Published online Apr 26, 2025. doi: 10.12998/wjcc.v13.i12.98768
Revised: October 24, 2024
Accepted: December 17, 2024
Published online: April 26, 2025
Processing time: 186 Days and 9.6 Hours
Posterior reversible encephalopathy syndrome (PRES) is a complex neurological disorder characterized by symptoms such as headaches, seizures, confusion, and visual disturbances. The pathophysiology of PRES involves endothelial dysfun
To elucidate the dual role of corticosteroids in the context of PRES by critically evaluating the existing literature. Specifically, it seeks to assess the results of PRES induced by corticosteroid therapy and the efficacy and safety of corticosteroids in the treatment of PRES. By synthesizing case reports and series, this review aims to provide a comprehensive understanding of the mechanisms, clinical presentations, and management strategies associated with corticosteroid-related PRES.
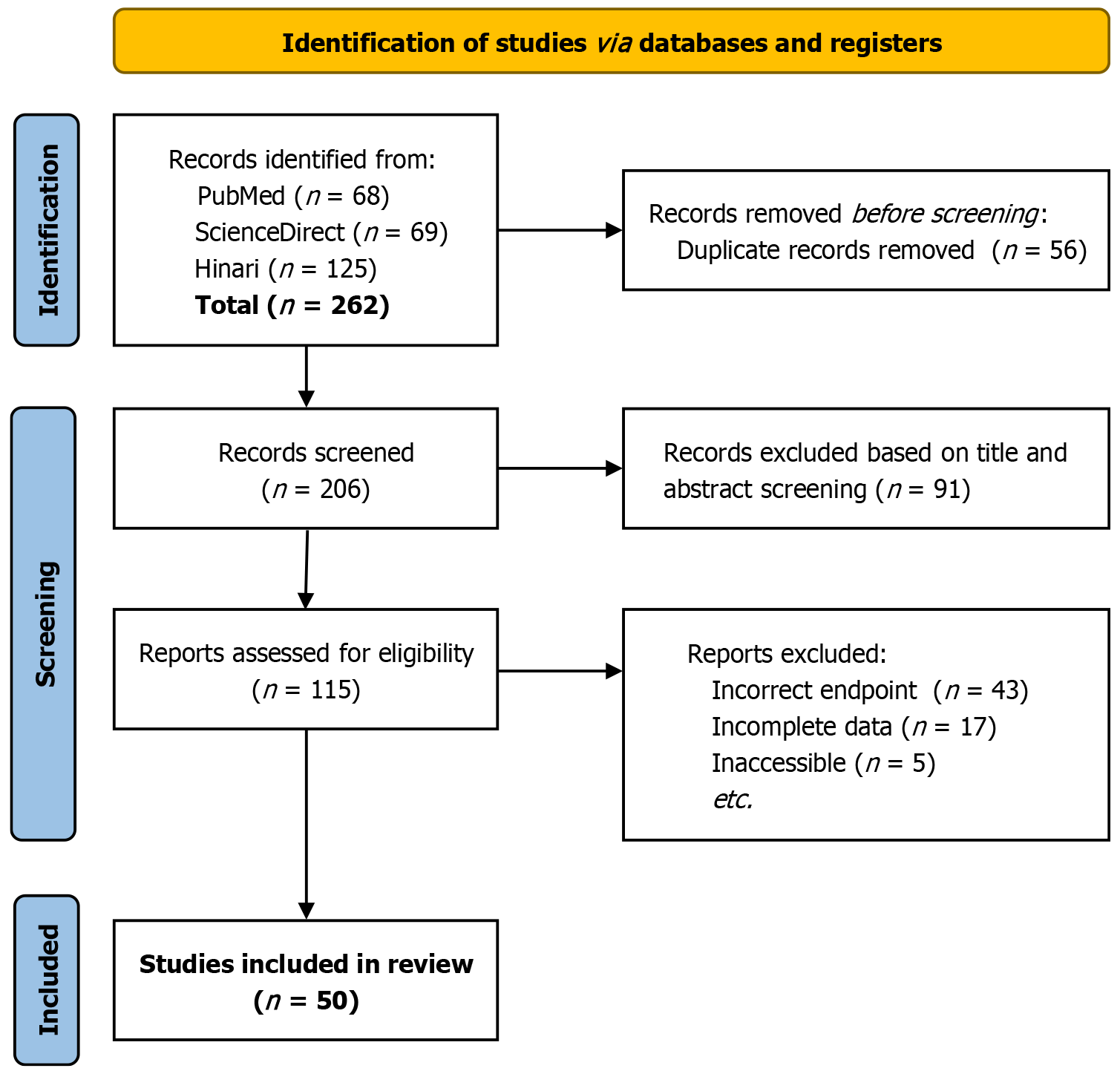
The review was carried out according to the PRISMA guidelines. The databases searched included Science Direct, PubMed, and Hinari. The search strategy encompassed terms related to corticosteroids and PRES. Studies were included if they were peer-reviewed articles examining corticosteroids in PRES, excluding non-English publications, reviews, and editorials. Data on patient demographics, clinical characteristics, imaging findings, corticosteroid regimens, and outcomes were extracted. The risk of bias was evaluated using the Joanna Briggs Institute tool for case reports.
A total of 56 cases of PRES (66.1% women, 33.9% men) potentially induced by corticosteroids and 14 cases in which corticosteroids were used to treat PRES were identified. Cases of PRES reportedly caused by corticosteroids showed a mean age of approximately 25.2 years, with seizures, headaches, hypertension, and visual disturbances being common clinical sequelae. Magnetic resonance findings typically revealed vasogenic edema in the bilateral parieto-occipital lobes. High-dose or prolonged corticosteroid therapy was a significant risk factor. On the contrary, in the treatment cases, corticosteroids were associated with positive outcomes, including resolution of vasogenic edema and stabilization of symptoms, particularly in patients with underlying inflammatory or autoimmune diseases.
Corticosteroids have a dual role in PRES, capable of both inducing and treating the condition. The current body of literature suggests that corticosteroids may play a greater role as a precipitating agent of PRES rather than treating. Corticosteroids may induce PRES through hypertension and subsequent increased cerebral blood flow and loss of autoregulation. Corticosteroids may aid in the management of PRES: (1) Enhancing endothelial stability; (2) Anti-inflammatory properties; and (3) Improving blood-brain barrier integrity. Mechanisms which may reduce or mitigate vasogenic edema formation.
Core Tip: Posterior reversible encephalopathy syndrome (PRES) is characterized by headaches, seizures, confusion, and visual disturbances due to endothelial dysfunction and vasogenic edema. Corticosteroids have a dual role in PRES, potentially inducing the condition through hypertension while also treating it by reducing inflammation and stabilizing the blood-brain barrier. This systematic review identifies 57 cases of corticosteroid-induced PRES and 14 cases where corticosteroids were used therapeutically. Understanding this dual role is crucial for optimizing patient care, highlighting the need for careful monitoring and tailored treatment strategies.
- Citation: Srichawla BS, Kaur T, Singh H. Corticosteroids in posterior reversible encephalopathy syndrome: Friend or foe? A systematic review. World J Clin Cases 2025; 13(12): 98768
- URL: https://www.wjgnet.com/2307-8960/full/v13/i12/98768.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v13.i12.98768
Posterior reversible encephalopathy syndrome (PRES) is a neurological disorder characterized by various symptoms, including headaches, seizures, altered mental status, and visual disturbances. Its pathophysiology remains incompletely understood but is believed to involve endothelial dysfunction leading to vasogenic edema[1]. The use of corticosteroids in PRES is controversial, with arguments both for and against their use based on their anti-inflammatory properties and the potential to exacerbate hypertension. PRES is a complex condition with variable clinical presentations, making its management difficult. Corticosteroids, widely used for their potent anti-inflammatory effects, have been proposed as a treatment option. However, its role in the management of PRES is debated, primarily due to concerns about adverse effects, including the potential for worsening hypertension, a key factor in the pathogenesis of PRES. Thus, understanding the impact of corticosteroids on PRES outcomes is crucial for optimizing patient care. This systematic review aims to critically evaluate the existing literature on the use of corticosteroids in PRES. We intend to assess the efficacy and safety of corticosteroids in the treatment of PRES, considering both clinical outcomes and potential adverse effects.
This systematic review was conducted in accordance with the PRISMA guidelines and the AMSTAR criteria[2,3]. The review protocol was registered with PROSPERO to ensure transparency and reproducibility. In this study, no original patient data was collected, and no attempt was made to identify individuals from the literature review. The systematic review was conducted following ethical considerations as outlined in the Declaration of Helsinki. We systematically searched the databases: Science Direct, PubMed, and Hinari. The search strategy was designed to encompass a wide range of terms and keywords related to corticosteroids and PRES. The search string included combinations of terms such as "corticosteroids", "Posterior Reversible Encephalopathy Syndrome", "PRES", "steroids", "treatment", "management", and their relevant synonyms and medical subject headings. The complete search string for each database is included in Table 1. A gray literature search was completed by reviewing the first ten pages of GoogleScholar.
