Published online May 6, 2025. doi: 10.12998/wjcc.v13.i13.101438
Revised: December 10, 2024
Accepted: December 23, 2024
Published online: May 6, 2025
Processing time: 119 Days and 9.1 Hours
The relationship between genetics and infectious diseases is important in shaping our understanding of disease susceptibility, progression, and treatment. Recent research shows the impact of genetic variations, such as heme-oxygenase promoter length, on diseases like malaria and sepsis, revealing both protective and inconclusive effects. Studies on vaccine responses highlight genetic markers like human leukocyte antigens, emphasizing the potential for personalized immunization strategies. The ongoing battle against drug-resistant tuberculosis (TB) illustrates the complexity of genomic variants in predicting resistance, highlighting the need for integrated diagnostic tools. Additionally, genome-wide association studies reveal antibiotic resistance mechanisms in bacterial genomes, while host genetic polymorphisms, such as those in solute carrier family 11 member 1 and vitamin D receptor, demonstrate their role in TB susceptibility. Advanced techniques like metagenomic next-generation sequencing promise detailed pathogen detection but face challenges in cost and accessibility. A case report involving a highly virulent Mycobacterium TB strain with the pks1 gene further highlights the need for genetic insights in understanding disease severity and developing targeted interventions. This evolving landscape emphasizes the role of genetics in infectious diseases, while also addressing the need for standardized studies and accessible technologies.
Core Tip: Genetics significantly impacts infectious disease outcomes, from susceptibility to treatment responses. Key genetic variations, such as those in heme-oxygenase-1 and vaccine-related markers, influence how individuals respond to infections and vaccinations. The study of drug-resistant tuberculosis highlights the complex role of genomic variants, requiring advanced diagnostic tools. Genome-wide association studies and host polymorphisms, like solute carrier family 11 member 1 and vitamin D receptor, provide insights into disease mechanisms and resistance.
- Citation: Bhowmik S, Hajra A, Bandyopadhyay D. Genetic insights in infectious diseases: Insights from a case report and implications for personalized medicine. World J Clin Cases 2025; 13(13): 101438
- URL: https://www.wjgnet.com/2307-8960/full/v13/i13/101438.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v13.i13.101438
The relationship between genetics and infectious diseases is a multifaceted area that has garnered considerable attention in recent years. Research on various pathogens and genetic markers has illuminated how genetic variations influence susceptibility, disease progression, and treatment outcomes. Traditional management relies on standardized, population-based methods, which can be inefficient due to their generalized nature. These methods fail to consider individual genetic makeup, lifestyle, environment, and health history, leading to variable treatment efficacy and increased healthcare costs. Personalized medicine represents a transformative approach in healthcare, tailoring treatments to individual genetic, environmental, and lifestyle factors.
Understanding the genetic factors that influence infectious disease outcomes is a growing field of research. A critical gene in this context is heme-oxygenase 1 (HMOX1), an important gene that helps the body respond to stress and has been linked to how we fight off infections by breaking down free heme into biliverdin, carbon monoxide, and iron, protecting against oxidative stress and inflammation during infections. This process regulates iron availability, limiting resources for pathogens while reducing iron-mediated tissue damage. The gene’s GT(n) repeat polymorphism influences its expression, with shorter repeats associated with higher expression, enhancing host defense mechanisms[1]. Hamilton et al[1] in 2022 reviewed the association between the length of an HMOX1 promoter and the incidence and outcomes of infectious diseases, such as malaria, human immunodeficiency virus, and sepsis. HMOX1 polymorphisms may serve as potential biomarkers and therapeutic targets, guiding interventions aimed at modulating immune responses and improving infection outcomes. The review presented mixed results: Some studies suggested longer alleles were associated with reduced risk of severe malaria and better outcomes in sepsis, while others showed no significant correlation. These findings highlight the complexity of gene-disease interactions and the necessity for larger, more standardized studies to draw definite conclusions. Similarly, Haslund et al[2] in 2023 explored the genetic determinants of antibody responses to the measles, mumps, and rubella vaccines. Their review identified significant genetic markers, including specific human leukocyte antigens (proteins on cell surface to help distinguish self and non-self-cells), CD-46 (cluster of differentiation), and cytokine genes that affect humoral immune response post-vaccination. These findings suggest that personalized immunization strategies tailored to genetic variability could enhance immunogenicity across diverse populations.
