Published online Jul 6, 2025. doi: 10.12998/wjcc.v13.i19.103585
Revised: February 16, 2025
Accepted: February 24, 2025
Published online: July 6, 2025
Processing time: 115 Days and 16.3 Hours
Several conditions may present with acute neurological symptoms, thus mimi
We present the case of a 63-year-old man who was brought to the emergency department after developing weakness of the left extremities, dizziness and a confusional state, which had lasted for approximately 30 minutes. The patient had a similar episode of a confusional state approximately two months earlier; at that time, a transient ischemic attack was suspected and he was started on aspirin. The initial clinical evaluation and imaging findings were unremarkable for stroke, but the patient’s symptoms, history of chronic alcohol abuse and abnormal liver function tests prompted the consideration of WE. Magnetic resonance imaging findings in subthalamic areas and electroencephalogram data of diffuse delta activity supported this diagnosis.
Through this case report, we aim to underscore the importance of considering WE as a differential diagnosis in patients presenting with symptoms suggestive of stroke, especially when the presentation is atypical or when risk factors for thiamine deficiency are present. Since intravenous thiamine significantly improves outcomes, delayed recognition and treatment in some cases might be deleterious.
Core Tip: Wernicke encephalopathy (WE) is a neurological disorder caused by the deficiency of thiamine (B1 vitamin), most often resulting from alcoholism, malnutrition, hyperemesis gravidarum or bariatric surgery. The diagnosis in a certain clinical setting might be easy, but in a few cases presenting with acute neurological deficits it can be particularly cha
- Citation: Roçi E, Mara E, Dodaj S, Vyshka G. Wernicke encephalopathy presenting as a stroke mimic: A case report. World J Clin Cases 2025; 13(19): 103585
- URL: https://www.wjgnet.com/2307-8960/full/v13/i19/103585.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v13.i19.103585
Stroke is one of the most common diseases, affecting one in four people during their lifetime. Several nonvascular medical conditions, such as stroke mimics, acute neurological deficits and acute ischemic stroke, have been described. Stroke mimics, especially when they correspond to a hypothetical vascular distribution, result in false-positive stroke cases[1,2]. Stroke mimics account for approximately 20%–30% of all suspected stroke presentations, of which more than three-quarters are of a neurological nature and only a few are of functional origin[3]. When symptoms are brief and resolve quickly, especially when magnetic resonance imaging (MRI) findings are normal, stroke mimics can be more challenging to differentiate from stroke. A hint at a diagnosis should be that suddenness at onset is not always evident, as is the fluctuating severity of symptoms and the presence of systemic signs such as altered levels of consciousness, drowsiness, agitation, fever, etc.[4]. Correct clinical evaluation and medical history, as well as the use of laboratory data and brain scan tomography, led to a lower percentage of stroke mimic admissions (4%–6.5%); the use of MRI, particularly diffusion weighted imaging, has reduced it to approximately 1%[5,6].
Wernicke encephalopathy (WE), first described in 1881, is a severe and potentially life-threatening neurological disorder caused by thiamine (vitamin B1) deficiency[7]. The prevalence among the general population is estimated to be 0.4%–2.8%, with a slight predominance in males[8]. Fifty percent of cases are caused by chronic alcohol consumption, and the rest are caused by malnutrition, hyperemesis, liver disease, gastrointestinal surgery, etc. The classical triad of WE is described as ophthalmoplegia and/or nystagmus, gait ataxia, and confusion or delirium[9,10]. It affects both the central and peripheral nervous systems. WE is associated with high rates of morbidity and mortality. Approximately 85% of patients with WE and associated Korsakoff syndrome may experience complications, and approximately 20% of them may die. Even though delirium is likely to resolve after thiamine replacement, ataxia and ophthalmoplegia may continue and sometimes be permanent[7]. Fewer than 10% of WE patients manage to recover after long-term hospitalization, whereas others may experience long-term neurological sequelae such as ataxia and Korsakoff psychosis, which significantly worsen their quality of life[7,10].
These findings highlight the importance of recognizing pathology and its early diagnosis to treat and prevent disease complications in time.