| Database | Search string |
| PubMed/PubMed Central/MEDLINE | (Corticosteroids [Title/Abstract]) OR (dexamethasone [Title/Abstract]) OR (methylprednisolone [Title/Abstract]) AND (posterior reversible encephalopathy syndrome [Title/Abstract]) |
| Science Direct | (Posterior reversible encephalopathy syndrome) AND (corticosteroids OR methylprednisolone OR dexamethasone) |
| Hinari | Corticosteroids OR dexamethasone OR methylprednisolone AND posterior reversible encephalopathy syndrome |
Studies were included if they were peer-reviewed articles that examined the use of corticosteroids in patients with PRES. We include case reports/series and observational studies. Exclusion criteria comprised non-English-language publications, case studies, reviews, editorials, and studies that did not report specific results related to corticosteroids in PRES. Records identified by database search were exported to EndNote, where duplicates were removed. The remaining records were then uploaded to the Rayyan QCRI web platform for screening[4]. Two authors (Kaur and Singh) inde
| Case No. | Ref. | Age (year) | Sex | Clinical sequela | BP (mmHg) | MRI findings | Vessel imaging | CSF studies | Cause | LOS (days) | Management | Outcomes |
| 1 | No author[23], 2016 | 33 | F | Subarachnoid hemorrhage | N/A | Although no magnetic resonance imaging and vessel imagining was performed, cranial imaging showed hypodense areas in small acute infarcts and temporal, parieto, and occipital regions. Later, a repeat cranial imaging showed delineation of hypodensities in temporal-parieto-occipital regions | N/A | N/A | Methylprednisolone pulse therapy | Not specified but > 11 | For seizures, anticonvulsants were administered. For SLE hydrocortisone along with mycophenolate mofetil. The patient was treated for pneumonia and underwent hemodialysis | Follow up: Normal brain parenchyma revealed by CT a year later. Outcome: Less edematous and seizures stopped |
| 2 | Alexander et al[24], 2013 | 16 | M | Three episodes of tonic clonic seizures, tubulitis, and increased systolic blood pressure | 220 | FLAIR MRI revealed bilateral multifocal subcortical hyperintensities in the parietal lobe. Furthermore, there was relative sparing in the left frontal lobe, which means that there were less severe hyperintense lesions there. There was involvement/lesions of the deep white matter of the ganglionic region and of the right internal capsule. Areas with high signal intensities reveal vasogenic edema, as shown by increased ADC values | N/A | N/A | Higher doses of tacrolimus, corticosteroids, and being of a young age were factors causing PRES. Additionally, an extended period of uremia before transplantation led to PRES | 9 | The patient received five oral antihypertensive drugs. This included antiepileptic levetiracetam and minoxidil. The dose of tacrolimus was increased | Follow up: 6 weeks later had complete resolution of brain abnormalities. Outcome: Fully recovered |
| 3 | Camara-Lemarroy et al[25], 2015 | 22 | F | Renal decline, severe bilateral occipital headache, seizures, confusion, hyperreflexia, reduced visual acuity | 170/100 | A T2 FLAIR of the occipital, parietal, and frontal lobes (subcortical) revealed bilateral hyperintensities. These findings are indicative of vasogenic edema with no diffusion restriction observed in DWI | N/A | N/A | Immunosuppressive therapy: Pulses of pulses of methylprednisolone | 8 | Hemodialysis, immunomodulation analgesic, IV diazepam (10 mg), IV nitroglycerin, IV phenytoin | Follow up: N/A. Outcome: After 24 hours, the patient recovered completely, the seizures stopped |
| 4 | Chennareddy et al[26], 2013 | 17 | F | Case 1: Headache, blurry vision, and tonic-clonic seizures | Case 1: 110/70 | Case 1: FLAIR hyperintense signal in the left occipital lobe. DWI: Showed no restriction in the left occipital lobe | N/A | N/A | Case 1: Endothelial dysfunction caused by SLE and pathologic dysregulation of cerebral blood flow that was exacerbated by methylprednisolone (in enhancing the vascular tone) caused PRES | Case 1: Estimated 5 days | Case 1: Analgesics and anticonvulsants | Follow up for Case 1: N/A. Outcome for Case 1: No flare-up of lupus, no more seizures, decreased headaches, well |
| 5 | Chennareddy et al[26], 2013 | 16 | F | Case 2: Headache, blurred vision | Case 2: 120/70 | Case 2: A hyperintense signal in the bilateral occipital areas that was suggestive of PRES | Case 2: N/A | Case 2: N/A | Case 2: N/A | Case 2: Estimated 7 days | Case 2: N/A | Follow up for Case 2: N/A. Outcome for Case 2: Headache went away, nephritis absent, and well |
| 6 | Wee et al[27], 2020 | 19 | M | Epilepticus, altered sensorium, HTN | 170/116 | The non-contrasted CT scan revealed hypodensities of white matter at the bifrontal, bilateral occipital, left cerebellum and left parietal lobe. Also, contrast CT did not reveal venous thrombosis | N/A | N/A | Methylprednisolone | 10 | For aspiration pneumonia, intravenous antibiotics and antiepileptics along with intermittent hemodialysis were administered | Follow up: N/A. Outcome: His GCS value returned to normal after one week in the ICU |
| 7 | Ding et al[28], 2015 | 40 | M | HTN, headache, two episodes of generalized tonic-clonic seizures | 159/93 | High signals seen on T2-weighted, FLAIR MRI, DWI, ADC. Comparable signals on T1 and enhanced sequence in the bilateral parieto-occipital lobes | N/A | Unrevealing | Methylprednisolone | Approximately 7 | Irbesartan and edaravone. A week later, he continued antihypertensive drugs with a tapering dose of methylprednisolone | Follow up: N/A. Outcome: Neurological symptoms improved. MRI showed a significant decrease in abnormal signals |
| 8 | Fujita et al[29], 2008 | 23 | F | Fever, headache, fatigue, vomiting, tonic-clonic seizures | 180/120 | T2WI/ADC: Increased bilateral parietal lobes. However, there were no significant signal alterations in DWI. Contrast MRI showed hyperintense signals on T2 and FLAIR images in the bilateral temporal–parietal–occipital lobes, left frontal lobe, and left cerebellar hemisphere. MRI showed more hyperintense signals on T2 and FLAIR images in the bilateral temporal–parietal– occipital lobes and left cerebellar hemisphere | The MRA did not show abnormalities in the cerebrovascular system except for a less important flow signal from the left vertebral artery. This is indicative of involvement of disease with the left vertebral artery. Cerebral angiography revealed no intracranial aneurysm | Normal | Pulse dose methylprednisolone | 60 | Diazepam, phenytoin, and nifedipine; Cyclophosphamide plasmapheresis; Thiopental; Betamethasone; Oral prednisolone; high-dose; methylprednisolone | Follow up: N/A. Outcome: A physical examination revealed no neurological deficits and CRP became negative, seizures disappeared |
| 9 | Fukuyama et al[30], 2011 | 6 | M | Generalized seizures, drowsiness, cortical blindness | 157/110 | MRI revealed high signal intensities in the posterior medial frontal regions consistent with vasogenic edema. ADC mappings and DWI revealed restricted diffusion in focal areas that were again consistent with vasogenic edema | N/A | Before developing PRES, patient had normal CSF levels | Methylprednisolone was listed as a risk factor for PRES in this patient. Also, the patient most likely developed PRES due to HSCT that included mPSL | N/A | mPSL, BMT, methotrexate, FK506, magnesium, intravenous nicardipine, myeloid engraftment, mycophenolate mofetil, VPA | Follow up: The patient died from gastrointestinal hemorrhagic shock. Outcome: N/A |
| 10 | Gera et al[31], 2014 | 5 | F | Headache, cortical blindness, seizure | 180/100 | MRI revealed lesions in the parietal and occipital lobe | N/A | N/A | 3 pulses of methylprednisolone | N/A | For renal disease and renal failure, timely correction of fluid electrolyte was performed along with acid base balance. For hypertension, antihypertensives and nitroglycerin drips were administered. IV albumin was administered for hypoalbuminemia. Lastly, HUS was treated using plasma exchanges and methylprednisolone medication dose adjustment | Follow up: Cranial images were normal after 4-5 weeks. Outcome: Recovery of complete renal and neurological function |
| 11 | El Hage et al[32], 2021 | 15 | M | Seizure, headache, HTN | 210/136 during PRES max. 150/82 | T2WI: Increased subcortical and bilateral occipital, parietal, and superior frontal areas | N/A | N/A | Corticosteroid was listed as a precipitating factor | N/A | Antihypertensives and antiepileptic medications | FU: Brain MRI was normal after three months. Outcome: Recovered |
| 12 | İncecik et al[33], 2013 | 8 | M | Headache, confusion, seizures, HTN | 150-90 - 160/100 | T2WI: Increased signal in the bilateral parieto-occipital lobe and left frontal lobe | N/A | N/A | Pulse IV methylprednisolone; HTN | N/A | Levetiracetam, pulse dose IVMP | Follow up: N/A. Outcome: Neurological symptoms improved and after 15 days, MRI showed significant reversal |
| 13 | Irvin et al[34], 2007 | 48 | F | Disoriented, agitated, autonomic instability, HTN | 190/100 | T2WI: Diffuse cortical and subcortical white matter signal abnormality in a symmetric bilateral distribution involving predominantly the occipital areas, superior frontal areas, the cerebellum, and some focal areas of the thalamus bilaterally, not evident on T1-weighted images | Same as left | Normal limits | Dexamethasone-induced PRES | N/A | Trazodone and zolpidem, and a single dose of haldol | Follow up: A repeat brain MRI 4 days later showed improving PRES. Outcome: Hospice was started and she did not receive radiation therapy to the brain |
| 14 | Ismail et al[35], 2021 | 57 | F | N/A | N/A | T2WI: Bihemispheric and extensive symmetric diffusion impairment (parieto-occipital, temporal, cerebellar, frontal, and temporal) hypersignal (cortical and subcortical) | N/A | N/A | Methylprednisolone | N/A | Levetiracetam and valproate | Follow up: N/A. Outcome: Died from PRES and confirmed from autopsy |
| 15 | Kamezaki et al[36], 2012 | 62 | F | High fever | 180/118 | T2WI: High signal intensity in the pons, cerebral hemisphere, basal ganglia, thalamus bilaterally. T2 star-weighted images show low intensity signals in the right thalamus. Diffusion-weighted images reveal high signal intensity in the same region | N/A | Normal | Chemotherapeutic agents which included methylprednisolone were listed as a possible factor | 120 | Unspecified but rehabilitation was used | Follow up: MRI revealed complete resolution of lesions. Outcome: N/A |
| 16 | Kamo et al[37], 2019 | 51 | F | Abducens palsy left eye, left-sided dysesthesia, dysarthria | 202/127 | MRI of the brain on DWI revealed isointense area in the pons. Additionally, it revealed a circumscribed hyperintense area on both the ADC and FLAIR. DWI, ADC map and FLAIR imaging showed vasogenic edema caused by PRES | N/A | N/A | IV methylprednisolone | N/A | Nicardipine with tranexamic acid and glycerol were administered. Plasmapheresis | Follow up: N/A. Outcome: Final NIHSS lowered to 3. From therapy, blindness improved to light perception. Sensory disturbance on the left side and cerebellar ataxia of both the lower and upper limbs persisted |
| 17 | Khan et al[38], 2018 | Patient 1: N/A; M Note: The mean age of all patients was 7 years | M | HTN; Note: Seizures and altered mental status were symptoms for > 90% of the symptoms for > 90% of cases although it was not specifically stated which cases had which symptoms | N/A | MRI revealed abnormalities in parietal cortex | N/A | WBC: 0; RBC: 1000; Cyto: Neg | Dexamethasone | N/A | Levetiracetam, methotrexate | Follow up: Alive. Outcome: Symptom resolution and PRES scan done after treatment showed resolvement |
