Published online Apr 16, 2025. doi: 10.12998/wjcc.v13.i11.99256
Revised: November 25, 2024
Accepted: December 10, 2024
Published online: April 16, 2025
Processing time: 161 Days and 0.3 Hours
Dystrophic epidermolysis bullosa is characterized by fragile ulcerations of the skin caused by mutations in specific genes. However, genetic typing of this con
An 11-year-old female suffered from recurrent fever, visible ulcerations of the entire skin, and severe malnutrition. Genetic testing revealed a frameshift mu
Dystrophic epidermolysis bullosa caused by a new framework shift mutation in COL7A1 should be taken seriously.
Core Tip: A new frameshift mutation in the COL7A1 gene caused dystrophic epidermolysis bullosa (DEB). Genetic testing of the patient showed a frameshift mutation in the coding region 4047 of the 35th intron of the COL7A1 gene, which was improved after symptomatic drug treatment. Indicating the role of genetic testing in DEB diagnosis and providing clinical data for DEB gene therapy.
- Citation: Yang Y, Guan ZW, Li QF. Dystrophic epidermolysis bullosa caused by novel frameshift mutation in the COL7A1 gene: A case report. World J Clin Cases 2025; 13(11): 99256
- URL: https://www.wjgnet.com/2307-8960/full/v13/i11/99256.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v13.i11.99256