We present the case of a 63-year-old man who was brought to the Emergency Department after he developed weakness of the left extremities, dizziness and confusion, which lasted approximately 30 minutes. His spouse noted that approximately two months earlier, the patient had a similar episode of confusion that lasted 20 minutes.
A neurological consultancy raised the suspicion of a transient ischemic attack (TIA), and the patient was given aspirin.
The patient did not report having a history of comorbidities including hypertension, diabetes mellitus, hyperlipidemia or stroke-inducing lifestyle behaviors, such as alcohol abuse and smoking.
His family history was significant for cerebrovascular disease, as his father had suffered a massive stroke in his 60s.
Upon arrival at the Emergency Department, the patient was alert but appeared slightly disoriented. He denied experiencing dizziness at the time of examination. His vital signs were stable, with a blood pressure of 138 mmHg/82 mmHg, heart rate of 78 beats per minute, respiratory rate of 16 breaths per minute, and oxygen saturation of 98% on room air.
Neurological examination revealed that his pupils were isochoric and reactive to light, with intact extraocular movements and no evidence of nystagmus. Remainder cranial nerve examination showed symmetrical facial movements, normal gag reflex, and preserved tongue mobility. Motor strength was graded as 5/5 in the right extremities and 4+/5 in the left upper and lower extremities. There was mild dysmetria and ataxia observed in the left upper extremity during finger-to-nose testing, and the patient had difficulty performing rapid alternating movements on the left side. The patient also exhibited a leftward drift in the outstretched arm test and showed an unsteady gait with a tendency to veer towards the left side. Romberg's test was mildly positive, indicating impaired balance. Deep tendon reflexes were symmetric with slightly brisk reflexes on the left side. Babinski’s sign was negative bilaterally. Sensory examination, including light touch, pain, temperature, and proprioception, was normal. No signs of meningeal irritation were present.
Computed tomography (CT) of the head and CT angiography of the supraaortic vessels did not reveal any acute lesions. Based on the acute presentation, a prior history of transient neurological deficits (probably due to TIA and acute stroke were suspected. Therefore, the patient was admitted to the neurology ward.
Laboratory investigations, including a complete blood count, basic metabolic panel, coagulation profile, and lipid panel, were unremarkable except for anemia with red blood cells 4.08 × 106/µL (normal range 4.4-5.9 × 106/µL), thrombocytopenia with platelet count 77 K/µL (150-400 K/µL). Coagulation tests showed prothrombin time (Quick time) at 59% (70%-110% without anticoagulation), international normalized ratio: 1.45 (0.85-1.15 without anticoagulation), and a D-Dimer level of 2.17 µg/mL (< 0.5 µg/mL).
Liver function tests were abnormal, with total bilirubin at 2.47 mg/dL (0.3-1.2 mg/dL), direct bilirubin at 1.16 mg/dL (0.1-0.5 mg/dL), alanine aminotransferase at 35 U/L (< 55 U/L), aspartate aminotransferase at 51 U/L (5-34 U/L), glutamyl transpeptidase at 83 U/L (12-64 U/L), and lactate dehydrogenase at 258 U/L (125-200 U/L). Renal function tests showed urea at 30.4 mg/dL (18-55 mg/dL) and creatinine at 0.61 mg/dL (0.72-1.25 mg/dL).
During the hospital stay, the patient experienced a few episodes of confusion lasting an average of 15–20 minutes, without accompanying signs or symptoms.
