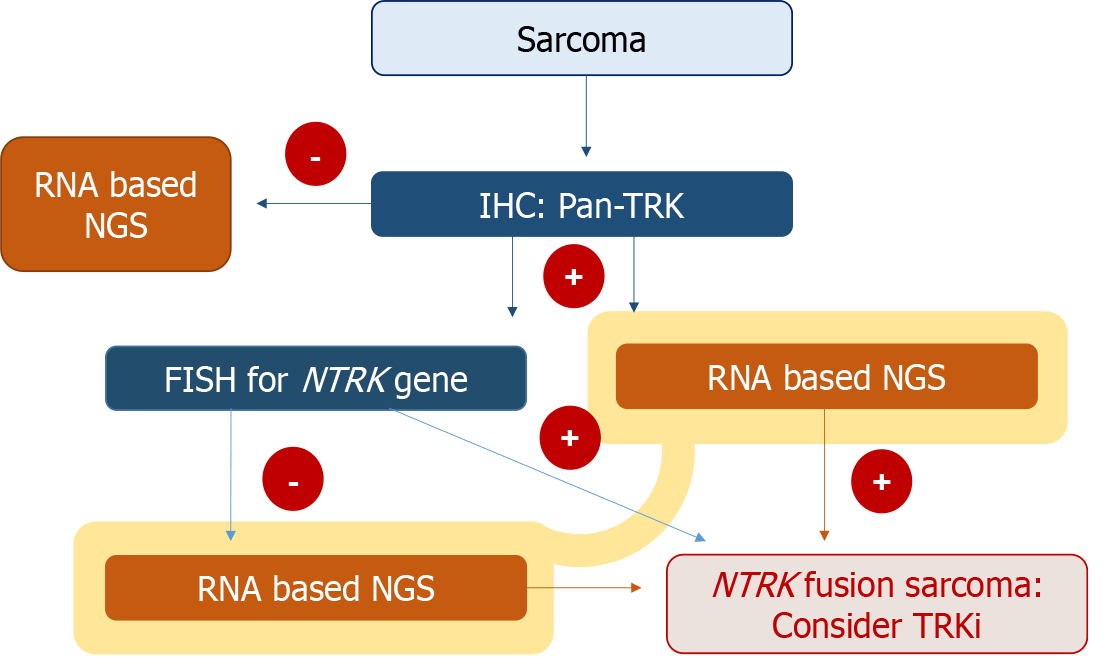
In cases like that of our patient, where pan-TRK IHC shows positivity, but NGS reveals negative results for NTRK gene fusion, it is imperative to corroborate the findings with NGS, even in the presence of positive IHC results. The diagnostic algorithm presented in Figure 6 outlines and approach for assessing NTRK gene fusion in sarcoma. It suggests that a positive pan-Trk IHC result, while indicative, necessitates further testing through RNA-based NGS (preferred for its sensitivity), DNA-based NGS, or FISH to definitively determine the NTRK fusion status for treatment decisions[19].
Sarcoma histotypes shows a relatively low incidence of NTRK rearrangement of less than 2%, necessitating a comprehensive, multi-step diagnostic process for accurate identification. Despite their rarity, the clinical significance of these tumors, particularly when located in the cervix, cannot be understated due to the potential effectiveness of TRK inhibitors in their treatment. Therefore, a two-tiered diagnostic approach will be beneficial for suspected NTRK gene fusion sarcomas. This approach should commence with pan-TRK IHC screening, followed by NGS in cases where protein expression is detected.