Based on the classification of inherited epidermolysis bullosa, dystrophic epidermolysis bullosa (DEB) is an inherited genetic variant with clinical manifestations of skin fragility and ulceration[1], especially in areas such as the hands, feet, and knees, where the skin is fragile and prone to blisters and scars after slight friction or trauma[2]. DEB is mainly caused by abnormal changes in the COL7A1 gene, which encodes a complex COL7 protein that maintains skin structure and function. Herein, we report a case of DEB associated with a novel frameshift mutation in the COL7A1 gene. This report aimed to draw doctors’ attention to genetic testing in DEB diagnosis and promote the progress of DEB gene drug therapy.
The patient presented with unexplained fever symptoms before admission, with a maximum body temperature of 39 °C and no convulsions. After oral treatment with ibuprofen, the body temperature decreased to normal levels but sub
An 11 years old female presented for a fever lasting 8 days.
The patient had a history of congenital epidermolysis bullosa for > 10 years.
The patient and her parents denied a family history of hereditary diseases.
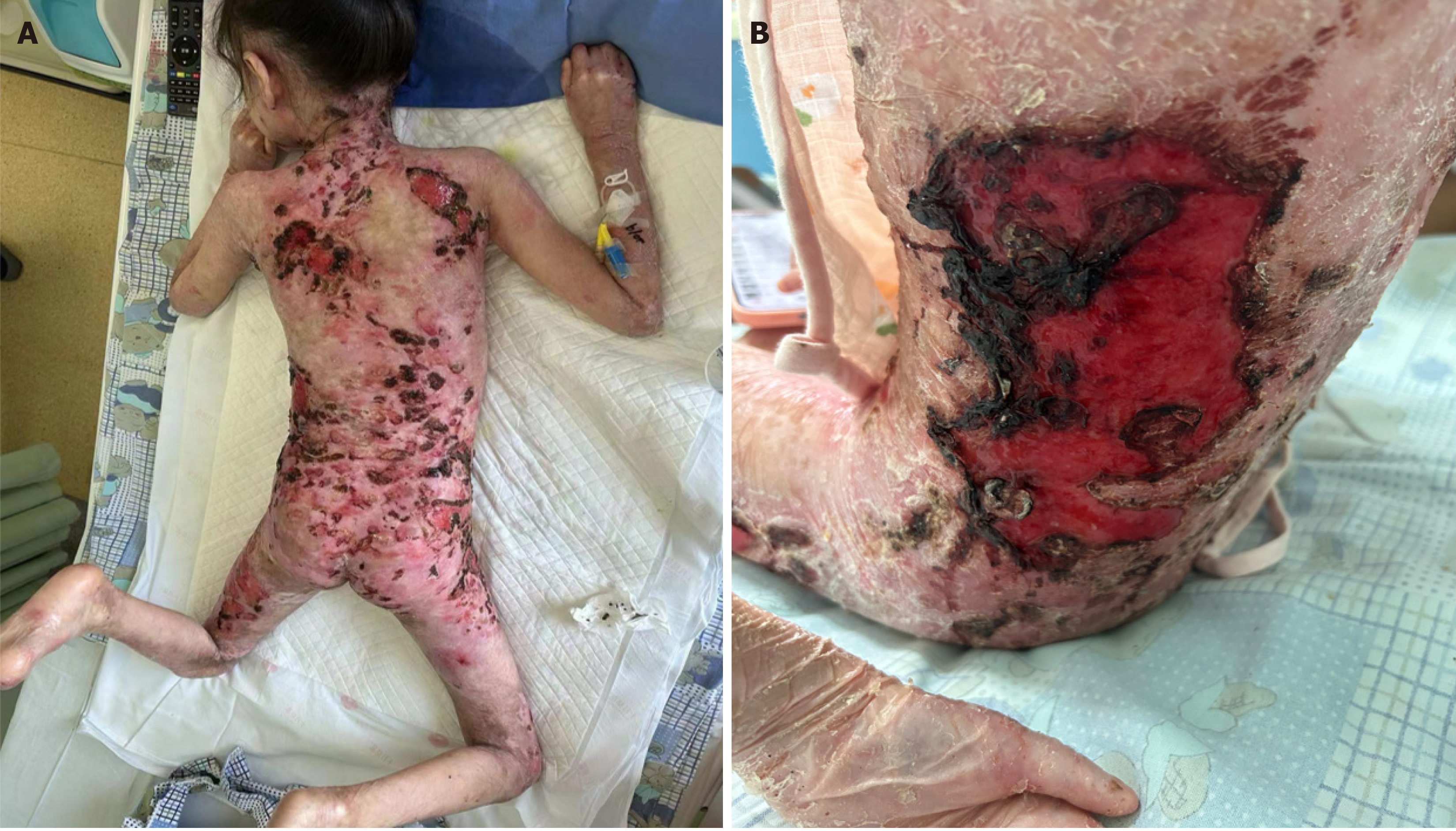