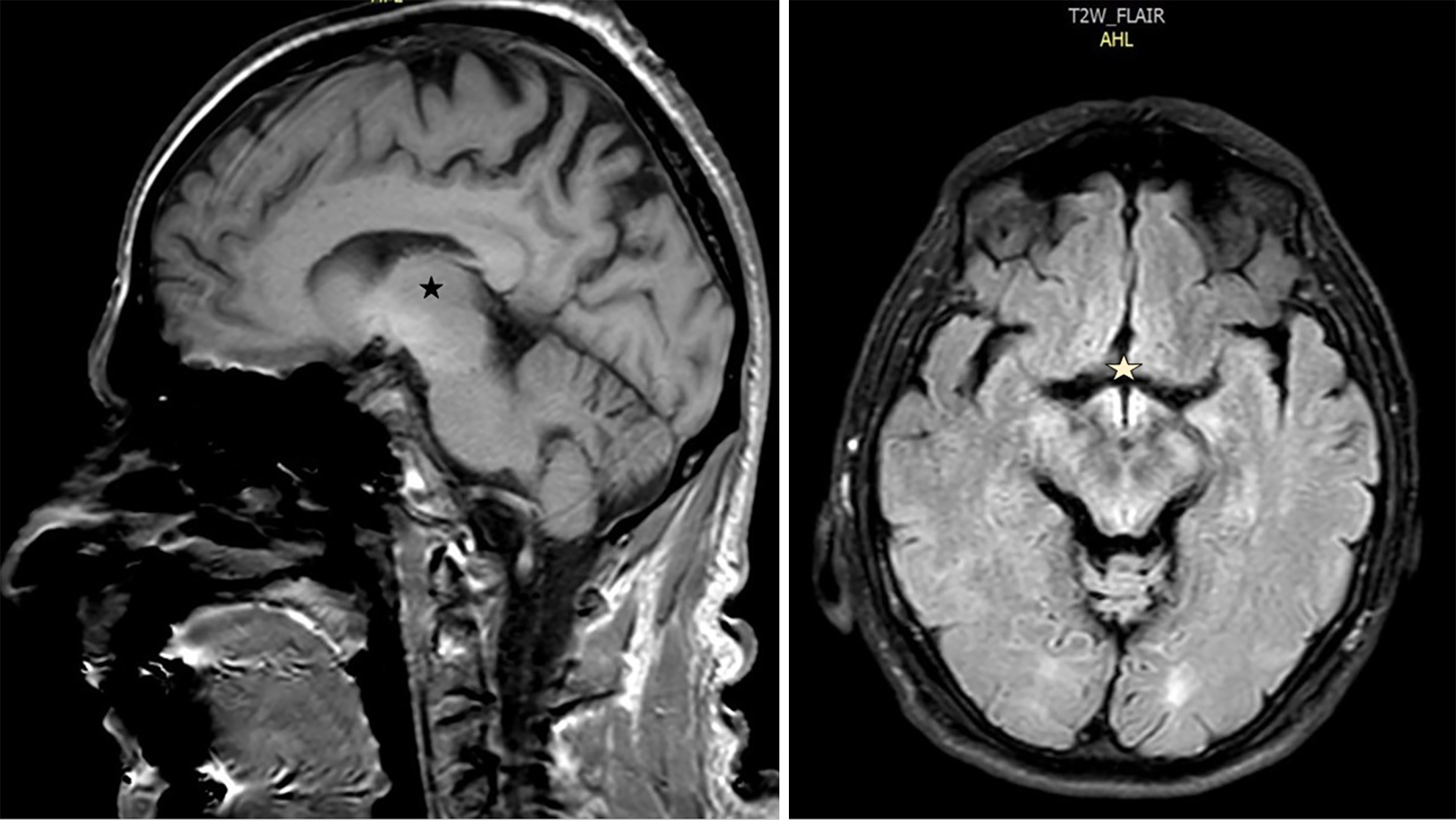
The results from an electroencephalogram examination revealed diffuse delta waves. Abdominal ultrasound revealed a coarse hepatic parenchyma, gallbladder sludge, ascites, and an enlarged spleen. Transthoracic echocardiogram revealed concentric hypertrophy of the left ventricle with type I diastolic dysfunction. MRI of the head revealed hyperintense signals in several sequences, such as those shown below, involving the mammillary and hypothalamic regions and adjacent midbrain and subthalamic nuclei (Figure 1).
His relatives reported that he had been recovering from alcoholism and that he had abused alcohol for the past 25 years. Past medical history, information and laboratory findings revealing elevated liver enzymes, elevated bilirubin, decreased prothrombin time, and low serum albumin and protein. An abdominal ultrasound revealed hepatic cirrhosis and ascites, and an MRI showed hyperintense signals bilaterally in the hypothalamic and subthalamic areas. Therefore, WE was diagnosed.