| 18 | Khan et al[38], 2018 | Patient 2: N/A | M | N/A | N/A | MRI revealed abnormalities in the parietal cortex and subcortical region | N/A | WBC: 1; RBC: 3; Cyto: Neg | Dexamethasone, steroid-induced hypertension that might lead to PRES | N/A | Levetiracetam, methotrexate | Follow up: Alive. Outcome: Symptom resolution and PRES scan after treatment showed resolvement |
| 19 | Khan et al[38], 2018 | Patient 3: N/A | M | HTN | N/A | MRI revealed abnormalities in parietal cortex, subcortical region, and anterior cortex | N/A | WBC: 0; RBC: 2; Cyto: Neg | Dexamethasone, steroid-induced hypertension that might lead to PRES | N/A | Methotrexate | Follow up: Dead. Outcome: No symptom resolution and no PRES scan done after treatment |
| 20 | Khan et al[38], 2018 | Patient 4: N/A | F | HTN | N/A | MRI revealed abnormalities in parietal cortex, subcortical region, and anterior cortex | N/A | WBC: 1; RBC: 1; Cyto: Neg | Dexamethasone | N/A | Levetiracetam, methotrexate | Follow up: Alive. Outcome: Unimproved |
| 21 | Khan et al[38], 2018 | Patient 5: N/A | F | HTN | N/A | MRI revealed abnormalities in parietal cortex, subcortical region, and anterior cortex | N/A | WBC: 0; RBC: 0; Cyto: Neg | Dexamethasone | N/A | Levetiracetam,methotrexate | Follow up: Deceased. Outcome: No symptom resolution and no PRES scan done after treatment |
| 22 | Khan et al[38], 2018 | Patient 8: N/A | M | HTN | N/A | MRI revealed abnormalities in the parietal cortex | N/A | WBC: 0; RBC: 1000; Cyto: Neg | Dexamethasone, steroid-induced hypertension that might lead to PRES | N/A | Phenytoin, methotrexate | Follow up: Dead. Outcome: No symptom resolution and no PRES scan done after treatment |
| 23 | Khan et al[38], 2018 | Patient 9: N/A | M | N/A | N/A | MRI revealed abnormalities in the parietal cortex and anterior cortex | N/A | WBC: 0; RBC: 2000; Cyto: Neg | Dexamethasone, steroid-induced hypertension that might lead to PRES | N/A | Levetiracetam | Follow up: Alive. Outcome: Symptom resolution but PRES scan didn’t show resolution after treatment |
| 24 | Khan et al[38], 2018 | Patient 10: N/A | M | HTN | N/A | MRI revealed abnormalities in parietal cortex, subcortical region, and anterior cortex | N/A | WBC: 0; RBC: 0; Cyto: Neg | Dexamethasone, steroid-induced hypertension that might lead to PRES | N/A | Methotrexate | Follow up: Alive. Outcome: Symptom resolution but no PRES scan done after treatment |
| 25 | Khan et al[38], 2018 | Patient 11: N/A | M | HTN | N/A | MRI revealed abnormalities in parietal cortex, subcortical region, and anterior cortex | N/A | WBC: 0; RBC: 0; Cyto: Neg | Dexamethasone, steroid-induced hypertension that might lead to PRES | N/A | Levetiracetam, phenytoin, and methotrexate | Follow up: Alive. Outcome: Symptom resolution and PRES scan after treatment showed resolvement |
| 26 | Khan et al[38], 2018 | Patient 14: N/A | M | HTN | N/A | MRI revealed abnormalities in the parietal cortex and anterior cortex | N/A | WBC: 5; RBC: 35; Cyto: Neg | Dexamethasone, steroid-induced hypertension that might lead to PRES | N/A | Phenytoin, methotrexate | Follow up: Dead. Outcome: No symptom resolution and no PRES scan done after treatment |
| 27 | Khan et al[38], 2018 | Patient 15: N/A | M | HTN | N/A | MRI revealed abnormalities in the parietal cortex and anterior cortex | N/A | WBC: 0; RBC: 0; Cyto: Neg | Dexamethasone, steroid-induced hypertension that might lead to PRES | N/A | Phenytoin | Follow up: Alive. Outcome: Symptom resolution but no PRES scan done after treatment |
| 28 | Khan et al[38], 2018 | Patient 18: N/A | M | HTN | N/A | MRI revealed abnormalities in the parietal cortex | N/A | WBC: 2; RBC: 1; Cyto: Neg | Dexamethasone, steroid-induced hypertension that might lead to PRES | N/A | Phenytoin, methotrexate | Follow up: Alive. Outcome: Symptom resolution and PRES scan done after treatment showed partial resolvement |
| 29 | Khan et al[38], 2018 | Patient 19: N/A | F | HTN | N/A | MRI revealed abnormalities in the parietal cortex | N/A | N/A | Dexamethasone, steroid-induced hypertension that might lead to PRES | N/A | Phenytoin, methotrexate | Follow up: Dead. Outcome: No symptom resolution and no PRES scan done after treatment |
| 30 | Khanjar et al[39], 2018 | Patient 1: 34 | F | Tonic-clonic seizures, headache, decreased vision, HTN. All patients reported headache and impaired vision | Case 1: 190/100; Cases 2, 3 and 4: N/A | T2WI: Patchy multi-focal increased signal within the parietal-occipital cortical and subcortical areas bilaterally | N/A | N/A | PRES was caused by the initiation of immunosuppressive therapy, mostly due to pulse methylprednisolone therapy | 6 | Management of blood pressure and seizures along with mechanical ventilation | Follow up: N/A. Outcome: Successfully managed |
| 31 | Khanjar et al[39], 2018 | Patient 2: 23 | F | Tonic-clonic seizures, HTN, and renal decline | N/A | T2WI: Patchy multi-focal increased signal within the parietal-occipital cortical and subcortical areas bilaterally | N/A | N/A | PRES was caused by the initiation of immunosuppressive therapy, mainly due to pulse methylprednisolone therapy | 16 | Intubation, antihypertensives, antiepileptics, mechanical ventilation | Follow up: N/A. Outcome: Improved dramatically |
| 32 | Khanjar et al[39], 2018 | Patient 3: 41 | F | N/A | N/A | T2WI: Patchy multi-focal increased signal within the parietal-occipital cortical and subcortical areas bilaterally | N/A | N/A | PRES was caused by the initiation of immunosuppressive therapy, mostly due to pulse methylprednisolone therapy | 16 | Intubation, hemodialysis, antihypertensive, antiepileptic, mechanical ventilation | Follow up: N/A. Outcome: Complete resolution |
| 33 | Khanjar et al[39], 2018 | Patient 4: 29 | F | Seizures, diffuse alveolar hemorrhage, hemophagocytic lymphocytosis | N/A | MRI indicated PRES | N/A | N/A | PRES was caused by the initiation of immunosuppressive therapy, mainly due to pulse methylprednisolone therapy | 20 | Rehab, mechanical ventilation | Follow up: N/A. Outcome: Patient required rehab but eventually recovered |
| 34 | Kitamura et al[40], 2022 | 84 | F | Fatigue, mental status deteriorated, leg numbness | 1288/58 | A DWI and MRI revealed vasogenic oedema after showing mild hyperintensity and higher signal of an ADC map of the parietal, bilateral occipital, frontal cortex, midbrain, and subcortical white matter. FLAIR-MRI findings also revealed an increased signal intensity of the previously mentioned regions, suggesting PRES | N/A | N/A | Methylprednisolone pulse therapy, prednisolone | At least 53 days | Rituximab, IV immunoglobulin, plasma exchange, hemodialysis, eculizumab, meningococcal vaccination, prednisolone was reduced | Follow up: 8 month follow up showed no relapse. Outcome: 56 days after admission. MRI showed PRES was resolved |
35 | Kumar and Rajam[41], 2011 | 6 | M | HTN, absence of visual fixation, seizures, cortical blindness, fever, macular rash | N/A | T2WI: bilateral focal hyperintensities in the occipital lobes involving cortical and subcortical white matter during the acute phase; mild diffuse brain atrophy | N/A | N/A | Pulse methylprednisolone therapy; HTN due to steroids | Approximately 3-4 months | High dose oral prednisolone; second course of methylprednisolone; cyclosporine, etoposide and dexamethasone (HLH-2004 protocol); ventilation | Follow up: N/A. Outcome: Full neurological recovery, no existing illness, disease control due to cyclosporine |
| 36 | Kurahashi et al[42], 2006 | 4 | F | Lethargic, convulsions, coma | 160/100 | T2WI: hyperintensities in the white matter of the bilateral occipital lobes | CSF no abnormalities | N/A | Methylprednisolone | At least 67 days | Intravenous hydrocortisone, continuous inhalation of b-2 stimulator, and additional oxygen; Sevoflurane; Furosemide; nicardipine, phenobarbital, and mannitol | Follow up: She presents no neurological sequelae 2 years after the event. Outcome: FLAIR image on the 67th hospital day shows resolution of hyperintensities |
| 37 | Dar et al[43], 2015 | 51 | F | Seizure, headache, HTN | N/A | MRI of the spine showed long segment myelitis. MRI of the brain revealed bilateral diffuse white matter edema and faint restricted diffusion in the subcortical and cortical regions. This was suggestive of PRES | N/A | N/A | IV methylprednisolone and IV vitamin B12 | N/A | Treatment with methylprednisolone and dexamethasone for 1 month | Follow up: Alert and coherent during activities. Outcome: Recovered significantly |
| 39 | Mimura et al[44], 2023 | 73 | F | Consciousness deteriorated | 140/80 | FLAIR MRI showed multiple lesions with high signal intensity | N/A | N/A | Steroid administration increases blood pressure due to fluid retention and can be a risk factor for the development of PRES | At least 11 days | Intravenous methylprednisolone oral prednisolone; Rituximab; Intravenous administration of nicardipine and fluid removal by hemodialysis | Follow up: N/A. Outcome: blood pressure decreased and her consciousness improved dramatically |
| 40 | Morrow et al[45], 2015 | 53 | F | Insomnia, dizziness, general malaise, and a headache, holocephalic headache | 199/110 | T2 hyperintensities were found in the cervical cord and brain that are suggestive of MS, same MRI results as six months earlier. In addition, there was an abnormal T2 signal in the posterior occipital, posterior white matter extending to the vertex, and in both thalami. Along with the new lesions, mild local sulcal effacement was observed. They found no diffusion restrictions based on the lesions. Overall, the findings were indicative of a diagnosis of PRES | N/A | N/A | Corticosteroids, HTN | N/A | High dose CR. 1250 mg oral prednisone. Hydrochlorothiazide and amlodipine. Labetalol and amlodipine | Follow up: 1 month follow-up neurologically stable. Outcome: One month later, the signal change resolved in the posterior cerebral hemispheres, consistent with the resolution of PRES |
| 41 | Nguyen et al[46], 2009 | 32 | F | Nausea, seizures, dizziness, forgetfulness | 112/85 | MRI revealed multifocal areas of increased non-enhancing T2-Weighted FLAIR signals. This was found within the white matter of both hemispheres | N/A | N/A | Dexamethasone | N/A | Aprepitant, palonosetron, lorazepam, oral dexamethasone, rescue prochlorperazine, ondansetron, prochlorperazine, diphenhydramine, Dronabinol, diazepam, and fosphenytoin | Follow up: 1 month MRI showed full resolution. Outcome: Returned to baseline in one week. Dexamethasone doses were tapered and eventually discontinued |
| 42 | Ozkok et al[47], 2012 | 22 | F | HTN | N/A | MRI from T2-weighted and FLAIR revealed lesions in the bilateral cortical occipital lobe | N/A | N/A | Hypertension and methylprednisolone were listed as the highest risk factors | Approximately 14 | 3 times a week, hemodialysis and antihypertensive treatment with amlodipine and doxazosin | Follow up: Her renal functions failed to improve and became anuric. However, she had no respiratory symptoms. Outcome: PRES resolved within 2 weeks |
| 43 | Shibata et al[48], 2022 | 51 | F | Headache, tonic-clonic seizures | 205/107 | FLAIR sequences MRI revealed a bilateral occipital subarachnoid hemorrhage, parenchymal hemorrhage in the left occipital lobe, and hyperintense lesions of the subcortical white matter of both occipital lobes. The lesion in the left occipital lobe showed hyperintensity on the ADC map suggesting vasogenic edema | MRA: A partial resolution of signal change and vasoconstrictions in the bilateral middle cerebral arteries and posterior cerebral arteries | N/A | Intravenous methylprednisolone pulse | 12 | Diazepam, phenytoin, levetiracetam, antihypertensive therapy | Follow up: N/A. Outcome: Discharged without any neurological deficits |