The global challenge of drug-resistant tuberculosis (TB) has necessitated genetic insights in developing effective treatment. Studies by Ismail et al[3] in 2021 and Nimmo et al[4] in 2024 investigated genomic variants linked to resistance against bedaquiline, a drug for treating rifampicin-resistant TB. Although several mutations were identified in genes such as Rv0678 and atpE, the correlation between these genetic changes and actual drug resistance was not always straightforward. This complexity highlights the necessity of integrating genomic data with phenotypic observations to develop reliable molecular diagnostic tools for TB.
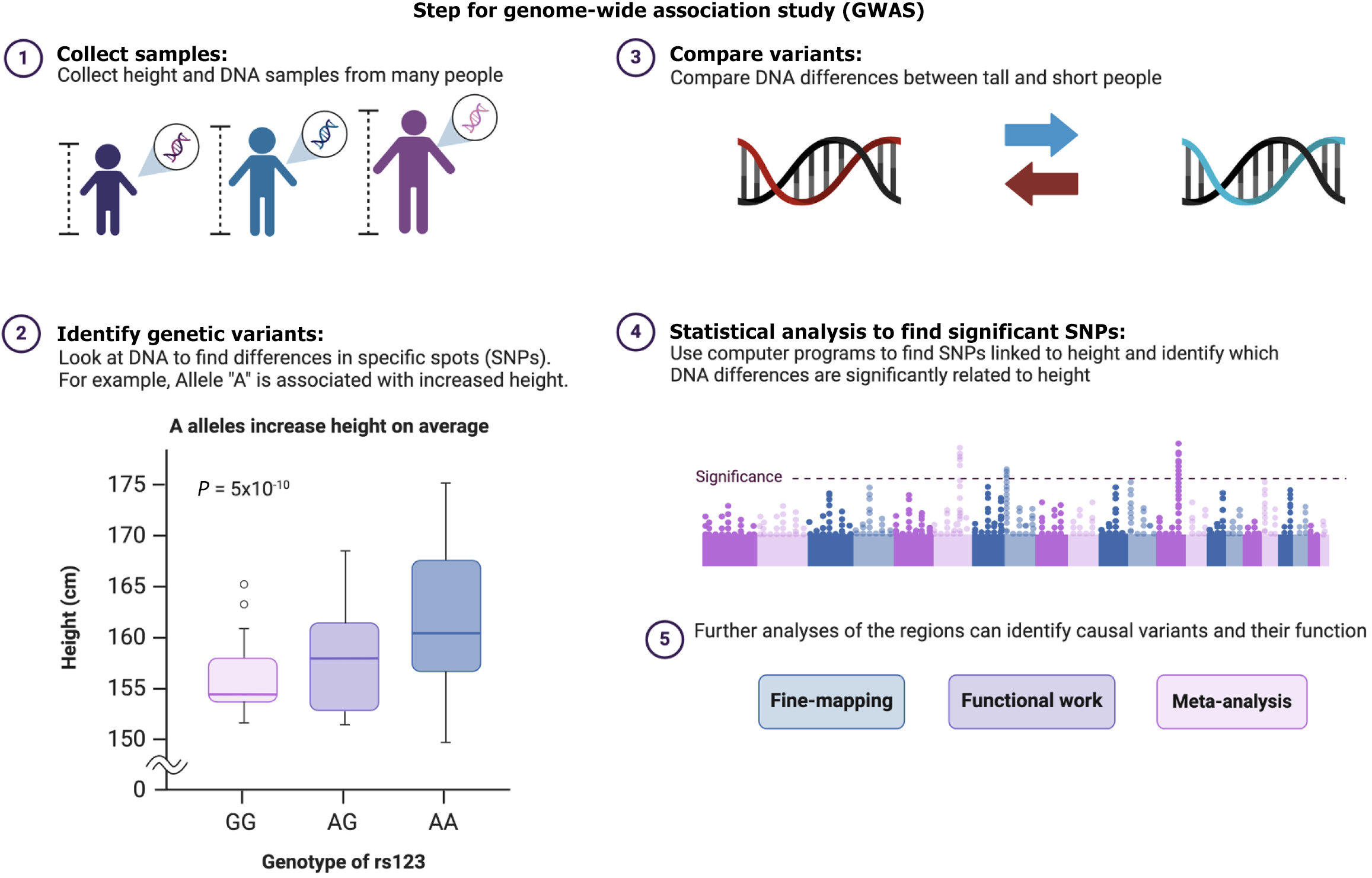
Complementing these efforts, Mosquera-Rendón et al[5] in 2023 used genome-wide association studies (GWAS) to examine the entire genetic makeup of bacteria, such as Mycobacterium TB (MTB) and Escherichia coli, to understand why some bacteria are resistant to antibiotics. GWAS examines the entire genome of organisms and helps researchers to pinpoint specific mutations linked to resistance and has emerged as a powerful tool. Figure 1 outlines and simplifies the key steps involved in GWAS. The insights from GWAS into resistance mechanisms can inform the development of new therapeutic targets. However, the complexity of bacterial genomes, including horizontal gene transfer and high genetic diversity, presents challenges, requiring continuous refinement of GWAS methodologies.