The patient received intravenous thiamine (500 mg every 8 hours for the first 24-48 hours, followed by 250 mg daily) for WE, diuretics for ascites (spironolactone 100mg/daily and furosemide 80 mg/daily), and benzodiazepines (diazepam 10 mg every 6-8 hours as needed).
On the third day of hospitalization, the patient experienced an episode of hematemesis. Emergent esophagogastroduodenoscopy revealed three esophageal varicose veins and one gastroesophageal varicosity, with no active bleeding. Ligation of the varicose veins was recommended and subsequently performed, with no surgical complications.
The patient was transferred to the intensive care unit (ICU) for one week. During his ICU stay, he continued the same treatment regimen, including thiamine, diuretics, and diazepam for alcohol withdrawal, with additional treatments as needed, such as fluids, electrolyte management, preemptive antibiotic use (Ceftriaxone 2 g/daily), and close monitoring of his liver function, coagulation status, inflammation and infection markers.
As treatment progressed, there was gradual improvement in his mental status, and he began to show more alertness and responsiveness. By the end of his ICU stay, there was significant improvement in both his neurological and systemic status. His confusion and disorientation continued to improve, although he still required monitoring for withdrawal symptoms. His liver function tests remained elevated, but the patient’s overall condition stabilized.
Upon discharge, he was stable and had no particular complaints. The medical team advised his referral to an outpatient rehabilitation service for long-term therapy for alcohol-dependent patients.
WE is a critical medical condition resulting from thiamine deficiency. Thiamine is a vitamin that is highly important for maintaining membrane integrity. After crossing the blood-brain barrier (BBB), it forms thiamine pyrophosphate, the active metabolite, which serves as a coenzyme for multiple enzymes in the Krebs cycle (tricarboxylic acid cycle) and the pentose phosphate pathway (PPP)[11-13].
Thiamine deficiency decreases intracellular thiamine diphosphate, leading to decreased activity of the Krebs cycle and PPP. Consequently, adenosine triphosphate (ATP) production decreases, as do DNA/RNA and nicotinamide adenine dinucleotide phosphate synthesis, increasing the vulnerability of cells to oxidative stress. Furthermore, there is an accumulation of toxic intermediates such as lactate, alanine and glutamate, decreasing the cellular pH and causing electrolytic disequilibrium, which ultimately results in cytotoxic edema[7,14]. Moreover, vasogenic edema is caused by disruption of the BBB because of tight junction malfunction, which allows intravascular fluid to penetrate the cerebral parenchymal extracellular space; this occurs because astrocytes are damaged following ATP depletion, oxidative stress and hyperexcitability due to excessive glutamate concentrations in synapses[13,15]. Some studies have focused on the inflammation process due to microglial hyperactivity and the production of proinflammatory cytokines in response to vitamin B1 deficiency. This overstimulation of microglia occurs due to a lack of inhibition by cholinergic neurons, since the bioavailability of acetylcholine may decrease in thiamine deficiency (Table 1)[16,17].