| 44 | Sinha and Hurley[49], 2008 | 16 | F | Disorientation, motor apraxia, blurred vision, headache | 150/80-160/90 | Axial FLAIR and turbo spin echo T-2 weighted images revealed increased signal intensity in the occipital and left parietal regions. DWI and ADC images did not reveal any restricted diffusion pattern | N/A | N/A | PRES was linked to corticosteroid | N/A | Cyclophosphamide and high oral dose | Follow up: MRI taken 6 weeks after starting treatment revealed resolution of the lesions. Outcome: N/A |
| 45 | Stârcea et al[50], 2018 | 10 | F | Seizures, respiratory distress, bilateral amaurosis, drooling, renal decline | 120/65 | T2FLAIR/DWI: Cortical hyperintensity in the parietal lobe, indicating damage to white matter; white matter damage; degeneration with diffuse demyelination in the parietal and posterior occipital lobes | N/A | Normal | Immunosuppressive therapy: Methylprednisolone | N/A | Mechanical ventilation, blood transfusions, platelet concentrate, antibiotic therapy, continuous veno-venous hemofiltration, nifedipine, clonidine, dialysis | Follow up: 6 months. Outcome: No neurological problems, fully recovered, regular hemodialysis sessions |
| 46 | Swarnalatha et al[51], 2012 | 11 | F | Generalized tonic clonic seizures | 110/60 | MRI of the brain revealed asymmetrical bilateral T2-weighted and FLAIR revealed hyperintense lesions in subcortical location of parieto-occipital, cortical, temporal lobes, cerebellum, and bilateral thalami indicative of PRES | N/A | CSF fluid analysis showed protein: 15 mg/dL, 3 cells/hpf, glucose: 56 mg/dL | Corticosteroid was listed as a possible factor leading to vasogenic edema, a characteristic of PRES | N/A | The patient was treated with antiepileptics and her dose was reduced to half of prednisolone | Follow up: N/A. Outcome: Recovered in 48 hours after the treatment listed in the left column |
| 47 | Tsukamoto et al[52], 2012 | 28 | F | Generalized tonic-clonic convulsions with loss of consciousness | 150/100 | Both FLAIR and DWI MRI revealed cortical and subcortical hyperintensities in the occipital lobes, left thalamus, and bilateral frontal lobe. ADC revealed high signal intensities in the lesions of T2 shine through | The MRA did not reveal vascular abnormalities | The CSF was normal without any infiltration of leukemic cells | Induction chemotherapy which included prednisone. It was also stated that corticosteroids along with l-asparaginase and vincristine may cause PRES | N/A | Nifedipine was used to control blood pressure and midazolam was used as an anticonvulsant. Consolidation therapy and intrathecal administration. Underwent BMT for ALL | Follow up: Completely recovered from PRES without leukemia relapse. Outcome: MRI revealed significant resolution of lesions but still had difficulty walking, leg pain, and somnolence |
| 48 | Ulutaş et al[53], 2020 | 39 | M | Tonic clonic seizures, severe headaches, HTN | 160/110 | White matter Hyperintense vasogenic edema lesions were found primarily on the right side of the posterior brain, as revealed by T2-weighted sequences | N/A | Nerve conduction studies and CSF analysis both revealed dysautonomic inflammatory demyelinating polyneuropathy and albuminocytologic dissociation, respectively | Patient developed PRES because of intensive immunosuppressive treatment that consisted of CyC and pulse steroid therapy | N/A | The patient was treated with antiedema therapy and antiepileptics. To control blood pressure, parenteral antihypertensive agents were administered | Follow up: Passed away. Outcome: No clinical improvement |
| 49 | Vernaza et al[54], 2021 | 34 | F | Holocranial pulsatile cephalalgia with photophobia, headache, tonic-clonic seizure | N/A | In both T2-weighted and FLAIR scans, there were hyperintensities in the cerebellar corticosubcorctical and parieto-occipital regions | Angiography and computed tomography showed a suboccluding thrombus in the right internal jugular vein and mild subarachnoid hemorrhage in both sides of the frontal lobe | No lumbar puncture | Methylprednisolone; immunosuppressive treatment | 17 days | Cyclosporine, IV artesunate, chloroquine and primaquine | Follow up: Died due to multiorgan failure. Outcome: 5 days later in the emergency department. Patient complained of gastrointestinal problems |
| 50 | Yang et al[55], 2022 | Patient 1: 48 | F | Sleepiness, diplopia | 120/74 | Lesions found in the parietal and occipital lobe | N/A | N/A | Intravenous methylprednisolone was found to be an inducing factor for PRES | N/A | No treatment for PRES | Follow up: N/A. Outcome: Partial brain lesion resolution |
| 51 | Yang et al[55], 2022 | Patient 2: 53 | F | Headache, giddiness | Normal (not specified) | Lesions found in the temporal, parietal, and occipital lobe | N/A | N/A | Intravenous methylprednisolone was found to be an inducing factor for PRES | N/A | Unknown | Follow up: N/A. Outcome: Unknown |
| 52 | Yang et al[55], 2022 | Patient 3: 35 | F | Sleepiness, Visual impairment, delirium | N/A | Lesions found in the temporal lobe | N/A | N/A | Intravenous methylprednisolone was found to be an inducing factor for PRES | N/A | Unknown | Follow up: N/A. Outcome: Partial brain lesion resolution |
| 53 | Yang et al[55], 2022 | Patient 4: 48 | F | Epilepsy, visual impairment | N/A | Lesions found in the semiovale, temporal and occipital lobe of the center | N/A | N/A | Intravenous methylprednisolone was found to be an inducing factor for PRES | N/A | Reduction/tapering of IVMP | Follow up: N/A. Unknown |
| 54 | Yang et al[55], 2022 | Patient 5: 51 | F | Headache | 202/127 | Lesions found in the brain stem | N/A | N/A | Intravenous methylprednisolone was found to be an inducing factor for PRES | N/A | Antihypertension and plasma exchange | Follow up: N/A. Complete resolution of brain lesion |
| 55 | Zekić et al[56], 2017 | 18 | F | The patient had fever, leg oedema, lupus nephritis, acute arthritis, and malaise | 160/100 | Transverse sequence T2 and FLAIR magnetic resonance imaging revealed subcortical and cortical hyperintensities of the right frontal lobe and both sides of the parietal brain lobe | N/A | No abnormalities were found by brain CT scans, cytological and bacteriological analysis of the CSF | Intravenous pulse therapy with methylprednisolone | N/A | Antihypertensive, antiedema, and antiepileptic therapy | Follow up: 1 year. Successfully delivered a child and was in complete clinical remission due to SLE. Outcome: Full neurological recovery |
| 56 | Zhang et al[57], 2018 | 22 | F | Sudden epileptic attacks, onset of seizures, HTN | 190/130 | MRI of cranium revealed vasogenic edema at both sides of the parietal region, occipital parietal regions, and centrum ovale | N/A | The CSF pressure was observed as 330 mm H2O, the protein level was normal, the white blood cell count was 0 cell/mm3, and no signs of infection | Intravenous methylprednisolone 80 mg daily induced hypertension which caused PRES | N/A | Corticosteroid impulse therapy was not administered. Drugs were administered to decrease intracranial pressure, intravenous drugs to lower blood pressure, anmidazolam for sedation | Follow up: N/A. Outcome: Recovered the next day after starting treatment |
The risk of bias in included case reports was assessed using the Joanna Briggs Institute (JBI) tool for case reports by two authors (Kaur and Singh). This tool evaluates various dimensions of bias, including the clarity of the diagnosis, the precision of the reported intervention, and the validity of the outcomes[5]. Descriptive statistics were used to summarize the findings. Quantitative synthesis was completed using R Studio (2023.06.0 + 421).
A PRISMA flow diagram is included in Figure 1. Based on data collected from various case studies, corticosteroids have been implicated as a potential causative agent in PRES. A total of 56 cases of PRES associated with corticosteroid use were identified and analyzed. Ages ranged from 4 to 84 years, with a mean age of approximately 25.2 years. A total of 37 women and 19 men were identified. The most common clinical sequelae included headaches (n = 22), seizures (n = 41), hypertension (n = 33), and visual disturbances (n = 10). The blood pressure readings at the time of presentation varied widely, with systolic pressures ranging from 110 to 220 mmHg and diastolic pressures from 58 to 136 mmHg. Magnetic resonance imaging (MRI) findings frequently revealed bilateral subcortical hyperintensities, primarily in the occipital, parietal, and frontal lobes, consistent with vasogenic edema as evident by an increased T2 weighted signal. Diffusion-weighted imaging (DWI) and apparent diffusion coefficient maps were used to confirm the presence of vasogenic edema without significant diffusion restriction. Methylprednisolone was the corticosteroid most involved, followed by dexamethasone (DEX) and prednisone. The doses and duration of corticosteroids therapy varied between cases, with some patients receiving high-dose pulse therapy. In several cases, corticosteroids were combined with other immunosuppressive treatments, such as cyclophosphamide, tacrolimus, and methotrexate.
Treatment strategies typically included discontinuation or reduction in corticosteroid use, along with antihypertensive and antiepileptic therapies. For some patients, additional treatments such as hemodialysis, immunosuppressive therapy, and supportive care for underlying conditions such as systemic lupus erythematosus (SLE) or renal failure were necessary. The outcomes varied, and many patients showed complete resolution of the symptoms related to PRES and the findings of the MRI after appropriate treatment. However, there were instances of severe complications and mortality, particularly in patients with concurrent severe systemic conditions or delayed treatment initiation. Follow-up data revealed that many patients achieved full recovery without long-term neurological deficits. However, there were instances of mortality attributed to complications such as gastrointestinal hemorrhagic shock and multiorgan failure. Among survivors, complete resolution of PRES was documented on follow-up imaging, and several cases reported normalization of blood pressure and cessation of seizures. A full summary of cases is provided in Table 2.
A total of 14 cases of PRES were identified and analyzed in which corticosteroids were used as part of the treatment regimen. The patient’s ages ranged from 11 to 75 years, with a mean age of approximately 37 years. The cohort consisted of 4 males and 10 females. The most common clinical sequelae included headaches (n = 6), seizures (n = 9), hypertension
Methylprednisolone was the most used corticosteroid, followed by DEX. The doses and duration of corticosteroids therapy varied between cases, with some patients receiving high-dose pulse therapy. Corticosteroids were administered as part of a broader management strategy that included antihypertensive agents, antiepileptics, and supportive care measures such as hemodialysis and mechanical ventilation. The length of hospital stays varied, with several patients requiring prolonged hospitalization due to severe clinical manifestations. Most of the patients showed significant clinical improvement after initiation of corticosteroid therapy, with MRI findings that demonstrate resolution of PRES-related abnormalities. Follow-up data revealed that most patients achieved full recovery without long-term neurological deficits. Among survivors, complete resolution of PRES was documented on follow-up imaging, and several cases reported normalization of blood pressure and cessation of seizures. The use of corticosteroids was associated with positive clinical outcomes, including stabilization of symptoms and resolution of vasogenic edema. A full summary of the cases is provided in Table 3.