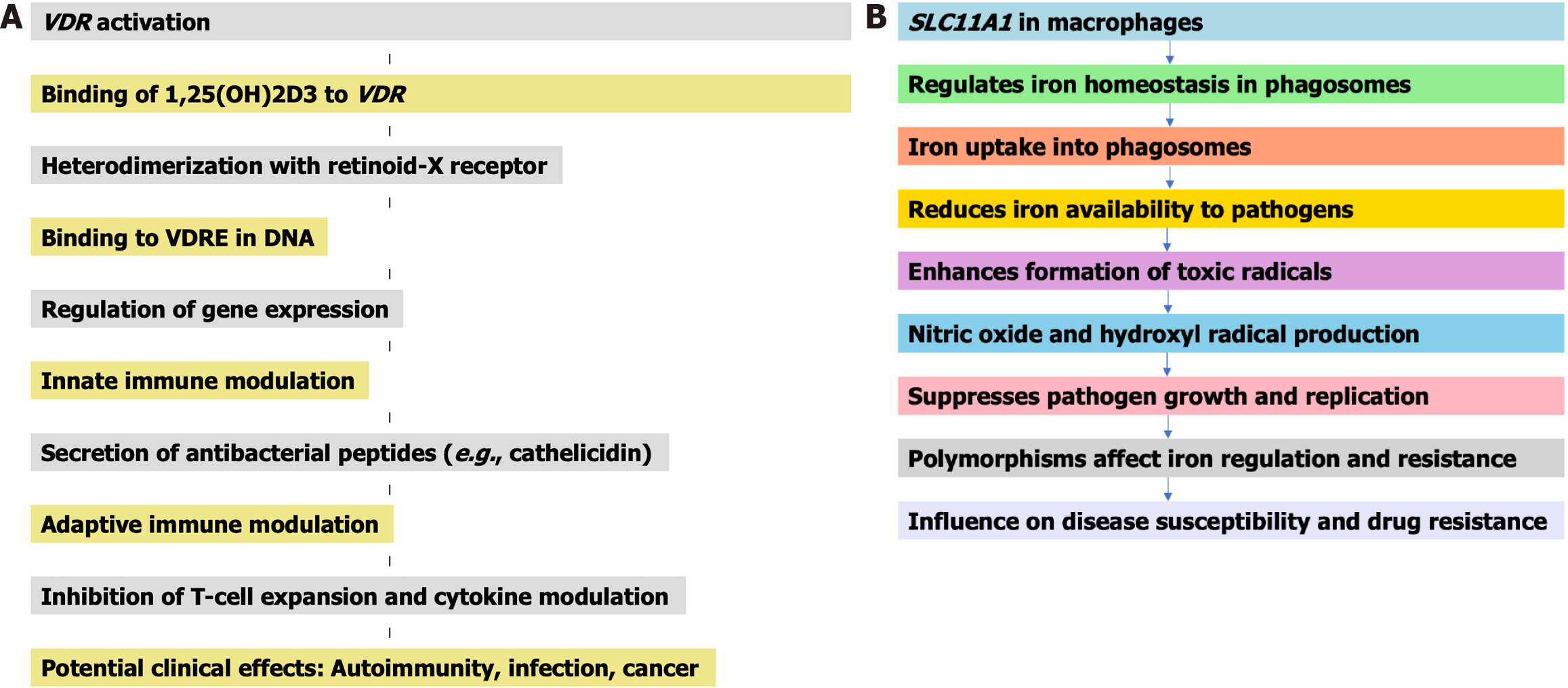
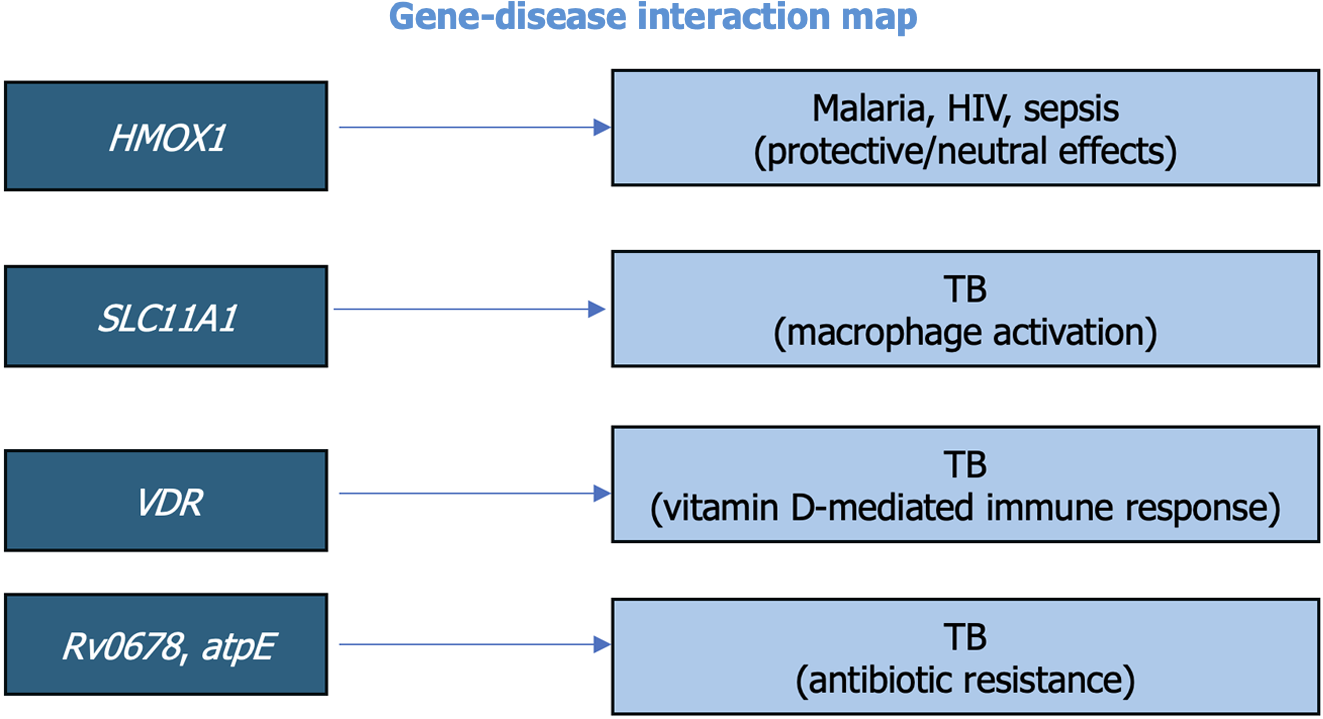
The role of host genetic polymorphisms in TB susceptibility has also been extensively studied. Li et al[6] in 2011 and Gao et al[7] in 2010 identified polymorphisms in genes such as solute carrier family 11 member 1 (SLC11A1) (SLC11A1 is also known as natural resistance-associated macrophage protein 1), and the vitamin D receptor (VDR) that are associated with increased TB risk. The SLC11A1 gene, also known as natural resistance-associated macrophage protein 1, is involved in regulating macrophage activation to combat intracellular infections like TB. For instance, polymorphisms in SLC11A1 were found to regulate macrophage activation and influence TB susceptibility, while VDR polymorphisms affected vitamin D-mediated immune responses. These findings support the hypothesis that genetic factors play a critical role in determining individual susceptibility to TB and could inform targeted interventions. To enhance the reader’s understanding, we have included two flowcharts summarizing their respective functions. Figure 2A illustrates the stepwise activation of the VDR and its downstream effects on innate and adaptive immunity, including antibacterial peptide secretion and cytokine modulation. Figure 2B outlines the role of SLC11A1 in macrophage iron homeostasis and its impact on pathogen suppression, with a particular emphasis on the consequences of genetic polymorphisms on disease outcomes. The gene-disease interaction map (Figure 3) visually organizes the connection between key genetic markers and infectious diseases to emphasize genetic complexity underlying susceptibility and treatment resistance.