| Ischemic stroke | WE | |
| Origin | Vascular | Metabolic |
| Mechanism | Thrombotic or embolic occlusion of a cerebral artery which interrupts blood flow depleting the brain from of oxygen and glucose, which leads to disrupted adenosine triphosphate synthesis and energy deficiency, as well as impaired ion homeostasis and acid-base imbalance. Cytotoxic edema is developed rapidly after ischemic stroke, followed by vasogenic and mixed edema | Krebs cycle (tricarboxylic acid cycle) and the pentose phosphate pathway due to thiamine deficiency leading to both vasogenic and cytotoxic edema |
| Associated risk factors | High blood pressure, atrial fibrillation, cardiac failure, diabetes mellitus, vasculopathies, hypercoagulability, carotid stenosis, dyslipidemia etc. | Chronic alcoholism, hyperemesis gravidarum, gastric surgery procedures, anorexia nervosa etc. |
| Clinical presentation | Depends on the vascular territory: Dysarthria, aphasia, hemiparesis, hemianopia, hemi paresthesia, ataxia etc. | Typical triad: Confusion, ophthalmoplegia, ataxia |
| Laboratory findings | No specific changes. Changes of international normalized ratio, prothrombin time, activated partial thromboplastin time if specific anticoagulation therapy is taken, elevated d-dimer or fibrinogen activity may be found | Low thiamine concentration in blood, low red blood cell transketolase activity. Elevated transaminases, bilirubin and glutamyl transpeptidase, and low serum concentration of hepatic proteins in chronic alcoholism |
| Radiological features | Vascular territory. CT perfusion: Hypoperfusion of the affected area. CT: Hypodensity. MRI findings: Hypointense in T1, Hyperintense in T2/FLAIR. High diffusion weighted imaging signal, with corresponding low ADC. Radiological findings corresponding to encephalomalacia of the affected area | Non-vascular territory. CT perfusion: Normal. CT is normal in the majority of WE cases in the acute phase of the disease. MRI findings: Symmetrical bilateral. Hyperintensities of medial thalami, mammillary bodies, and periaqueductal region in T2/FLAIR. Atrophy of the mammillary bodies may be absent initially but is a typical finding. ADC varies from normal to reduced but less than that in most cases of ischemic stroke. Reversal of radiological findings with the adequate treatment |
| Treatment | Anticoagulants, antiplatelet drugs, symptomatic measures such as antihypertensive and/or antidiabetic agents etc. | Thiamine replacement |
Antemortem and postmortem studies have shown a tendency of WE to affect specific regions of the brain, and the most affected areas are the thalamus and periventricular region in almost 85% of cases, the periaqueductal area in 59%–65% of cases, the mammillary bodies in approximately 45%, the midbrain tectum in 37% of cases, the cranial nerve nuclei (18%), and, less frequently, the cerebellum (5%)[14,18,19]. Butterworth et al[20] suggested that these areas were more sensitive to vitamin B1 deficiency due to their high rates of oxidative and thiamine-related metabolism.
Similar to these reports, the MRI findings of our patient revealed involvement of the mammillary body, subthalamic and adjacent midbrain areas.
Owing to the severity of WE symptoms, if not properly treated, complications such as Korsakoff psychosis and even death may occur. However, it is not always easy to detect and diagnose the disease in time. Many factors contribute to non-timely diagnosis, such as the variability of symptoms. In fact, the classical triad of WEs is present in only one-third of cases[12]. Similarly, alcoholism, which is the condition most closely related to WE, is associated with only half of WE patients[21]. Therefore, nonalcoholic WE patients are more likely to present different symptoms and atypical MRI findings. In addition, in nonalcoholic patients, an altered level of consciousness is the only clinical presentation, which can lead to delayed or missed diagnoses[18,19].
In fact, histopathological studies admit that many cases of WE may not be diagnosed[22,23]. In addition to Korsakoff psychosis, WE-associated complications include hepatic encephalopathy, epileptic seizures, central pontine myelinolysis, and posterior reversible encephalopathy syndrome[9]. Unusual sites of brain lesions on MRI can also be confusing for doctors and delay diagnosis. Moreover, bilaterally symmetrical lesions of the medial thalami, which are typical of WE, can be misdiagnosed as top-of-the-basilar syndrome, deep cerebral venous thrombosis, Creutzfeldt–Jakob disease, metronidazole-induced encephalopathy or paramedian thalamic syndrome[7,22,23].
Even though WE patients in the acute phase present with delirium, to the best of our knowledge, most guidelines on delirium do not include thiamine deficiency as one of the possible causes. Furthermore, in the alcohol disorders guidelines, delirium is mentioned mostly in relation to alcohol withdrawal syndrome, leading to misdiagnosis of WE delirium, which can also present similar attention deficits, speech difficulty, altered levels of consciousness, and gait ataxia. Concomitant conditions in WE patients, such as infections, can also mask the typical clinical presentation of WE[10,24,25].
Neurological examination can be difficult to perform correctly on these patients, and diagnostic confirmation is often delayed. Repeated clinical evaluations, a high degree of clinical suspicion, a good medical history and recognition of the predisposing conditions are needed to prompt the diagnosis and initiate treatment with thiamine as soon as possible[9].