| Case No. | Ref. | Age (year) | Sex | Clinical sequela | BP (mmHg) | MRI findings | Vessel imaging | CSF studies | Cause | LOS (days) | Management | Outcomes |
| 1 | Aridon et al[58], 2011 | 53 | M | Elevated blood pressure, gait disturbance, dizziness, urinary incontinence, and lethargy | 260/180 | MRI showed signals in both sides of the white matter of the cerebral and cerebellar hemispheres with involvement of the cerebellar peduncles and midbrain. A triventricular hypotheses hydrocephalus, a result of brainstem oedema, was observed | N/A | A lumbar puncture was performed. It revealed a normal cell count (0.8/mm3) along with limpid and uncolored CSF. Also slightly higher total protein concentration (77mmg/mL, normal is less than 40 mg/mL) | TTP | N/A | High-dose methylprednisolone and plasma exchange therapy | Follow up: 6 months later the neurological examination was normal and 24 months later he did not relapse and is still healthy. Outcome: Brain magnetic resonance imaging revealed almost complete resolution of brainstem oedema and changes in abnormal T2 signals |
| 2 | Christofidis et al[59], 2019 | 20 | F | Generalized arthralgia, high grade fever (up to 39 degrees C), and severe headaches | 140/70 | DWI was normal with no sign of restricted diffusion. The MRI revealed leptomeningeal enhancement and bilateral subcortical lesions in the parietal-occipital regions on T2/FLAIR images, indicating vasogenic edema was present there | MRA demonstrating constriction and vasospasm of the cerebral arteries | Both PCR tests of CSF and blood were found to be positive for Neisseria meningitis (serogroup B). Both culture and Gram stain were negative for CSF. The white blood cell count was 15 K/uL, indicative of neutrophilic pleocytosis. The glucose level was low at 1 mg/dL and elevated protein concentrations at 352.01 mg/dL | Infection, sepsis | 23 | The patient was given mannitol, dexamethasone, the antiepileptic drug levetiracetam, and Ceftriaxone. Endotracheal intubation was also performed | Follow up: Follow-up MRI of both T2 and FLAIR images showed on the 23rd day complete resolution of the hyperintense and diffuse lesions in the parietal-occipital brain regions. The MRA follow-up was normal. Outcome: Mental status was completely restored 3-4 days after initiation of treatment, rapid recovery |
| 3 | Hao et al[60], 2021 | 28 | F | Anasarca and tachycardia | 107/79 | MRI showed an abnormal high signal intensity in T2-weighted imaging, FLAIR, and ADC maps. In addition, low signal intensities on T1-weighted imaging and DWI were revealed. These were found in the parietal, bilateral frontal, occipital, temporal, basal ganglia, and cerebellar hemispheres | N/A | Increased protein levels | Autoimmune inflammation or ischemic changes that resulted from SLE (such as vasculitis, embolism, thrombus, and vasospasm) lead to endothelial dysfunction and, subsequently, to PRES. The author acknowledges that PRES could have been caused by corticosteroids, but ultimately was used to help treat PRES | Approximately 12 | The patient was administered corticosteroids with methylprednisolone at 160 mg/day for inflation. She was sedated and also administered antiepileptic, acid suppression medications, and antipyretic. Once symptoms returned, methylprednisolone was administered 1000 mg/day for 3 days, 20 mg of dexamethasone, 10 mg of intrathecal injection and 60 mg of prednisone tablets in the morning. Immunosuppressant agents were administered hydroxychloroquine sulfate at 0.2 g twice a day, r-globulin at 20 g once a day for 5 days, and cyclophosphamide at 0.4 g per week as needed | Follow up: Condition stable. Outcome: Lesions were mostly resolved |
| 4 | Hosseini[61], 2022 | 40 | F | Severe and refractory headache with multiple convulsive events | 160/90 | T2WI: Vasogenic edema in occipito-parieto-frontal lobes white matter compatible with PRES. Hyperintensities in occipito-parieto-frontal white matter with predominance in occipital lobes, without any restriction in diffusion weighted sequences (DWI), compatible with brain edema. Cervical cord MRI was normal too | MRV was normal | Although the IgG index in CSF was 0.7, CSF analysis was normal and the oligoclonal bands were negative | N/A | N/A | Adjuvant levetiracetam.IVMP, 1000 mg/day | Follow up: N/A. Outcome: After three days of IVMP, her headache completely subsided and a control brain MRI showed resolution of most hyperintense regions |
| 5 | Islam et al[62], 2021 | 29 | F | Seizures, drowsiness, fever, occasional headache, muscle weakness | N/A | T2WI: Symmetrical hyperintensities over posterior brain regions in T2 and fluid attenuated inversion recovery images with no restricted diffusion in diffusion weighted image suggestive of PRES | N/A | Normal cell count 5/mm3 (n = 0–10), elevated protein 69 g/dL (n = 20–40). She also had a normal sugar and antideaminase level | N/A | N/A | Methylprednisolone and monthly cyclophosphamide (patient responded well to these). Levetiracetam, O2, normal saline. Low dose of oral prednisolone Hydroxychloroquine | Follow up: N/A. Outcome: Improvement in all aspects |
| 6 | Jadib et al[63], 2021 | 11 | F | Tonic-clonic seizures, nausea, abdominal pain, headaches, and a more recent onset of blurred vision, HTN | 161/109 | Low signal on T1-weighted images and high signal on T2-weighted images, high apparent diffusion coefficient with no hemorrhage | N/A | N/A | Left renal artery stenosis due to TA | N/A | Intrarectal diazepam Nicardipine Amlodipine and propranolol azathioprine and corticosteroid given | Follow up: 11 months later MRI revealed total resolution. Outcome: Showed neurological improvement |
| 7 | Mai et al[64], 2018 | 55 | F | Confused, speech slurred, convulsions, upgoing plantar reflex, vertical gaze palsy | 140/90 | T2WI: Involving periventricular and deep cerebral white matter. Repeat MRI showed resolution | CSF: Opening pressure of 12 cm H2O, cell count of 7 cells/ µL, a red cell count of 4 cells/µL, protein level of 4.4 g/ dL, a glucose level of 4.76 mmol/L (10.4 mmol/L in blood), and a lactate level of 3.25 mmol/L. Dengue virus IgM was detected in CSF | N/A | Dengue infection | 59 days | Intravenous methylprednisolone, oral prednisolone, and Phenobarbital given | Follow up: N/A. Outcome: Patient was discharged for rehab and a repeat MRI showed almost complete resolution |
| 8 | Min et al[65], 2006 | 22 | F | Headaches, blurred vision, vertigo, nausea, vomiting, and altered mental function, binocular blindness, seizure, and lethargic | 200/110 | T2WI: Cerebellum, brainstem, basal ganglia, and central white matter. These abnormalities resolved | MRA was normal | 20 RBC/L, 1 WBC/L, protein 106 mg/dL (normal range 10 to 40 mg/dL), and glucose 46 mg/dL (normal range 40 to 70 mg/dL) | Hypertension is possible in this context | 14 days at least | Cyclophosphamide, methylprednisolone, dialysis, diazepam, fosphenytoin, Phenytoin, mycophenolate mofetil, and dialysis were performed. Methylprednisolone 1 g IV for 2 days. Plasmapheresis | Follow up: N/A. Outcome. Stopped hemodialysis after renal function improved |
| 9 | Ortega-Carnicer et al[66], 2005 | 24 | M | Generalized seizures, deep coma, flaccid tetraplegia, and fixed dilated pupils | 225/120 | N/A | N/A | N/A | PRES was induced by immunoglobulin administration due to the temporal relationship between immunoglobulin administration and the onset of neurological symptoms of PRES | Approximately 25 | Treatment with IVMP (1000 mg/24 hours) resulted in neurological improvement | Follow up: N/A. Outcome: In good neurological condition |
| 10 | Sato et al[67], 2011 | 42 | F | Throbbing headache, drowsiness | N/A | MRI T2-weighted imaging revealed hyperintense lesions in the occipital and temporal-parietal lobes. Diffusion weighted images revealed bilateral and symmetric hyperintense lesions in the occipital lobe | The right posterior cerebral artery experienced vasoconstriction according to MRA | N/A | Treatment with methylprednisolone helped reverse cerebral edema | 68 | Treatment included methylprednisolone and glycerin | Follow up: MRA returned to normal 6 months after discharge. Complete recovery of the lesions on day 64. Outcome: N/A |
| 11 | Symeonidis et al[68], 2021 | 75 | M | Mental status decline, lethargic, disoriented, episodes of hypertensive crisis | 180/100 | MRI FLAIR and T2-weighted images revealed signal hyperintensity in the bilateral areas of thalamus, fibers of reticular formation, hypothalamus, mild edema of left parahippocampal gyrus, and anterior section of cerebral vermis | N/A | The lumbar puncture revealed negative cytology for metastatic cells. Gram-positive and negative bacteria, herpes zoster, EBV, HSV, BK, CMV, JC, influenza, adenovirus, fungal causes, tuberculosis, listeria, borrelia, and other cultures were negative | Oxaliplatin was the main cytotoxic agent that led to PRES | N/A | Treatment was with dexamethasone and antiepileptics | Follow up: Neurological resolution of PRES after 3-4 weeks. Outcome: Clinical improvement |
| 12 | Tetsuka and Nonaka[69], 2017 | 38 | F | Drowsy but conscious, delirium and fluctuating mental stability. | 150/90 | T2WI/ADC: Revealed hypersignal intense lesions in the cortical and subcortical white matter in the basal ganglia, callosal splenium, and occipital lobes | N/A | N/A | Corticosteroids helped reduce vasogenic edema | 21 | Intravenous corticosteroids and nifedipine | Follow up: MRI two weeks after her initial MRI revealed complete resolution. Outcome: Laboratory results and MRI became normal |
| 13 | Xia and Lv[70], 2022 | 58 | M | Drowsiness, left hemiparesis, lethargy, impaired attention and memory | 169/94 | T2WI: Subcortical hyperintensities in the left and right hemispheres, mainly in the temporal and occipital lobe. ADC revealed increased diffusivity of legions that represent vasogenic edema. SWI: Cortical and subcortical CMBs in the bilateral temporal lobes | N/A | CSF: 13 cells/mm3 (mononuclear cells-10 cells/mm3, polynuclear cells-3 cells/mm3). Elevated total protein concentration revealed 207 mg/dL and an opening pressure of 400 mmH2O. No oligoclonal IgB bands were detected in the analysis. However, both immunoglobulin IgG and albumin increased significantly at 464 mg/L and 1.57 g/L, respectively. The IgG index was slightly higher at .89 in comparison to normal, which is less than or equal to 0.70 | PRES was caused by shock wave lithotripsy and HTN | 24 | High-dose oral methylprednisolone (500 mg/day) for 5 days, dehydration therapy, prednisolone 60mg/day with a decrease dose of 5 mg every 10 days | Follow up: MRI 3 months after discharge revealed most resolution of white matter hyperintensities without CMB in SWI |
| 14 | Xu et al[71], 2021 | 14 | F | Tonic clonic seizures, auditory hallucinations, disorder of thought | 150/100 | T2-weighted and FLAIR revealed hyperintense lesions in the parietal, occipital, temporal, and frontal lobe, subcortex, and cerebral cortex. Diffusion-weighted magnetic resonance imaging and apparent diffusion coefficient revealed isointense and hyperintense lesions | MRA was normal | N/A | Methylprednisolone was used to treat MPA, which in return cured the PRES | N/A | 200 mg of methylprednisolone and 7 courses of plasma exchang | Follow up: No PRES relapse. Outcome: 17 days later, reduced gyrus swelling was observed |
The risk of bias in the included studies was assessed using the JBI tool for case reports and series. This tool assesses various dimensions of bias, including the clarity of diagnosis, the precision of the reported intervention, and the validity of the results. The results of this assessment are summarized below (Table 4). Of the 70 case reports and series included in this review, the majority were found to have a low risk of bias. Specifically, 57 cases were rated as having a low risk of bias, 12 cases were rated as having a moderate risk of bias, and one study was rated as having a high risk of bias. Overall, the risk of bias assessment highlights the variability in the quality of reporting among the included studies. Although many of the studies were of high quality, the presence of studies with moderate and high risk of bias underscores the need for more standardized and comprehensive reporting in future research on PRES and corticosteroids.