After admission, physical examination revealed scattered ulcerations and erosions of the child’s entire skin; peeling of the skin on the face, shoulders, back, waist, and thighs, with local exudation and visible scabbing; the gap between the palms and fingers of both hands disappeared; and both toes were missing (Figure 1). The child was 124 cm tall and weighed 18.5 kg, showing significant physical underdevelopment, poor nutritional status, and a relatively thin and weak physique.
No significant abnormality was found in biochemical routine, calcitoninogen, blood sedimentation, liver and kidney function, coagulation function. Immunoglobulin E: 281.7 IU/mL; chest X-ray showed: Heavy texture in both lungs. Electrocardiogram showed non-specific changes in the T-wave, along with incomplete right bundle branch block. Echocardiography showed no significant abnormalities.
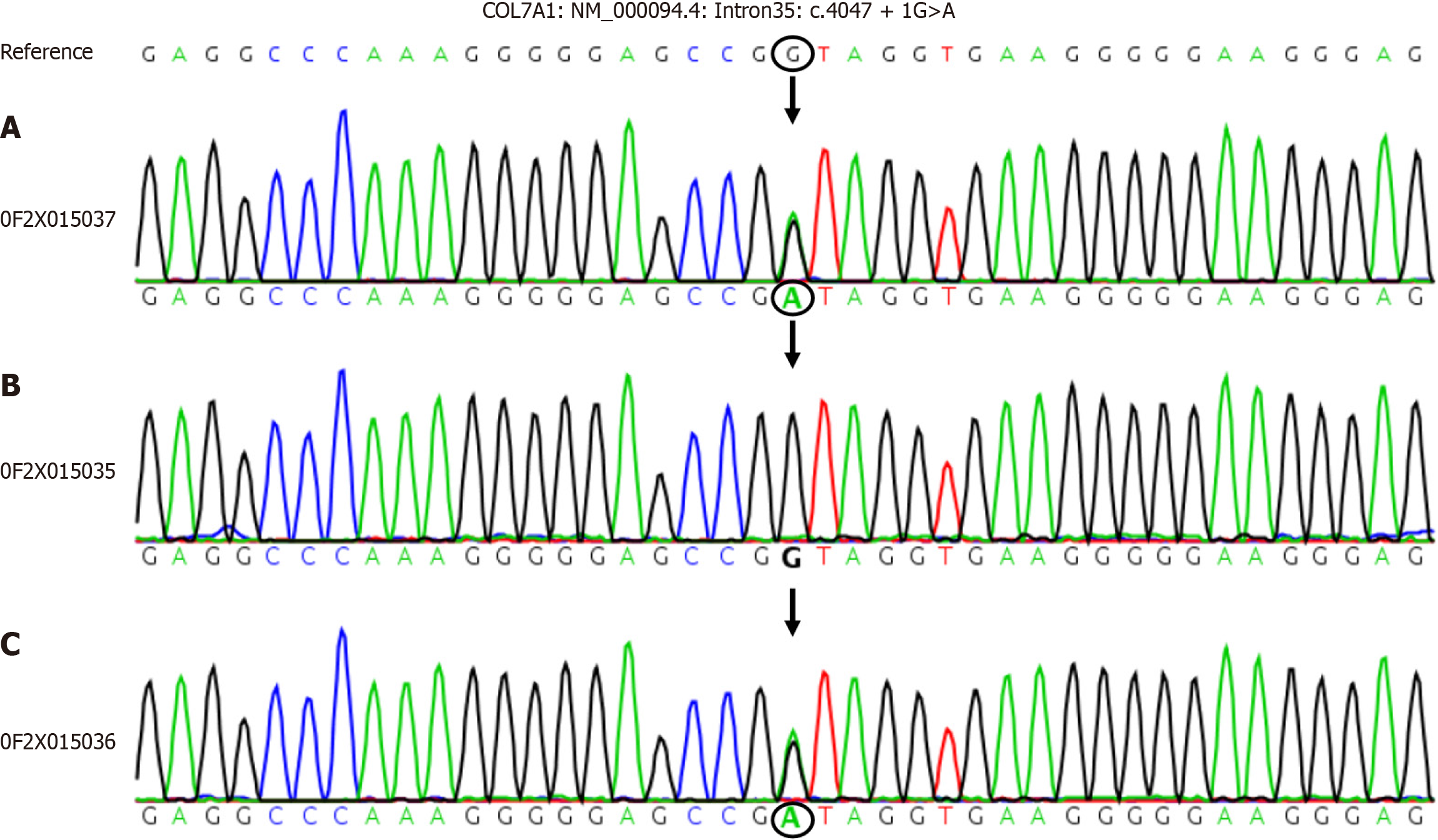
Genetic testing revealed an abnormal change in the sequence of COL7A1 with NM-000094.4: Intron35: C.4047 + 1 guanine (G) > adenine (A) (the nucleotide in the coding region 4047 changed from G to A). The mother harbored a heterozygous mutation at this site, whereas the father did not (Figure 2).
Based on the patient’s clinical symptoms and genetic test results, the final diagnosis was malnourity-type bullous epidermal lysis.