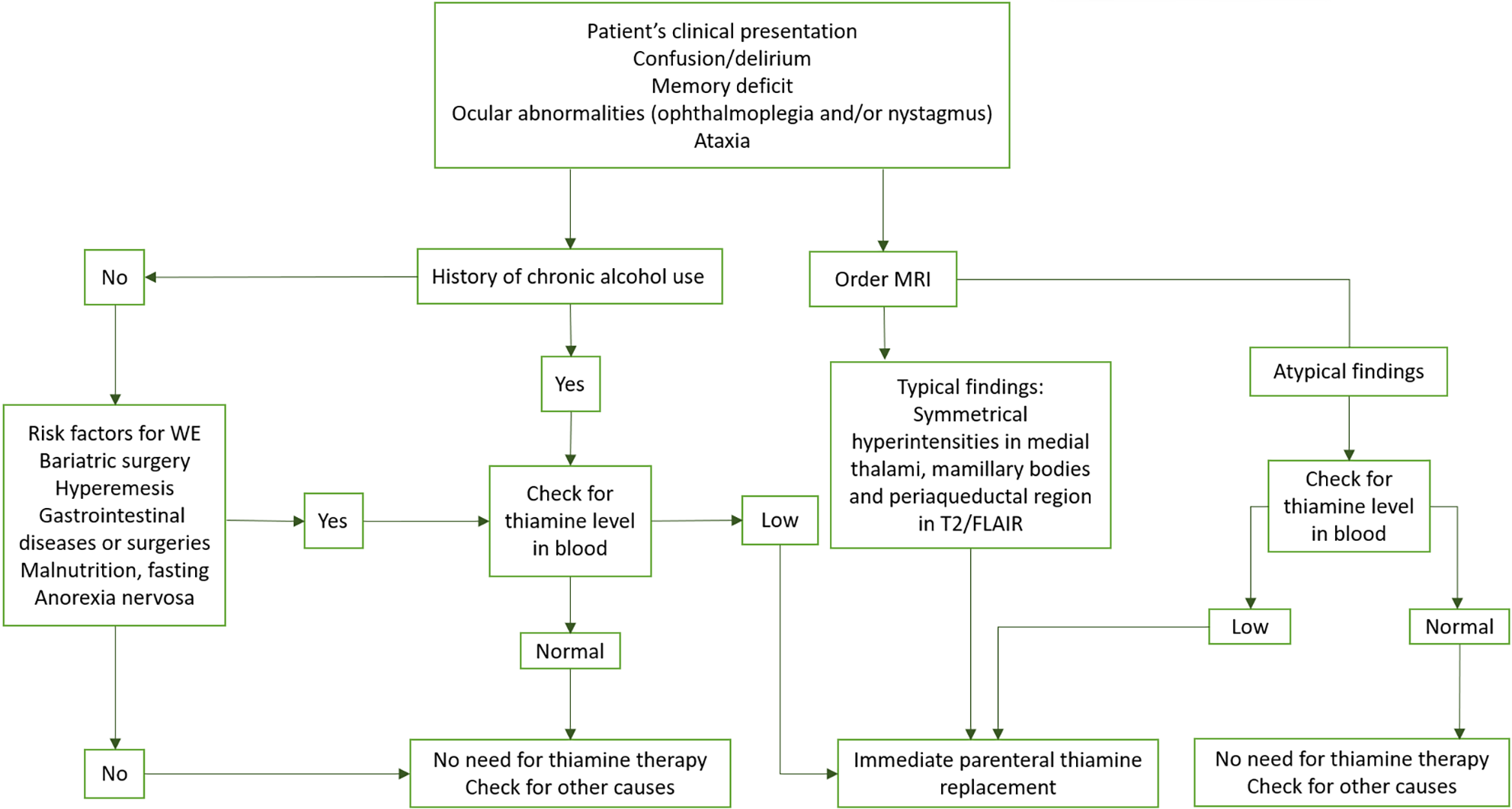
The European Federation of Neurological Societies guidelines recommend the administration of 200 mg intravenous thiamine diluted with 100 mL of normal saline or 5% dextrose, given over 30 minutes three times per day[26]. The recommended treatment in the United States is 100 mg of parenteral thiamine three to seven days in the treatment phase, followed by oral thiamine for as long as the patient continues consuming alcohol. In the United Kingdom, the proposed treatment is intravenous thiamine 500 mg every 8 hours or 12 hours for 2 days to 3 days[27]. Parenteral administration of 250 mg thiamine for 5 days should be maintained in patients with neuropsychiatric symptoms. Higher doses are not correlated with better outcomes than intermediate or lower doses are[28]. Even if the duration is not fixed, it is suggested that intravenous thiamine replacement should be prolonged in comatose patients. Oral vitamin B1 at a dose of 50 mg to 100 mg must be continued in patients with alcohol addiction or those who have undergone bariatric surgery. Magnesium supplementation should also be administered because of its deficiency in alcohol-dependent WE patients[12]. These guidelines are summarized in the below algorithm (Figure 2).