| Ref. | Q1 | Q2 | Q3 | Q4 | Q5 | Q6 | Q7 | Q8 | Overall | Risk |
| No author[23], 2016 | Y | N | Y | Y | Y | Y | Y | Y | 7 | Low |
| Alexander et al[24], 2013 | N | Y | Y | Y | Y | Y | Y | Y | 7 | Low |
| Aridon et al[58], 2011 | Y | Y | Y | Y | Y | Y | Y | Y | 8 | Low |
| Camara-Lemarroy et al[25], 2015 | Y | Y | Y | Y | Y | N | U | Y | 6 | Mod |
| Chennareddy et al[26], 2013 | Y | Y | Y | Y | U | U | Y | Y | 6 | Mod |
| Christofidis et al[59] ,2019 | Y | U | Y | Y | Y | Y | Y | Y | 7 | Low |
| Ding et al[28], 2015 | Y | Y | Y | Y | Y | Y | Y | Y | 8 | Low |
| Fujita et al[29], 2008 | Y | Y | Y | Y | Y | Y | Y | Y | 8 | Low |
| Fukuyama et al[30], 2011 | Y | Y | Y | Y | Y | Y | Y | Y | 8 | Low |
| Gera et al[31], 2014 | Y | N | N | Y | N | N | N | Y | 3 | High |
| El Hage et al[32], 2021 | Y | Y | Y | Y | Y | Y | Y | Y | 8 | Low |
| Hao et al[60], 2021 | N | Y | Y | Y | Y | Y | Y | Y | 7 | Low |
| Hosseini[61], 2022 | Y | N | Y | Y | Y | N | Y | Y | 6 | Mod |
| İncecik et al[33], 2013 | Y | N | Y | Y | Y | Y | Y | Y | 7 | Low |
| Irvin et al[34], 2007 | Y | N | Y | Y | Y | N | Y | Y | 6 | Mod |
| Islam et al[62], 2021 | Y | Y | Y | Y | Y | Y | Y | Y | 8 | Low |
| Ismail et al[35], 2021 | Y | N | N | Y | Y | N | N | Y | 4 | Mod |
| Jadib et al[63], 2021 | Y | N | Y | Y | Y | Y | Y | Y | 7 | Low |
| Kamezaki et al[36], 2012 | Y | Y | Y | Y | N | Y | Y | Y | 6 | Mod |
| Kamo et al[37], 2019 | Y | U | Y | Y | Y | Y | Y | Y | 7 | Low |
| Khanjar et al[39], 2018 | Y | N | U | N | Y | N | Y | Y | 4 | Mod |
| Khan et al[38], 2018 | Y | Y | N | Y | Y | N | Y | Y | 6 | Mod |
| Kitamura et al[40], 2022 | Y | Y | Y | N | Y | Y | Y | Y | 7 | Low |
| Kumar and Rajam[41], 2011 | Y | N | N | Y | Y | Y | Y | Y | 6 | Mod |
| Kurahashi et al[42], 2006 | Y | Y | Y | Y | Y | Y | Y | Y | 8 | Low |
| Mai et al[64], 2018 | Y | U | Y | Y | Y | Y | Y | Y | 7 | Low |
| Dar et al[43], 2015 | Y | Y | Y | Y | Y | Y | Y | Y | 8 | Low |
| Mimura et al[44], 2023 | Y | N | Y | N | Y | N | Y | Y | 5 | Mod |
| Min et al[65], 2006 | Y | U | Y | Y | Y | Y | Y | Y | 7 | Low |
| Morrow et al[45], 2015 | Y | U | Y | U | Y | Y | Y | Y | 6 | Mod |
| Nguyen et al[46], 2009 | Y | N | Y | Y | Y | Y | Y | Y | 7 | Low |
| Ortega-Carnicer et al[66], 2005 | Y | Y | Y | Y | Y | N | Y | Y | 7 | Low |
| Ozkok et al[47], 2012 | Y | Y | Y | Y | Y | Y | Y | Y | 8 | Low |
| Sato et al[67], 2011 | Y | Y | Y | Y | Y | Y | Y | Y | 8 | Low |
| Shibata et al[48], 2022 | Y | U | Y | Y | Y | Y | Y | Y | 7 | Low |
| Sinha and Hurley[49], 2008 | Y | Y | Y | Y | Y | Y | Y | Y | 8 | Low |
| Stârcea et al[50], 2018 | Y | N | Y | Y | Y | Y | Y | Y | 7 | Low |
| Swarnalatha et al[51], 2012 | Y | Y | Y | Y | Y | N | Y | Y | 7 | Low |
| Symeonidis et al[68], 2021 | Y | Y | Y | Y | Y | Y | Y | Y | 8 | Low |
| Tetsuka and Nonaka[69], 2017 | Y | Y | Y | Y | Y | Y | Y | Y | 8 | Low |
| Tsukamoto et al[52], 2012 | Y | Y | Y | Y | Y | Y | Y | Y | 8 | Low |
| Ulutaş et al[53], 2020 | Y | Y | Y | Y | Y | Y | Y | Y | 8 | Low |
| Vernaza et al[54], 2021 | Y | Y | Y | Y | Y | Y | Y | Y | 8 | Low |
| Yang et al[55], 2022 | N | N | N | Y | Y | Y | Y | Y | 6 | Mod |
| Xia and Lv[70], 2022 | Y | Y | Y | Y | Y | Y | Y | Y | 8 | Low |
| Xu et al[71], 2021 | Y | Y | Y | Y | Y | Y | Y | Y | 8 | Low |
| Zekić et al[56], 2017 | Y | Y | Y | Y | Y | Y | Y | Y | 8 | Low |
| Zhang et al[57], 2018 | Y | Y | Y | Y | Y | N | Y | Y | 7 | Low |
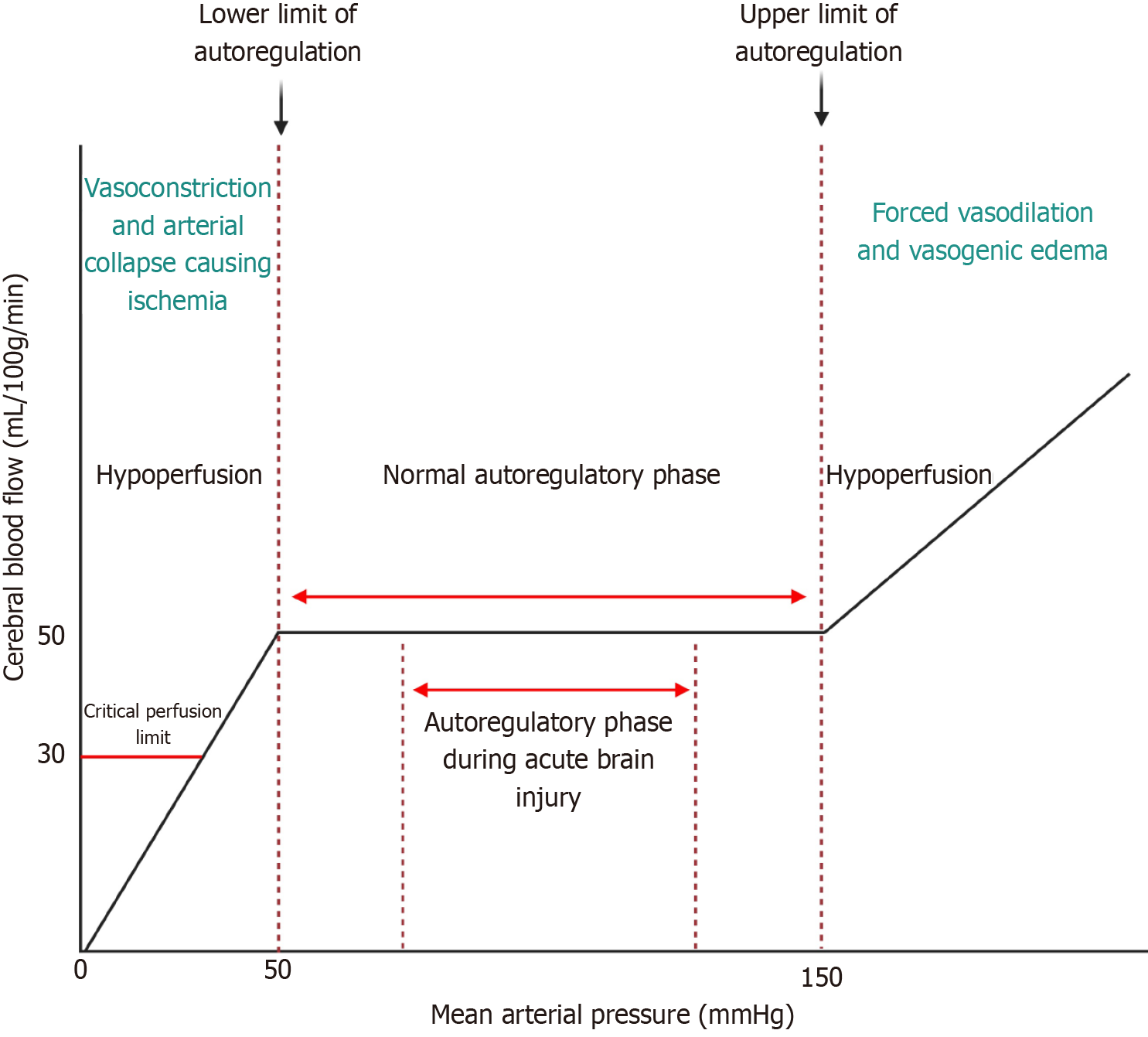
The underlying pathophysiology of PRES is believed to involve a combination of endothelial dysfunction and altered cerebral autoregulation leading to vasogenic edema[6]. Hypertension is a well-recognized precipitating factor, which can overwhelm the autoregulatory capacity of cerebral vessels, particularly in the posterior circulation due to its relatively sparse sympathetic innervation. This results in hyperperfusion, capillary leakage, and the formation of vasogenic edema primarily in the parieto-occipital regions of the brain. Endothelial dysfunction plays a critical role in the development of PRES. Various conditions such as eclampsia, autoimmune diseases, renal failure, and immunosuppressive therapies can damage endothelial cells, leading to increased permeability and subsequent edema formation[7]. The role of cytokines and other inflammatory mediators in promoting endothelial dysfunction and disrupting the blood-brain barrier (BBB) is also significant, contributing further to the pathology of PRES[8]. Frequent neurological and vital checks every 4 hours for the first 24-48 hours after the diagnosis of PRES may be beneficial to prevent both hyperperfusive and hypoperfusive events. Hypertensive crises are best managed with the initiation of an intravenous infusion such as nicardipine or clevidipine which also have anti-spasmodic effects.
In our review, we identified 57 cases of PRES associated with corticosteroid use. These cases highlight the potential for long-term or high-dose corticosteroids to induce PRES, particularly in patients with underlying conditions such as SLE, renal disease, and post-transplant immunosuppression. The pathophysiology of corticosteroid-induced PRES likely involves endothelial dysfunction, leading to vasogenic edema. Hypertension, a common side effect of corticosteroids, is a key factor in the development of PRES, as evidenced by elevated blood pressure readings in many of the cases reviewed[9,10]. The clinical manifestations in these cases were diverse, with seizures, headaches, hypertension, and visual disturbances being the most common. MRI findings consistently showed bilateral subcortical hyperintensities, predominantly in the occipital, parietal, and frontal lobes. Treatment strategies varied, but reduction or cessation of corticosteroid therapy often resulted in significant clinical improvement and resolution of MRI abnormalities.
On the contrary, we identified 14 cases where corticosteroids were used as part of the treatment regimen for PRES. These cases predominantly involved patients with underlying autoimmune or inflammatory conditions, where corticosteroids were used for their potent anti-inflammatory effects. The therapeutic use of corticosteroids in PRES was associated with positive results, including stabilization of symptoms and resolution of vasogenic edema, as evidenced by follow-up MRI. The rationale for using corticosteroids in these cases is based on their ability to reduce inflammation and stabilize the BBB, thus mitigating the vasogenic edema characteristic of PRES. This approach is particularly relevant in cases where PRES is precipitated by an inflammatory or autoimmune process[11]. However, the use of corticosteroids in the treatment of PRES requires careful monitoring to balance their benefits against the risk of exacerbating hypertension.
Corticosteroids are known for their potent anti-inflammatory and immunosuppressive effects, which are beneficial in the treatment of various inflammatory and autoimmune conditions[12]. However, its use can influence the pathophysiology of PRES in several ways. Corticosteroids can lead to hypertension through mechanisms such as sodium and water retention, increased vascular sensitivity to catecholamines, and altered renal function. This hypertensive effect can exacerbate the cerebral hyperperfusion seen in PRES, overwhelming the autoregulatory capacity of the brain's vasculature and leading to vasogenic edema (Figure 2). While corticosteroids have anti-inflammatory effects that can stabilize the endothelium, they can also induce endothelial dysfunction when used in high doses or over prolonged periods. This paradoxical effect can contribute to the development of PRES, particularly in patients with underlying vulnerabilities such as renal disease or pre-existing hypertension[13]. In cases where PRES is driven by an autoimmune or inflammatory process, corticosteroids can help mitigate the inflammatory response and stabilize the BBB, thus reducing edema and improving clinical outcomes[14]. This dual role underscores the complexity of corticosteroid use in PRES, where they can be both a precipitant and a treatment.