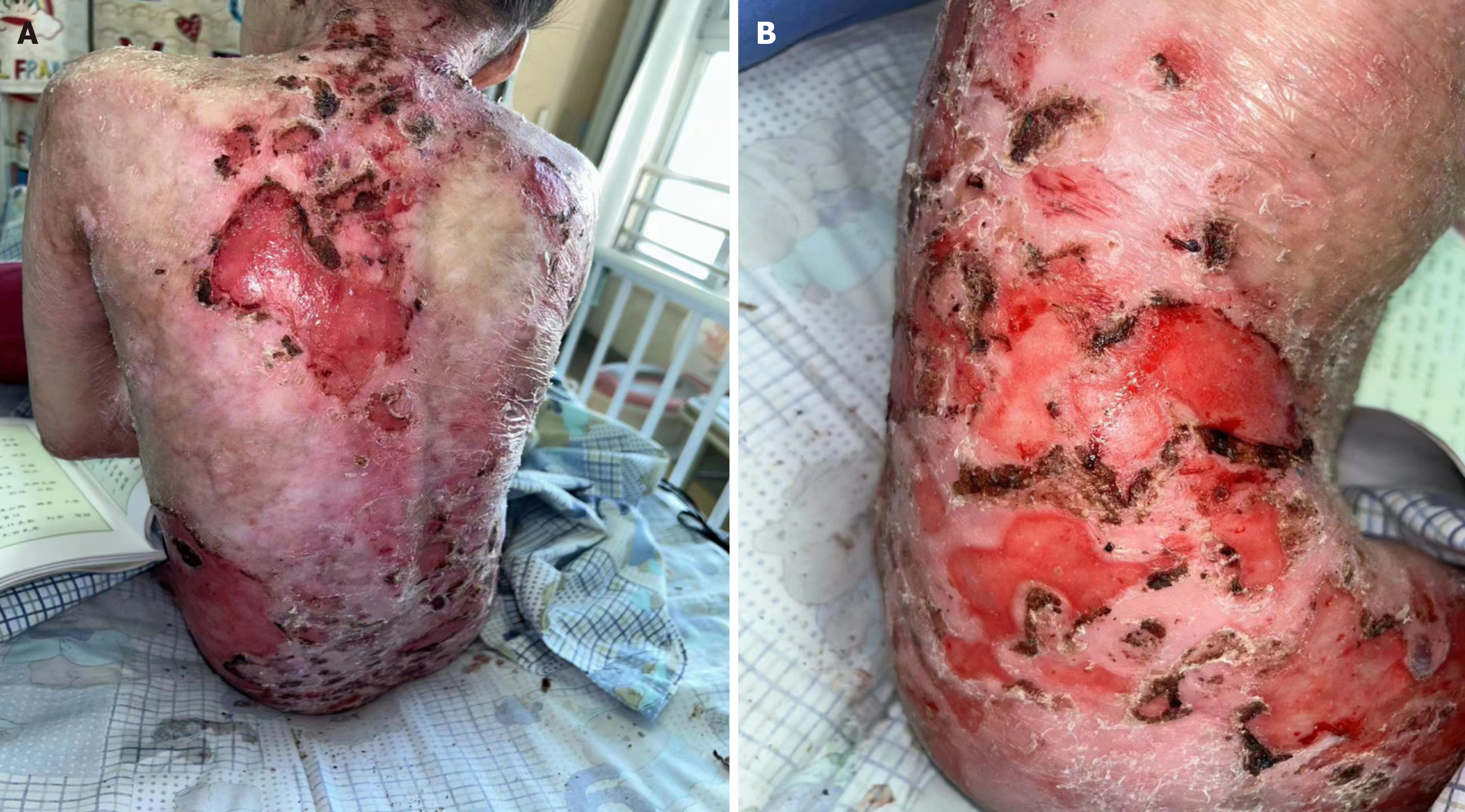
After the patient was admitted to the hospital, she was given linezolid and ertapenem for one week to treat recurrent high fever. Drug treatment: The patient was treated with immunoglobulin at approximately 0.4 g/kg, albumin was given to maintain blood volume and blood pressure, fresh frozen plasma was used to treat coagulation disorders and supplement protein, and ulinastatin was used to reduce inflammation and promote wound healing. External treatment: External medications, laser, wet compresses, medication baths, scab removal, dressing changes of the patient’s wounds, iodine disinfection, physiological saline flushing, silver ion dressing (antibacterial), and external application of freeze-dried recombinant human acidic fibroblast growth factor and mupirocin ointment were also done. On the 20th day of admission, the patient’s chest X-ray showed consolidation shadows in the right middle lung field, and she was treated with a cephalosporin laxative. However, the child had a persistent cough and was treated with nebulization, an oral solution of eucalyptus palmatum, and ambromoterol oral solution for symptomatic cough relief. The child’s immunoglobulin E levels were > 2500 IU/mL in the serum, allergy level to milk in allergen testing is at level 2. Cetirizine hydrochloride drops were also administered for anti-allergy purposes. The patient’s mother was also encouraged to show the patient love and affection to help balance gut microbiota. After treatment, the patient’s overall skin exudation decreased, the area of injury became smaller, and the locally visible skin was fresh (Figure 3). The patient was sub
The patient was discharged after 28 days of hospitalization, and the wound improved significantly upon discharge. The patient was advised to take home care measures to prevent infection. Regular follow-up visits are conducted through communication such as phone calls.