| 1. | Wright JD, Rosenblum K, Huettner PC, Mutch DG, Rader JS, Powell MA, Gibb RK. Cervical sarcomas: an analysis of incidence and outcome. Gynecol Oncol. 2005;99:348-351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 45] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 2. | Mariño-Enríquez A, Bovée JV. Molecular Pathogenesis and Diagnostic, Prognostic and Predictive Molecular Markers in Sarcoma. Surg Pathol Clin. 2016;9:457-473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 42] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 3. | Chiang S. S100 and Pan-Trk Staining to Report NTRK Fusion-Positive Uterine Sarcoma: Proceedings of the ISGyP Companion Society Session at the 2020 USCAP Annual Meeting. Int J Gynecol Pathol. 2021;40:24-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 4. | Martin-Zanca D, Hughes SH, Barbacid M. A human oncogene formed by the fusion of truncated tropomyosin and protein tyrosine kinase sequences. Nature. 1986;319:743-748. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 520] [Cited by in RCA: 595] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 5. | Siozopoulou V, Smits E, De Winne K, Marcq E, Pauwels P. NTRK Fusions in Sarcomas: Diagnostic Challenges and Clinical Aspects. Diagnostics (Basel). 2021;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 35] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 6. | Drilon A, Laetsch TW, Kummar S, DuBois SG, Lassen UN, Demetri GD, Nathenson M, Doebele RC, Farago AF, Pappo AS, Turpin B, Dowlati A, Brose MS, Mascarenhas L, Federman N, Berlin J, El-Deiry WS, Baik C, Deeken J, Boni V, Nagasubramanian R, Taylor M, Rudzinski ER, Meric-Bernstam F, Sohal DPS, Ma PC, Raez LE, Hechtman JF, Benayed R, Ladanyi M, Tuch BB, Ebata K, Cruickshank S, Ku NC, Cox MC, Hawkins DS, Hong DS, Hyman DM. Efficacy of Larotrectinib in TRK Fusion-Positive Cancers in Adults and Children. N Engl J Med. 2018;378:731-739. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1577] [Cited by in RCA: 2072] [Article Influence: 259.0] [Reference Citation Analysis (0)] |
| 7. | Hong DS, DuBois SG, Kummar S, Farago AF, Albert CM, Rohrberg KS, van Tilburg CM, Nagasubramanian R, Berlin JD, Federman N, Mascarenhas L, Geoerger B, Dowlati A, Pappo AS, Bielack S, Doz F, McDermott R, Patel JD, Schilder RJ, Tahara M, Pfister SM, Witt O, Ladanyi M, Rudzinski ER, Nanda S, Childs BH, Laetsch TW, Hyman DM, Drilon A. Larotrectinib in patients with TRK fusion-positive solid tumours: a pooled analysis of three phase 1/2 clinical trials. Lancet Oncol. 2020;21:531-540. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 335] [Cited by in RCA: 757] [Article Influence: 126.2] [Reference Citation Analysis (0)] |
| 8. | Kummar S, Mascarenhas L, Geoerger B, Turpin B, Cox MC, Yu S, Nanda S, Hiemeyer F, Keating KN, Chirila C, Gnanasakthy A, Davenport E, Hong DS, Drilon AE. Patient-reported outcomes from two global multicenter clinical trials of children and adults with tropomyosin receptor kinase (TRK) fusion cancer receiving larotrectinib. JCO. 2019;37:6602-6602. [RCA] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 9. | Demetri GD, De Braud F, Drilon A, Siena S, Patel MR, Cho BC, Liu SV, Ahn MJ, Chiu CH, Lin JJ, Goto K, Lee J, Bazhenova L, John T, Fakih M, Chawla SP, Dziadziuszko R, Seto T, Heinzmann S, Pitcher B, Chen D, Wilson TR, Rolfo C. Updated Integrated Analysis of the Efficacy and Safety of Entrectinib in Patients With NTRK Fusion-Positive Solid Tumors. Clin Cancer Res. 2022;28:1302-1312. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 150] [Article Influence: 37.5] [Reference Citation Analysis (0)] |
| 10. | Drilon A. TRK inhibitors in TRK fusion-positive cancers. Ann Oncol. 2019;30:viii23-viii30. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 166] [Cited by in RCA: 201] [Article Influence: 28.7] [Reference Citation Analysis (0)] |