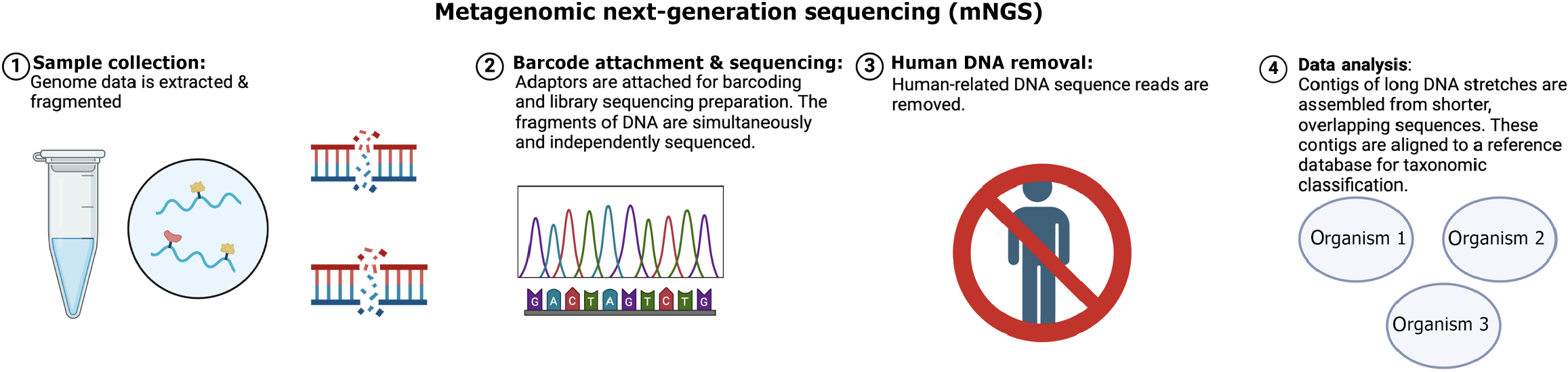
Advanced genetic testing methods, like metagenomic next-generation sequencing (mNGS), can detect TB bacteria and other infections by analyzing their genetic material in detail. Unlike targeted assays, which focus on predefined pathogens, mNGS provides an unbiased approach to identifying all genetic material present in a clinical sample. Figure 4 illustrates the sequential workflow of mNGS to emphasize its role in advancing diagnostics for infectious diseases. While mNGS shows promise in detecting multiple drug resistance sites and offering precise treatment options, its high cost compared to conventional assays limits its widespread use. Li et al[8] in 2022 discussed its potential to detect MTB and co-infection pathogens, providing a detailed genetic landscape of infections.
A compelling case report on this issue further underlines the role of genetic factors in infectious diseases[9]. This case involves a young woman with normal immune function presented with severe respiratory symptoms indicative of pulmonary TB. Despite her normal immune status, the disease progressed rapidly, raising concerns about potential underlying factors contributing to its severity. Upon genetic analysis, the MTB strain infecting the patient possessed the pks1 gene, hypothesized to increase virulence and dissemination.
The pks1 gene in MTB encodes a polyketide synthase protein involved in the biosynthesis of complex lipids that are critical to the pathogen's virulence and survival. These lipids, such as dimycocerosates and mycolic acids, form part of the bacterial cell wall, contributing to its impermeability, immune evasion, and resistance to antimicrobial agents. Polyketide synthases like pks1 operate via modular mechanisms, facilitating stepwise elongation and functional modification of lipid precursors. Mutations in the pks1 gene can enhance MTB transmissibility by altering lipid biosynthesis, which may strengthen the pathogen’s ability to evade host immune responses and thrive within hostile environments, such as macrophages. Studies have identified such mutations as potential markers for higher transmission and as promising targets for novel therapeutic interventions aimed at disrupting the production of key virulence factors[10].