DEB is a rare congenital genetic disorder caused by mutations in many genes, mostly occurs in children, which manifests clinically as dystrophica and a tendency to form blisters and vesicles when subjected to minor trauma or friction, prognosis difficulties, which greatly affects the quality of life of patients[3]. DEB is classified into malnourished, borderline, and simple types based on genetic patterns and lesion depth. Among these, DEB is a relatively rare type caused by the absence or abnormal morphology of anchored fibers in the dermal connective tissue beneath the dense plate of the basement membrane[4]. From a genetic perspective, DEB is caused by abnormal changes in type VII collagen (COL7A1), the main component of anchor fibers[5]. The COL7A1 gene is located at position 3p21.1 on the human chromosome and is approximately three times the length of the mRNA encoding type VII collagen. The subsequence of this gene is relatively short and the arrangement of exons is relatively compact, which plays an important role in the function of the protein it encodes. However, in patients with DEB, early termination codon mutations or glycine substitution mutations in COL7A1 cause qualitative and quantitative changes in type VII collagen, thereby affecting the molecular biological characteristics of anchor fibers and leading to instability of the true epidermal junction structure, which can make skin tissue extremely sensitive to friction and pressure; even a slight touch can lead to blisters and skin tearing. However, the condition described in this case is relatively rare. Congenital EB is typically diagnosed during infancy. At the age of 11, an abnormal fever may lead to severe illness. In our case, the patient’s skin was ulcerated, especially on the limbs, face, shoulder, back, waist, and thighs. Local exudation and scabbing were observed, the pro
Harmful missense mutations and gene function loss mutations can disrupt gene function, thereby reducing an individual’s adaptability to survive in the existing environment and leading to a genetic burden. Dang et al[6] pointed out that 23 different COL7A1 allelic variants, nine of which were novel, which involves glycine substitutions within the triple helix of COL7A1. Liu and Wang[7] reported COL7A1 frameshift mutations at C2005T and G7922A, which were identified as autosomal recessive malnutrition bullous epidermal lysis. Han et al[8] described cases of heterozygous frameshift mutations occurring in two exon positions of COL7A1 that were identified and found to be associated with Bart syndrome. However, the mutations identified in our study were different from those mentioned above. The DEB cases in this study were unique and caused by a newly discovered COL7A1 frameshift mutation. This association was determined by analyzing the clinical manifestations and genotypes of the affected children. From a clinical perspective, the patient presented with systemic lesions, severe ulceration, and loss of both toes. As patients with this condition age, they exhibit worsening skin damage accompanied by severe malnutrition and delayed growth and development. From a genotypic perspective, a heterozygous frameshift mutation was found in the coding region 4047 of the 35th intron of the COL7A1 gene. The mutation in the coding region 4047 resulted in a substitution of the first nucleotide from G to A, followed by the formation of a stop codon. Therefore, the triple-helix region of type VII collagen in COL7A1 undergoes changes, synthesizing mutated peptides that affect the recognition of the ultrastructure of anchored fibrils.
Many methods are currently available for treating DEB. Venugopal et al[9] used allogeneic fibroblasts cultured in suspension to treat DEB wounds. Gurevich et al[10] applied beremagene geperpavec containing COL7A1 to treat skin injuries in patients with DEB. This treatment method delivers the normal COL7A1 gene to the patient’s skin cells through a viral vector to restore or enhance the function of type VII collagen, thereby improving skin condition. However, there is no fundamental and widespread treatment for DEB that mainly focuses on alleviating symptoms and preventing complications. In terms of treatment, this article highlights the use of immunoglobulin, albumin, fresh frozen plasma, and ulinastatin for the treatment of affected children to improve coagulation disorders, supplement proteins, and promote wound healing. It also highlighted the use of external treatments such as silver ion dressings supplemented with growth factors and mupirocin ointment for wound scab removal and dressing changes. In our case, the patient’s condition worsened due to repeated fever, which affected the gastrointestinal and respiratory tracts. Therefore, treatments such as fever reduction, cough relief, and nebulization may be necessary. After treatment, the patient’s symptoms improved, and she was discharged without complications.