| 11. | Suh K, Carlson JJ, Xia F, Williamson T, Sullivan SD. Comparative effectiveness of larotrectinib versus entrectinib for the treatment of metastatic NTRK gene fusion cancers. J Comp Eff Res. 2022;11:1011-1019. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 12. | Demetri GD, Antonescu CR, Bjerkehagen B, Bovée JVMG, Boye K, Chacón M, Dei Tos AP, Desai J, Fletcher JA, Gelderblom H, George S, Gronchi A, Haas RL, Hindi N, Hohenberger P, Joensuu H, Jones RL, Judson I, Kang YK, Kawai A, Lazar AJ, Le Cesne A, Maestro R, Maki RG, Martín J, Patel S, Penault-Llorca F, Premanand Raut C, Rutkowski P, Safwat A, Sbaraglia M, Schaefer IM, Shen L, Serrano C, Schöffski P, Stacchiotti S, Sundby Hall K, Tap WD, Thomas DM, Trent J, Valverde C, van der Graaf WTA, von Mehren M, Wagner A, Wardelmann E, Naito Y, Zalcberg J, Blay JY. Diagnosis and management of tropomyosin receptor kinase (TRK) fusion sarcomas: expert recommendations from the World Sarcoma Network. Ann Oncol. 2020;31:1506-1517. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 101] [Cited by in RCA: 121] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 13. | Doebele RC, Drilon A, Paz-Ares L, Siena S, Shaw AT, Farago AF, Blakely CM, Seto T, Cho BC, Tosi D, Besse B, Chawla SP, Bazhenova L, Krauss JC, Chae YK, Barve M, Garrido-Laguna I, Liu SV, Conkling P, John T, Fakih M, Sigal D, Loong HH, Buchschacher GL Jr, Garrido P, Nieva J, Steuer C, Overbeck TR, Bowles DW, Fox E, Riehl T, Chow-Maneval E, Simmons B, Cui N, Johnson A, Eng S, Wilson TR, Demetri GD; trial investigators. Entrectinib in patients with advanced or metastatic NTRK fusion-positive solid tumours: integrated analysis of three phase 1-2 trials. Lancet Oncol. 2020;21:271-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 574] [Cited by in RCA: 1233] [Article Influence: 176.1] [Reference Citation Analysis (0)] |
| 14. | Durzyńska M, Michałek IM. Pan-TRK immunohistochemistry as a tool in the screening for NTRK gene fusions in cancer patients. Oncol Clin Pract. 2024;20:15-21. [DOI] [Full Text] |
| 15. | Church AJ, Calicchio ML, Nardi V, Skalova A, Pinto A, Dillon DA, Gomez-Fernandez CR, Manoj N, Haimes JD, Stahl JA, Dela Cruz FS, Tannenbaum-Dvir S, Glade-Bender JL, Kung AL, DuBois SG, Kozakewich HP, Janeway KA, Perez-Atayde AR, Harris MH. Recurrent EML4-NTRK3 fusions in infantile fibrosarcoma and congenital mesoblastic nephroma suggest a revised testing strategy. Mod Pathol. 2018;31:463-473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 121] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 16. | Zehir A, Benayed R, Shah RH, Syed A, Middha S, Kim HR, Srinivasan P, Gao J, Chakravarty D, Devlin SM, Hellmann MD, Barron DA, Schram AM, Hameed M, Dogan S, Ross DS, Hechtman JF, DeLair DF, Yao J, Mandelker DL, Cheng DT, Chandramohan R, Mohanty AS, Ptashkin RN, Jayakumaran G, Prasad M, Syed MH, Rema AB, Liu ZY, Nafa K, Borsu L, Sadowska J, Casanova J, Bacares R, Kiecka IJ, Razumova A, Son JB, Stewart L, Baldi T, Mullaney KA, Al-Ahmadie H, Vakiani E, Abeshouse AA, Penson AV, Jonsson P, Camacho N, Chang MT, Won HH, Gross BE, Kundra R, Heins ZJ, Chen HW, Phillips S, Zhang H, Wang J, Ochoa A, Wills J, Eubank M, Thomas SB, Gardos SM, Reales DN, Galle J, Durany R, Cambria R, Abida W, Cercek A, Feldman DR, Gounder MM, Hakimi AA, Harding JJ, Iyer G, Janjigian YY, Jordan EJ, Kelly CM, Lowery MA, Morris LGT, Omuro AM, Raj N, Razavi P, Shoushtari AN, Shukla N, Soumerai TE, Varghese AM, Yaeger R, Coleman J, Bochner B, Riely GJ, Saltz LB, Scher HI, Sabbatini PJ, Robson ME, Klimstra DS, Taylor BS, Baselga J, Schultz N, Hyman DM, Arcila ME, Solit DB, Ladanyi M, Berger MF. Mutational landscape of metastatic cancer revealed from prospective clinical sequencing of 10,000 patients. Nat Med. 2017;23:703-713. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2768] [Cited by in RCA: 2619] [Article Influence: 291.0] [Reference Citation Analysis (0)] |
| 17. | Solomon JP, Hechtman JF. Detection of NTRK Fusions: Merits and Limitations of Current Diagnostic Platforms. Cancer Res. 2019;79:3163-3168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 141] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 18. | Chiang S, Cotzia P, Hyman DM, Drilon A, Tap WD, Zhang L, Hechtman JF, Frosina D, Jungbluth AA, Murali R, Park KJ, Soslow RA, Oliva E, Iafrate AJ, Benayed R, Ladanyi M, Antonescu CR. NTRK Fusions Define a Novel Uterine Sarcoma Subtype With Features of Fibrosarcoma. Am J Surg Pathol. 2018;42:791-798. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 184] [Article Influence: 26.3] [Reference Citation Analysis (0)] |
| 19. | Weiss LM, Funari VA. NTRK fusions and Trk proteins: what are they and how to test for them. Hum Pathol. 2021;112:59-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 32] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/