Published online Apr 6, 2025. doi: 10.12998/wjcc.v13.i10.98390
Revised: October 29, 2024
Accepted: December 5, 2024
Published online: April 6, 2025
Processing time: 176 Days and 16.1 Hours
Thrombophilia contributes to a significant increased risk of venous thromboembolism and can be either inherited or acquired. Hereditary thrombophilia may arise from various gene mutations, some of which have not even been adequately reported or poorly understood. Previous studies reported a rare and novel missense mutation in the prothrombin gene (p.Arg596Gln), known as proth
We present the case of a 26-year-old woman with recurrent systemic thrombosis induced by prothrombin Belgrade mutation. The patient suffered from cerebral venous sinus thrombosis that rapidly progressed to systemic thrombosis, along
This case strengthens our understanding about hereditary basis of thrombophilia and provokes considerations for therapeutic options on prothrombin Belgrade mutation.
Core Tip: The aim of our present study is to display a novel missense mutation in the prothrombin gene (p.Arg596Gln) in a Chinese patient with onset of cerebral venous thrombosis. Considering that prothrombin Belgrade mutation mechanism and treatment are still not fully elucidated, we provide the understanding about hereditary risk factors of cerebral venous thrombosis and trigger the further exploration of effective and exact therapeutic options and management on cerebral venous thrombosis caused by prothrombin Belgrade mutation.
- Citation: Wu YF, Huang Y, Weng BH, Deng S, Pan LY, Li Z. Systemic thrombosis with prothrombin Belgrade mutation in a Chinese patient: A case report. World J Clin Cases 2025; 13(10): 98390
- URL: https://www.wjgnet.com/2307-8960/full/v13/i10/98390.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v13.i10.98390
Thrombophilia is characterized by abnormal blood coagulation, with tendency toward an increased risk of thromboembolism, particularly in the venous system[1]. Universally recognized risk factors for thrombophilia are composed of inherited conditions (e.g., deficiencies of protein C, protein S, and antithrombin, as well as the factor V “Leiden” and prothrombin G20210A mutation) and acquired ingredients (e.g., antiphospholipid syndrome, myeloproliferative neoplasms, cancer)[2]. These elements may contribute to heightened coagulation pathways, irregular anticoagulant functions, or issues with fibrinolysis[3,4]. These complex factors and mechanism play a significant role in clarifying and defining the etiology and pathogenesis of deep vein thrombosis, pulmonary embolism and cerebral venous thrombosis.
Regarding the hereditary factor, common gain-of-function mutations like the factor V “Leiden” and prothrombin (factor II) G20210A mutation, have been extensively studied[5]. Additionally, a novel and rare c.1787G>T prothrombin mutation was first reported in two Serbian families with recurrent thrombosis at a young age a decade ago, resulting from a substitution of arginine with glutamine at position 596 (p.Arg596Gln) in the gene encoding prothrombin (called “prothrombin Belgrade”)[6]. The mutation contributes to substantially impairment of thrombin-antithrombin binding and an increased risk of thrombophilia and antithrombin resistance (ATR)[7]. In this instructive case report, we exhibit a case that a Chinese patient was presented with onset of cerebral venous sinus thrombosis and further evolved into systemic thrombosis due to the prothrombin Belgrade mutation. We hope to raise awareness of this disease and provide new insights into the pathogenesis and potential treatment strategies of this novel genetic mutation.
A 26-year-old woman with severe paroxysmal headache accompanied by nausea and vomiting for twelve days.
The patient suffered from severe paroxysmal headache accompanied by nausea and vomiting for twelve days. On October 19, 2022, the patient developed a burst headache without paying attention. On October 23, 2022, the headache recurred, accompanied by nausea, vomiting and left neck pain. The head computed tomography scan revealed no obvious abnormalities, while carotid duplex ultrasound indicated left internal jugular vein occlusion.
Apart from family history, the patient had no risk factors for thromboembolic events like pregnancy, hormone replacement therapy, or infections. Furthermore, she presented no history of hypertension, coronary artery disease, or diabetes mellitus.
The patient was a non-smoker and non-drinker. Family history revealed thrombotic conditions in the sense that her father and sister had the record of cerebral thrombosis. No other family members had undergone genetic testing.
On admission, the patient exhibited left hemi-cranial distending pain with cervical unsymmetric radicular pain. The rest of the general physical and neurological examination was normal.
Laboratory findings on the complete blood count revealed the presence of neutrophilia, lymphopenia and monocytosis. Coagulation tests showed elevated D-dimer levels. Protein C, antithrombin III, lupus anticoagulant, anti-cardiolipin antibodies were normal, except for a mildly low value of protein S. Liver and renal function tests, along with serum electrolytes, were within normal limits. In consideration of her family history and onset age, whole genome sequencing was performed on the patient, pointing out a c.1787G>T (p.Arg596Gln) heterozygous missense mutation in the prothrombin gene.