The field of genetic medicine has developed rapidly in recent years. With the accumulation of clinical manifestations and genetic testing data for DEB, the precise classification of DEB and methods based on cell and gene therapies may become a reality. Gene therapy differs from traditional therapy, which involves treating hereditary diseases by adding, repairing, replacing, or silencing specific genes. Therefore, it is necessary to study novel heterozygous frameshift muta
We present a case of DEB caused by novel frameshift mutation in the COL7A1 gene, which not only plays a key role in the development of complex diseases such as hereditary dermatological disorders and cancers, but may also directly affect the patient’s responsiveness to targeted drugs. In the future, we will conduct in-depth research on relevant case genes to provide clinical trial data and theoretical support for DEB gene therapy.
| 1. | Tang JY, Marinkovich MP, Lucas E, Gorell E, Chiou A, Lu Y, Gillon J, Patel D, Rudin D. A systematic literature review of the disease burden in patients with recessive dystrophic epidermolysis bullosa. Orphanet J Rare Dis. 2021;16:175. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 71] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 2. | Guide SV, Gonzalez ME, Bağcı IS, Agostini B, Chen H, Feeney G, Steimer M, Kapadia B, Sridhar K, Quesada Sanchez L, Gonzalez F, Van Ligten M, Parry TJ, Chitra S, Kammerman LA, Krishnan S, Marinkovich MP. Trial of Beremagene Geperpavec (B-VEC) for Dystrophic Epidermolysis Bullosa. N Engl J Med. 2022;387:2211-2219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 122] [Article Influence: 30.5] [Reference Citation Analysis (0)] |
| 3. | Yadav RS, Jayswal A, Shrestha S, Gupta SK, Paudel U. Dystrophic Epidermolysis Bullosa. JNMA J Nepal Med Assoc. 2018;56:879-882. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 4. | Samuelov L. Dystrophic epidermolysis bullosa: from disease biology to biologic therapy. Br J Dermatol. 2024;191:159-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 5. | Hou PC, Del Agua N, Lwin SM, Hsu CK, McGrath JA. Innovations in the Treatment of Dystrophic Epidermolysis Bullosa (DEB): Current Landscape and Prospects. Ther Clin Risk Manag. 2023;19:455-473. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 40] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 6. | Dang N, Klingberg S, Marr P, Murrell DF. Review of collagen VII sequence variants found in Australasian patients with dystrophic epidermolysis bullosa reveals nine novel COL7A1 variants. J Dermatol Sci. 2007;46:169-178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 7. | Liu J, Wang L. A Case Report of an Infant with Autosomal Recessive Dystrophic Epidermolysis Bullosa: COL7A1 Gene Mutations at C2005T and G7922A. Acta Dermatovenerol Croat. 2021;29:164-166. [PubMed] |
| 8. | Han YM, Lee N, Byun SY, Cheon SJ, Ko HC. Bart's Syndrome with Novel Frameshift Mutations in the COL7A1 Gene. Fetal Pediatr Pathol. 2019;38:72-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 9. | Venugopal SS, Yan W, Frew JW, Cohn HI, Rhodes LM, Tran K, Melbourne W, Nelson JA, Sturm M, Fogarty J, Marinkovich MP, Igawa S, Ishida-Yamamoto A, Murrell DF. A phase II randomized vehicle-controlled trial of intradermal allogeneic fibroblasts for recessive dystrophic epidermolysis bullosa. J Am Acad Dermatol. 2013;69:898-908.e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 85] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 10. | Gurevich I, Agarwal P, Zhang P, Dolorito JA, Oliver S, Liu H, Reitze N, Sarma N, Bagci IS, Sridhar K, Kakarla V, Yenamandra VK, O'Malley M, Prisco M, Tufa SF, Keene DR, South AP, Krishnan SM, Marinkovich MP. In vivo topical gene therapy for recessive dystrophic epidermolysis bullosa: a phase 1 and 2 trial. Nat Med. 2022;28:780-788. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 107] [Article Influence: 26.8] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/