Corticosteroids have been used to treat brain edema for over six decades. However, its clinical utility is often in the management of vasogenic edema from intracranial neoplasms and bacterial meningitis[15,16]. A high concentration of steroids inhibits active ion transport mediated by the Na+-K+-ATPase channel[17]. And the rate of sodium transport from the blood to the parenchyma determines the rate of cerebral edema formation. Therefore, corticosteroids may decrease the amount of cerebral edema formation via Na+-K+-ATPase channel inhibition[18]. On the contrary, a meta-analysis of eight randomized controlled trials showed no benefit of corticosteroids in the management of intracerebral hemorrhage and subarachnoid hemorrhage[16]. Corticosteroids have also been shown to decrease peri-contusional vasogenic edema in patients with traumatic brain injury. However, long-term functional outcomes were not measured[19].
McMahon et al[20] explored the use of post-sonication DEX to mitigate risks associated with the delivery of therapeutic agents across the BBB using focused ultrasound and microbubbles (FUS + MB). The study involved adult male rats and assessed BBB permeability, inflammatory protein expression, blood vessel growth, and astrocyte activation. The findings revealed that DEX administration expedited the restoration of BBB integrity and reduced inflammation-related protein production shortly after sonication. Furthermore, DEX decreased astrocyte activation and vascular growth at a later stage compared to controls. These results suggest that DEX can enhance the safety profile of FUS + MB-mediated drug delivery by modulating BBB permeability and reducing inflammation-induced tissue damage, especially in treatments aimed at preserving neural function and requiring multiple sonications.
Bastin et al[21] studied the effects of DEX on cerebral perfusion and water diffusion in patients with glioblastoma using dynamic susceptibility contrast-MRI and diffusion tensor-MRI. The study measured cerebral blood flow (CBF), cerebral blood volume (CBV), mean transit time (MTT) and mean diffusivity (<D>) in enhancing tumors, peritumoral edematous brain, and normal contralateral white matter. The results indicated that there were no significant changes in tumor CBF, CBV, or MTT after DEX treatment. However, there was a notable increase in CBF (11.6%; P = 0.05) in the edematous brain. Additionally, <D> was significantly reduced in both enhancing tumor (-5.8%; P = 0.001) and edematous brain
Cabañas et al[22] investigated the effects of DEX on cerebral and ocular blood flow velocities in ten ventilated preterm infants with lung disease. Using color Doppler flow imaging, they measured blood flow velocities in the internal carotid, anterior cerebral, and ophthalmic arteries before and after multiple doses of DEX. The results showed that DEX progressively increased blood flow velocities and decreased resistance indices, with the most significant changes in end diastolic flow velocities, especially after the fifth dose. Arterial blood pressure changes mirrored increases in blood flow velocities. This study suggests that DEX significantly influences cerebral and ocular hemodynamics in preterm infants.
This systematic review has several limitations that must be acknowledged. Most of the data comes from case reports and series, which can introduce bias and limit the generalizability of the findings. Additionally, some cases may have been missed. These types of studies often lack control groups, making it difficult to establish causality. There was significant variability in the reporting of clinical and imaging findings, corticosteroid doses, and treatment regimens. This heterogeneity complicates the synthesis of data and the drawing of definitive conclusions. Many cases lacked long-term follow-up data, which limits our understanding of the enduring effects of corticosteroids on PRES outcomes. Many cases were confounded with concomitant usage of immunosuppressive therapies which are also implicated in PRES[8]. Future research should aim to address these limitations through more robust study designs and comprehensive data collection. Well-designed prospective cohort studies and randomized controlled trials are needed to better elucidate the relationship between corticosteroids and PRES. These studies should aim to establish clearer causal links and assess the efficacy and safety of corticosteroid use in the treatment of PRES. Further research is essential into the pathophysiological mechanisms by which corticosteroids influence PRES development and resolution. Understanding these mechanisms at the molecular level could inform the development of targeted therapies. Long-term follow-up studies are needed to assess the sustained effects of corticosteroids on PRES and to determine the best practices for preventing recurrence and managing chronic sequela.
PRES is a complex neurological disorder with a multifaceted pathophysiology that involves endothelial dysfunction, altered cerebral autoregulation, and vasogenic edema. This systematic review has explored the dual role of corticosteroids in the context of PRES, revealing their potential to induce and treat this condition. Our findings indicate that high-dose or prolonged corticosteroid therapy can contribute to the development of PRES, particularly in patients with underlying risk factors such as hypertension, kidney disease, and autoimmune conditions. On the contrary, corticosteroids also play a crucial therapeutic role in the treatment of PRES, especially in cases driven by inflammatory or autoimmune mechanisms. Their anti-inflammatory and endothelial stabilizing properties can mitigate the vasogenic edema characteristic of PRES, leading to significant clinical improvement and resolution of imaging abnormalities. The clinical implications of these findings underscore the need for a balanced approach to corticosteroid use in patients at risk of or presenting with PRES. Vigilant monitoring of blood pressure and neurological status is essential to optimize patient outcomes. A deeper understanding of the pathophysiological mechanisms and clinical factors involved will be crucial in developing evidence-based guidelines on the use or avoidance of corticosteroids in PRES.
| 1. | Triplett JD, Kutlubaev MA, Kermode AG, Hardy T. Posterior reversible encephalopathy syndrome (PRES): diagnosis and management. Pract Neurol. 2022;22:183-189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 67] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 2. | Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hróbjartsson A, Lalu MM, Li T, Loder EW, Mayo-Wilson E, McDonald S, McGuinness LA, Stewart LA, Thomas J, Tricco AC, Welch VA, Whiting P, Moher D. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44932] [Cited by in RCA: 51767] [Article Influence: 10353.4] [Reference Citation Analysis (2)] |
| 3. | Shea BJ, Reeves BC, Wells G, Thuku M, Hamel C, Moran J, Moher D, Tugwell P, Welch V, Kristjansson E, Henry DA. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ. 2017;358:j4008. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3100] [Cited by in RCA: 6517] [Article Influence: 724.1] [Reference Citation Analysis (0)] |
| 4. | Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. Rayyan-a web and mobile app for systematic reviews. Syst Rev. 2016;5:210. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5711] [Cited by in RCA: 14469] [Article Influence: 1446.9] [Reference Citation Analysis (1)] |
| 5. | Zeng X, Zhang Y, Kwong JS, Zhang C, Li S, Sun F, Niu Y, Du L. The methodological quality assessment tools for preclinical and clinical studies, systematic review and meta-analysis, and clinical practice guideline: a systematic review. J Evid Based Med. 2015;8:2-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1492] [Cited by in RCA: 1529] [Article Influence: 139.0] [Reference Citation Analysis (0)] |
| 6. | Parasher A, Jhamb R. Posterior reversible encephalopathy syndrome (PRES): presentation, diagnosis and treatment. Postgrad Med J. 2020;96:623-628. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 46] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 7. | Shaikh N, Nawaz S, Ummunisa F, Shahzad A, Hussain J, Ahmad K, Almohannadi HS, Sharara HA. Eclampsia and posterior reversible encephalopathy syndrome (PRES): A retrospective review of risk factors and outcomes. Qatar Med J. 2021;2021:4. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 8. | Srichawla BS, Presti K, Kipkorir V, Berrios Morales I. Chemotherapy-associated hemorrhagic posterior reversible encephalopathy syndrome (PRES) with considerations for circle of Willis variants on cerebral blood flow and autoregulation: A case report. Medicine (Baltimore). 2024;103:e37250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 9. | Shen JZ, Young MJ. Corticosteroids, heart failure, and hypertension: a role for immune cells? Endocrinology. 2012;153:5692-5700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 10. | Srichawla BS, Quast J. Magnesium deficiency: An overlooked key to the puzzle of posterior reversible encephalopathy syndrome (PRES) and reversible cerebral vasoconstriction syndrome (RCVS)? J Neurol Sci. 2023;453:120796. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 11. | Witt KA, Sandoval KE. Steroids and the blood-brain barrier: therapeutic implications. Adv Pharmacol. 2014;71:361-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 52] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 12. | Slominski AT, Mahata B, Raman C, Bereshchenko O. Editorial: Steroids and Secosteroids in the Modulation of Inflammation and Immunity. Front Immunol. 2021;12:825577. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 13. | Buchman AL. Side effects of corticosteroid therapy. J Clin Gastroenterol. 2001;33:289-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 370] [Cited by in RCA: 479] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 14. | Salvador E, Shityakov S, Förster C. Glucocorticoids and endothelial cell barrier function. Cell Tissue Res. 2014;355:597-605. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 83] [Cited by in RCA: 122] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 15. | Majd NK, Dasgupta PR, de Groot JF. Immunotherapy for Neuro-oncology. Adv Exp Med Biol. 2021;1342:233-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 16. | Cook AM, Morgan Jones G, Hawryluk GWJ, Mailloux P, McLaughlin D, Papangelou A, Samuel S, Tokumaru S, Venkatasubramanian C, Zacko C, Zimmermann LL, Hirsch K, Shutter L. Guidelines for the Acute Treatment of Cerebral Edema in Neurocritical Care Patients. Neurocrit Care. 2020;32:647-666. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 152] [Cited by in RCA: 243] [Article Influence: 40.5] [Reference Citation Analysis (0)] |
| 17. | Chaplin ER, Free RG, Goldstein GW. Inhibition by steroids of the uptake of potassium by capillaries isolated from rat brain. Biochem Pharmacol. 1981;30:241-245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 22] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 18. | Betz AL, Ennis SR, Schielke GP. Blood-brain barrier sodium transport limits development of brain edema during partial ischemia in gerbils. Stroke. 1989;20:1253-1259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 39] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 19. | Moll A, Lara M, Pomar J, Orozco M, Frontera G, Llompart-Pou JA, Moratinos L, González V, Ibáñez J, Pérez-Bárcena J. Effects of dexamethasone in traumatic brain injury patients with pericontusional vasogenic edema: A prospective-observational DTI-MRI study. Medicine (Baltimore). 2020;99:e22879. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 20. | McMahon D, Oakden W, Hynynen K. Investigating the effects of dexamethasone on blood-brain barrier permeability and inflammatory response following focused ultrasound and microbubble exposure. Theranostics. 2020;10:1604-1618. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 49] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 21. | Bastin ME, Carpenter TK, Armitage PA, Sinha S, Wardlaw JM, Whittle IR. Effects of dexamethasone on cerebral perfusion and water diffusion in patients with high-grade glioma. AJNR Am J Neuroradiol. 2006;27:402-408. [PubMed] |