This case highlights the importance of considering WE in the differential diagnosis of any patient presenting with acute neurological symptoms, especially when a background of alcohol abuse or risk factors for thiamine deficiency are identified. The overlapping clinical features of stroke and WE, such as confusion, ataxia, and focal neurological deficits, can easily lead to misdiagnosis. Awareness of the pathophysiology, clinical presentation, laboratory abnormalities, and characteristic MRI features of WE are essential for early recognition and treatment. The immediate administration of thiamine is crucial, as delays can result in irreversible damages. Encephalopathy gradually diminishes, but residual neurologic deficits such as ocular abnormalities and memory deficit, are very common. Therefore, multidisciplinary teams and specialized centers are so much needed as well as educating and encouraging alcohol abstinence is very important.
| 1. | H Buck B, Akhtar N, Alrohimi A, Khan K, Shuaib A. Stroke mimics: incidence, aetiology, clinical features and treatment. Ann Med. 2021;53:420-436. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 65] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 2. | Vilela P. Acute stroke differential diagnosis: Stroke mimics. Eur J Radiol. 2017;96:133-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 73] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 3. | Sookdeo A, Shaikh YM, Bhattacharjee M, Khan J, Alvi WA, Arshad MS, Tariq AH, Muzammil M. Current understanding of stroke and stroke mimics in adolescents and young adults: a narrative review. Int J Emerg Med. 2024;17:180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 4. | Moulin S, Leys D. Stroke mimics and chameleons. Curr Opin Neurol. 2019;32:54-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 47] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 5. | Vroomen PC, Buddingh MK, Luijckx GJ, De Keyser J. The incidence of stroke mimics among stroke department admissions in relation to age group. J Stroke Cerebrovasc Dis. 2008;17:418-422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 44] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 6. | Huff JS. Stroke mimics and chameleons. Emerg Med Clin North Am. 2002;20:583-595. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 45] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 7. | Habas E, Farfar K, Errayes N, Rayani A, Elzouki AN. Wernicke Encephalopathy: An Updated Narrative Review. Saudi J Med Med Sci. 2023;11:193-200. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 8. | Chandrakumar A, Bhardwaj A, 't Jong GW. Review of thiamine deficiency disorders: Wernicke encephalopathy and Korsakoff psychosis. J Basic Clin Physiol Pharmacol. 2018;30:153-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 88] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 9. | Ota Y, Capizzano AA, Moritani T, Naganawa S, Kurokawa R, Srinivasan A. Comprehensive review of Wernicke encephalopathy: pathophysiology, clinical symptoms and imaging findings. Jpn J Radiol. 2020;38:809-820. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 100] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 10. | Wijnia JW. A Clinician's View of Wernicke-Korsakoff Syndrome. J Clin Med. 2022;11:6755. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 35] [Reference Citation Analysis (0)] |
| 11. | Kohnke S, Meek CL. Don't seek, don't find: The diagnostic challenge of Wernicke's encephalopathy. Ann Clin Biochem. 2021;58:38-46. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 45] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 12. | Sechi G, Serra A. Wernicke's encephalopathy: new clinical settings and recent advances in diagnosis and management. Lancet Neurol. 2007;6:442-455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 729] [Cited by in RCA: 761] [Article Influence: 40.1] [Reference Citation Analysis (0)] |