Further studies on the pks1 gene and its role in virulence and disease progression could enhance our understanding of highly pathogenic MTB strains. Additionally, expanding research to identify and characterize similar virulence factors in other infectious diseases may uncover broader mechanisms underlying pathogen-host interactions. This MTB case highlights the use of genetic factors in pathogenicity, prognosis, and understanding of infectious diseases. It also emphasizes the potential for personalized medicine to enhance public health strategies. For example, profiling the pks1 gene in MTB strains can: (1) Enhance awareness and monitoring of highly virulent strains; (2) Aid in epidemiological tracking and targeted containment strategies; and (3) Inform research efforts towards developing targeted immunization and novel therapeutics.
Such integration of genetic data into the study of infectious diseases provides potential for personalized medicine and targeted interventions. The diverse research findings and the case report discussed highlight the importance of understanding genetic factors in disease susceptibility, immunization responses, and drug resistance. However, to fully realize the benefits of personalized medicine, challenges such as data heterogeneity, the need for larger standardized studies, and cost considerations must be addressed. Additionally, efforts to improve the accessibility and affordability of advanced genetic testing methods, such as mNGS, are critical to ensuring equitable implementation of precision health solutions globally.
| 1. | Hamilton FW, Somers J, Mitchell RE, Ghazal P, Timpson NJ. HMOX1 genetic polymorphisms and outcomes in infectious disease: A systematic review. PLoS One. 2022;17:e0267399. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 2. | Haslund MM, Sørensen JK, Graff Stensballe L. Genetics and measles, mumps and rubella vaccine response in childhood and adolescence-A systematic review. Scand J Immunol. 2023;97:e13266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 3. | Ismail N, Rivière E, Limberis J, Huo S, Metcalfe JZ, Warren RM, Van Rie A. Genetic variants and their association with phenotypic resistance to bedaquiline in Mycobacterium tuberculosis: a systematic review and individual isolate data analysis. Lancet Microbe. 2021;2:e604-e616. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 45] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 4. | Nimmo C, Bionghi N, Cummings MJ, Perumal R, Hopson M, Al Jubaer S, Naidoo K, Wolf A, Mathema B, Larsen MH, O'Donnell M. Opportunities and limitations of genomics for diagnosing bedaquiline-resistant tuberculosis: a systematic review and individual isolate meta-analysis. Lancet Microbe. 2024;5:e164-e172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 10] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 5. | Mosquera-Rendón J, Moreno-Herrera CX, Robledo J, Hurtado-Páez U. Genome-Wide Association Studies (GWAS) Approaches for the Detection of Genetic Variants Associated with Antibiotic Resistance: A Systematic Review. Microorganisms. 2023;11:2866. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 6. | Li X, Yang Y, Zhou F, Zhang Y, Lu H, Jin Q, Gao L. SLC11A1 (NRAMP1) polymorphisms and tuberculosis susceptibility: updated systematic review and meta-analysis. PLoS One. 2011;6:e15831. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 82] [Cited by in RCA: 81] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 7. | Gao L, Tao Y, Zhang L, Jin Q. Vitamin D receptor genetic polymorphisms and tuberculosis: updated systematic review and meta-analysis. Int J Tuberc Lung Dis. 2010;14:15-23. [PubMed] |
| 8. | Li Y, Jiao M, Liu Y, Ren Z, Li A. Application of Metagenomic Next-Generation Sequencing in Mycobacterium tuberculosis Infection. Front Med (Lausanne). 2022;9:802719. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 9. | Wu F, Yang B, Xiao Y, Ren L, Chen H, Hu X, Pan Y, Chen Y, Li H. psk1 virulence gene-induced pulmonary and systemic tuberculosis in a young woman with normal immune function: A case report. World J Clin Cases. 2024;12:6826-6833. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 10. | Wang TT, Hu YL, Li YF, Kong XL, Li YM, Sun PY, Wang DX, Li YY, Zhang YZ, Han QL, Zhu XH, An QQ, Liu LL, Liu Y, Li HC. Polyketide synthases mutation in tuberculosis transmission revealed by whole genomic sequence, China, 2011-2019. Front Genet. 2023;14:1217255. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/