| 22. | Cabañas F, Pellicer A, García-Alix A, Quero J, Stiris TA. Effect of dexamethasone therapy on cerebral and ocular blood flow velocity in premature infants studied by colour Doppler flow imaging. Eur J Pediatr. 1997;156:41-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 23. | Abstracts of the 18th Asia Pacific League of Associations for Rheumatology Congress (APLAR), 26-29 September 2016, Shanghai, China. Int J Rheum Dis. 2016;19 Suppl 2:3-293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 24. | Alexander S, David VG, Varughese S, Tamilarasi V, Jacob CK. Posterior reversible encephalopathy syndrome in a renal allograft recipient: A complication of immunosuppression? Indian J Nephrol. 2013;23:137-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 25. | Camara-Lemarroy CR, Cruz-Moreno MA, Gamboa-Sarquis RN, Gonzalez-Padilla KA, Tamez-Perez HE, Galarza-Delgado DA. Goodpasture syndrome and posterior reversible encephalopathy syndrome. J Neurol Sci. 2015;354:135-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 26. | Chennareddy S, Adapa R, Kishore BK, Rajasekhar L. Posterior reversible encephalopathy syndrome in systemic lupus erythematosus following methylprednisolone: report of two cases. Int J Rheum Dis. 2013;16:786-788. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 27. | Wee CS, Yeoh WC, Low IJ. Posterior Reversible Encephalopathy Syndrome – A Rare Complication after Methylprednisolone. Bangla J Med. 2020;31:41-42. [DOI] [Full Text] |
| 28. | Ding D, Li K, Li G, Long X. Posterior reversible encephalopathy syndrome following paroxysmal nocturnal hemoglobinuria: a case report and literature review. Int J Clin Exp Med. 2015;8:11617-11620. [PubMed] |
| 29. | Fujita M, Komatsu K, Hatachi S, Yagita M. Reversible posterior leukoencephalopathy syndrome in a patient with Takayasu arteritis. Mod Rheumatol. 2008;18:623-629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 30. | Fukuyama T, Tanaka M, Nakazawa Y, Motoki N, Inaba Y, Higuchi T, Koike K. Prophylactic treatment for hypertension and seizure in a case of allogeneic hematopoietic stem cell transplantation after posterior reversible encephalopathy syndrome. Pediatr Transplant. 2011;15:E169-E173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 31. | Gera DN, Patil SB, Iyer A, Kute VB, Gandhi S, Kumar D, Trivedi HL. Posterior reversible encephalopathy syndrome in children with kidney disease. Indian J Nephrol. 2014;24:28-34. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 32. | El Hage S, Akiki D, Khalife L, Assaf E, Jaoude MA. Rapid clinical recovery of posterior reversible encephalopathy syndrome in two cases of IgA nephropathy disease and nephrotic syndrome type 9 post-renal transplant. Transpl Immunol. 2021;68:101450. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 33. | İncecik F, Hergüner MÖ, Yıldızdaş D, Yılmaz M, Mert G, Horoz ÖO, Altunbaşak Ş. Posterior reversible encephalopathy syndrome due to pulse methylprednisolone therapy in a child. Turk J Pediatr. 2013;55:455-457. [PubMed] |
| 34. | Irvin W, MacDonald G, Smith JK, Kim WY. Dexamethasone-induced posterior reversible encephalopathy syndrome. J Clin Oncol. 2007;25:2484-2486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 40] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 35. | Ismail FS, van de Nes J, Kleffner I. A broad spectrum of posterior reversible encephalopathy syndrome - a case series with clinical and paraclinical characterisation, and histopathological findings. BMC Neurol. 2021;21:386. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 36. | Kamezaki M, Kakimoto T, Takeuchi T, Akuta K, Kasahara H, Yamamoto K, Ujiie H, Sugahara H, Nishinaka K, Udaka F, Sakoda H. Reversible posterior leukoencephalopathy syndrome of bilateral thalamus in acute lymphoblastic leukemia. Leuk Lymphoma. 2012;53:2083-2084. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 37. | Kamo H, Ueno Y, Sugiyama M, Miyamoto N, Yamashiro K, Tanaka R, Yokoyama K, Hattori N. Pontine hemorrhage accompanied by neuromyelitis optica spectrum disorder. J Neuroimmunol. 2019;330:19-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 38. | Khan SJ, Arshad AA, Fayyaz MB, Ud Din Mirza I. Posterior Reversible Encephalopathy Syndrome in Pediatric Cancer: Clinical and Radiologic Findings. J Glob Oncol. 2018;4:1-8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 39. | Khanjar I, Abdulmomen I, Yahia YM, Al-Allaf AW. Posterior Reversible Encephalopathy Syndrome (PRES) in Rheumatic Autoimmune Disease. Eur J Case Rep Intern Med. 2018;5:000866. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 40. | Kitamura F, Yamaguchi M, Nishimura M, Katsuno T, Ito M, Sugiyama H, Iwagaitsu S, Nobata H, Kinashi H, Ishimoto T, Banno S, Ito Y. Anti-neutrophil cytoplasmic antibody-associated vasculitis complicated by thrombotic microangiopathy with posterior reversible encephalopathy syndrome successfully treated with eculizumab: A case report. Mod Rheumatol Case Rep. 2022;6:254-259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 41. | Kumar S, Rajam L. Posterior reversible encephalopathy syndrome (PRES/RPLS) during pulse steroid therapy in macrophage activation syndrome. Indian J Pediatr. 2011;78:1002-1004. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 42. | Kurahashi H, Okumura A, Koide T, Ando Y, Hirata H, Magota M, Watabane K. Posterior reversible encephalopathy syndrome in a child with bronchial asthma. Brain Dev. 2006;28:544-546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 43. | Dar AM, Kumar M, Malik AH, Saikia V, Goel K, Mukherji JD. Posterior reversible encephalopathy syndrome (PRES) associated with i.v Methyl prednisolone and Methylcobalamine. Annals of Indian Academy of Neurology. 2015;18:S44. |
| 44. | Mimura A, Fujii K, Sugi W, Kato A, Yoshifuji A, Komatsu M, Ryuzaki M. PS-R06-4: Posterior reversible encephalopathy syndrome triggered by fluid retention and hypertension after steroid initiation in patients with ANCA-associated vasculitis. Hypertens. 2023;41 Suppl 1:e508. [RCA] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 45. | Morrow SA, Rana R, Lee D, Paul T, Mahon JL. Posterior Reversible Encephalopathy Syndrome due to High Dose Corticosteroids for an MS Relapse. Case Rep Neurol Med. 2015;2015:325657. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 46. | Nguyen MT, Virk IY, Chew L, Villano JL. Extended use dexamethasone-associated posterior reversible encephalopathy syndrome with cisplatin-based chemotherapy. J Clin Neurosci. 2009;16:1688-1690. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 47. | Ozkok A, Elcioglu OC, Bakan A, Atilgan KG, Alisir S, Odabas AR. Reversible posterior leukoencephalopathy in the course of Goodpasture syndrome. Ren Fail. 2012;34:254-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 48. | Shibata T, Hashimoto N, Mase M. Intracranial hemorrhage in posterior reversible encephalopathy syndrome due to corticosteroid pulse therapy. Brain Disord. 2022;7:100040. [RCA] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 49. | Sinha R, Hurley RM. Posterior reversible encephalopathy syndrome in SLE nephritis. Postgrad Med J. 2008;84:56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 50. | Stârcea M, Gavrilovici C, Munteanu M, Miron I. Focal segmental glomerulosclerosis in children complicated by posterior reversible encephalopathy syndrome. J Int Med Res. 2018;46:1172-1177. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 51. | Swarnalatha G, Ram R, Pai BH, Dakshinamurty KV. Posterior reversible encephalopathy syndrome in minimal change disease. Indian J Nephrol. 2012;22:153-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 52. | Tsukamoto S, Takeuchi M, Kawajiri C, Tanaka S, Nagao Y, Sugita Y, Yamazaki A, Kawaguchi T, Muto T, Sakai S, Takeda Y, Ohwada C, Sakaida E, Shimizu N, Yokote K, Iseki T, Nakaseko C. Posterior reversible encephalopathy syndrome in an adult patient with acute lymphoblastic leukemia after remission induction chemotherapy. Int J Hematol. 2012;95:204-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 53. | Ulutaş F, Çobankara V, Karasu U, Baser N, Akbudak IH. A Rare Case with Systemic Lupus Erythematosus Manifested by two Different Neurologic Entities; Guillain Barre Syndrome and Posterior Reversible Encephalopathy Syndrome. Mediterr J Rheumatol. 2020;31:358-361. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 54. | Vernaza A, Pinilla-Monsalve G, Cañas F, Carrillo D, David López J, Flórez N, Gomez-Mesa JE. Malaria and encephalopathy in a heart transplant recipient: A case report in the context of multiorgan donation. Transpl Infect Dis. 2021;23:e13565. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 55. | Yang B, Guo L, Yang X, Yu N. The pathogenesis and treatment of posterior reversible encephalopathy syndrome after neuromyelitis optica spectrum disorder: a case report and literature review. BMC Neurol. 2022;22:493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 56. | Zekić T, Benić MS, Antulov R, Antončić I, Novak S. The multifactorial origin of posterior reversible encephalopathy syndrome in cyclophosphamide-treated lupus patients. Rheumatol Int. 2017;37:2105-2114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 13] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 57. | Zhang XH, Deng XR, Li F, Zhu Y, Zhang ZL. [Posterior reversible encephalopathy syndrome in systemic lupus erythematosus: a case report]. Beijing Daxue Xuebao Yixueban. 2018;50:1102-1107. [PubMed] [DOI] [Full Text] |
| 58. | Aridon P, Ragonese P, Mazzola MA, Quintini G, Lo Re M, Talamanca S, Terruso V, D'Amelio M, Savettieri G. Reversible posterior leukoencephalopathy syndrome in a patient with thrombotic thrombocytopenic purpura. Neurol Sci. 2011;32:469-472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 59. | Christofidis C, Anastasiou A, Krasnikova E, Mandros C, Potolidis E. Posterior reversible encephalopathy syndrome as a complication of bacterial meningitis. Hippokratia. 2019;23:131-134. [PubMed] |
| 60. | Hao DL, Yang YL, Zhou LM, Liu QH, Liu R, Xu K, Zhang GL, Zhang LY. Systemic lupus erythematosus complicated with reversible posterior encephalopathy syndrome: a case report. J Int Med Res. 2021;49:3000605211029766. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 61. | Hosseini S. Posterior reversible encephalopathic syndrome in systemic lupus erythematous: A case report. J Res Clin Med. 2022;10:31. [RCA] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 62. | Islam R, Das S, Chattopadhyay S, Basu S. Neuropsychiatric lupus with posterior reversible encephalopathy syndrome: a rare presentation. BMJ Case Rep. 2021;14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 63. | Jadib A, Salam S, Harmoumi Y, Chahidi El Ouazzani L, Soussi O, Laoudiyi D, Chbani K, Ouzidane L. Posterior reversible encephalopathy syndrome revealing Takayasu's arteritis in a child. Radiol Case Rep. 2021;16:3969-3972. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 64. | Mai NTH, Phu NH, Nghia HDT, Phuong TM, Duc DT, Chau NVV, Wills B, Lim CCT, Thwaites G, Simmons CP, Yacoub S. Dengue-Associated Posterior Reversible Encephalopathy Syndrome, Vietnam. Emerg Infect Dis. 2018;24:402-404. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 65. | Min L, Zwerling J, Ocava LC, Chen IH, Putterman C. Reversible posterior leukoencephalopathy in connective tissue diseases. Semin Arthritis Rheum. 2006;35:388-395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 52] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 66. | Ortega-Carnicer J, Ambrós A, Diarte JI. Reversible posterior leukoencephalopathy syndrome in a young trauma patient. Resuscitation. 2005;64:119-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 67. | Sato Y, Hirose M, Inoue Y, Komukai D, Takayasu M, Kawashima E, Koiwa F, Yoshimura A. Reversible posterior leukoencephalopathy syndrome after blood transfusion in a patient with end-stage renal disease. Clin Exp Nephrol. 2011;15:942-947. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 25] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 68. | Symeonidis DG, Liatsos AD, Mazlimoglou EK, Geraki EC, Kosmas C. Posterior Reversible Encephalopathy Syndrome Associated with Oxaliplatin Use for Pancreatic Adenocarcinoma. Case Rep Oncol. 2021;14:838-844. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 69. | Tetsuka S, Nonaka H. Importance of correctly interpreting magnetic resonance imaging to diagnose posterior reversible encephalopathy syndrome associated with HELLP syndrome: a case report. BMC Med Imaging. 2017;17:35. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 70. | Xia C, Lv Y. Cerebral amyloid angiopathy-related inflammation with posterior reversible encephalopathy syndrome-like presentation: a case report. BMC Neurol. 2022;22:449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 71. | Xu J, Ding Y, Qu Z, Yu F. Posterior Reversible Encephalopathy Syndrome in a Patient With Microscopic Polyangiitis: A Case Report and Literature Review. Front Med (Lausanne). 2021;8:792744. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/