| 13. | Manzo G, De Gennaro A, Cozzolino A, Serino A, Fenza G, Manto A. MR imaging findings in alcoholic and nonalcoholic acute Wernicke's encephalopathy: a review. Biomed Res Int. 2014;2014:503596. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 106] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 14. | Jung YC, Chanraud S, Sullivan EV. Neuroimaging of Wernicke's encephalopathy and Korsakoff's syndrome. Neuropsychol Rev. 2012;22:170-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 88] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 15. | Stokum JA, Kurland DB, Gerzanich V, Simard JM. Mechanisms of astrocyte-mediated cerebral edema. Neurochem Res. 2015;40:317-328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 120] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 16. | Abdou E, Hazell AS. Thiamine deficiency: an update of pathophysiologic mechanisms and future therapeutic considerations. Neurochem Res. 2015;40:353-361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 79] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 17. | van Gool WA, van de Beek D, Eikelenboom P. Systemic infection and delirium: when cytokines and acetylcholine collide. Lancet. 2010;375:773-775. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 358] [Cited by in RCA: 410] [Article Influence: 25.6] [Reference Citation Analysis (0)] |
| 18. | Zuccoli G, Santa Cruz D, Bertolini M, Rovira A, Gallucci M, Carollo C, Pipitone N. MR imaging findings in 56 patients with Wernicke encephalopathy: nonalcoholics may differ from alcoholics. AJNR Am J Neuroradiol. 2009;30:171-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 182] [Article Influence: 10.1] [Reference Citation Analysis (1)] |
| 19. | Zuccoli G, Gallucci M, Capellades J, Regnicolo L, Tumiati B, Giadás TC, Bottari W, Mandrioli J, Bertolini M. Wernicke encephalopathy: MR findings at clinical presentation in twenty-six alcoholic and nonalcoholic patients. AJNR Am J Neuroradiol. 2007;28:1328-1331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 119] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 20. | Butterworth RF, Kril JJ, Harper CG. Thiamine-dependent enzyme changes in the brains of alcoholics: relationship to the Wernicke-Korsakoff syndrome. Alcohol Clin Exp Res. 1993;17:1084-1088. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 137] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 21. | Ogershok PR, Rahman A, Nestor S, Brick J. Wernicke encephalopathy in nonalcoholic patients. Am J Med Sci. 2002;323:107-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 80] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 22. | Martin PR, Singleton CK, Hiller-Sturmhöfel S. The role of thiamine deficiency in alcoholic brain disease. Alcohol Res Health. 2003;27:134-142. [PubMed] |
| 23. | Moizé V, Ibarzabal A, Sanchez Dalmau B, Flores L, Andreu A, Lacy A, Vidal J. Nystagmus: an uncommon neurological manifestation of thiamine deficiency as a serious complication of sleeve gastrectomy. Nutr Clin Pract. 2012;27:788-792. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 24. | Bush SH, Marchington KL, Agar M, Davis DH, Sikora L, Tsang TW. Quality of clinical practice guidelines in delirium: a systematic appraisal. BMJ Open. 2017;7:e013809. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 35] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 25. | Caine D, Halliday GM, Kril JJ, Harper CG. Operational criteria for the classification of chronic alcoholics: identification of Wernicke's encephalopathy. J Neurol Neurosurg Psychiatry. 1997;62:51-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 293] [Cited by in RCA: 308] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 26. | Latt N, Dore G. Thiamine in the treatment of Wernicke encephalopathy in patients with alcohol use disorders. Intern Med J. 2014;44:911-915. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 104] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 27. | Thomson AD, Cook CC, Touquet R, Henry JA; Royal College of Physicians, London. The Royal College of Physicians report on alcohol: guidelines for managing Wernicke's encephalopathy in the accident and Emergency Department. Alcohol Alcohol. 2002;37:513-521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 264] [Cited by in RCA: 254] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 28. | Galvin R, Bråthen G, Ivashynka A, Hillbom M, Tanasescu R, Leone MA; EFNS. EFNS guidelines for diagnosis, therapy and prevention of Wernicke encephalopathy. Eur J Neurol. 2010;17:1408-1418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 375] [Cited by in RCA: 409] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/