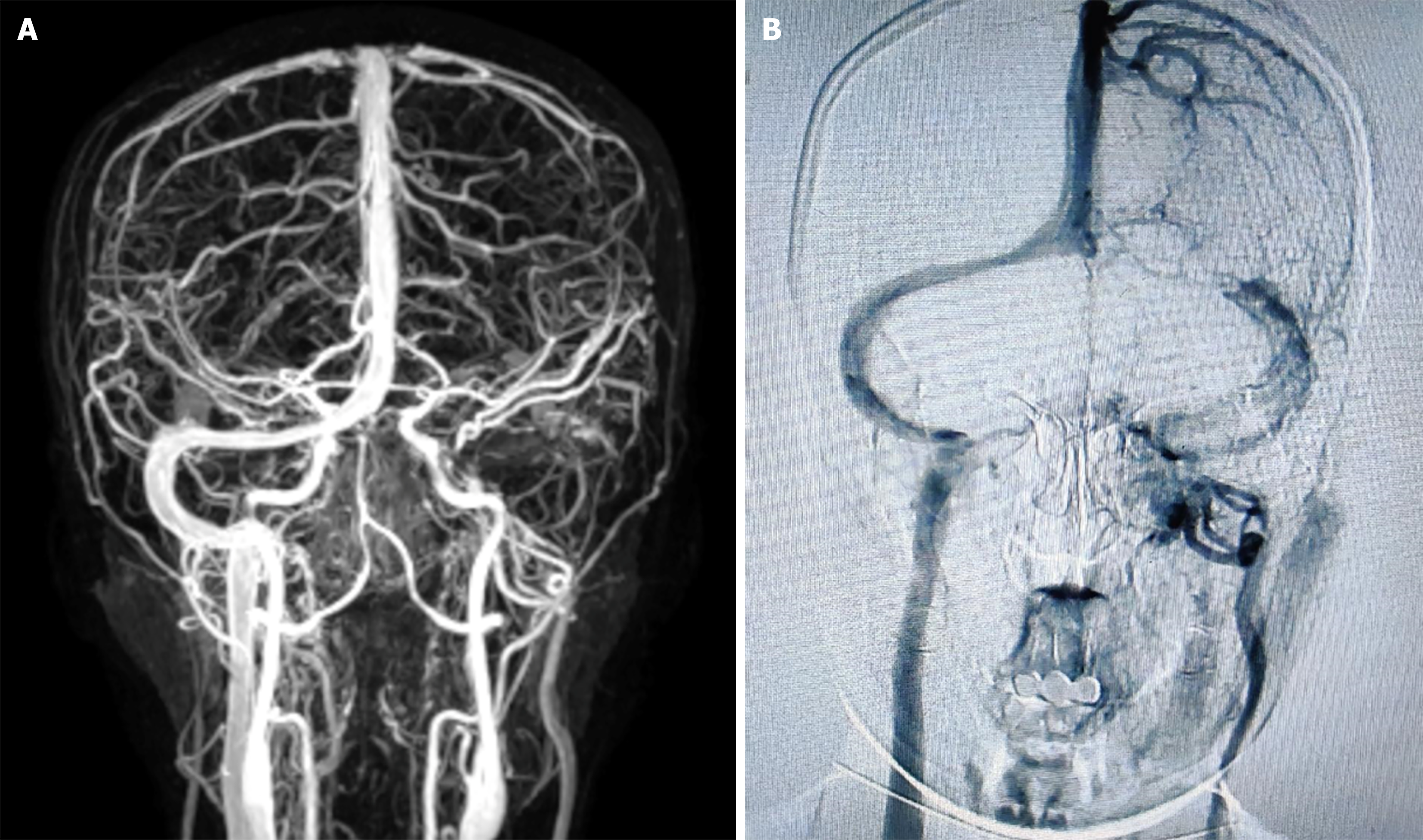
The cerebral computed tomography scan showed a hypodense shadow in the left cerebellar hemisphere, suggesting venous cerebral infarction. Cerebral magnetic resonance angiography was performed and revealed complete thrombosis of the left transverse sinus (Figure 1A). Further cerebral vascular angiography further confirmed thrombosis and occlusion of left transverse sinus and internal jugular vein (Figure 1B).
A final diagnosis of cerebral venous sinus, thrombosis upper extremity venous thrombosis, and lower extremity deep venous thrombosis was made.
The patient received subcutaneous low molecular weight heparin as well as symptomatic treatment during hospitalization and rivaroxaban therapy after discharge.
However, she returned to the hospital with numbness and pain in her left upper limb in October 2023. After inquiring about medication compliance, she reported having stopped taking anticoagulants for three months. The ultrasound suggested thrombosis of the left internal jugular vein, superficial veins of left upper limb, left axillary vein and left subclavian vein, as well as bilateral thrombosis of the common femoral vein, superficial femoral vein, deep femoral vein, and popliteal vein. The computed tomographic pulmonary angiography was performed and revealed partial pulmonary artery branch embolism in the lower lobes of bilateral lungs. The reexamination of cerebral magnetic resonance angiography indicated residual but decreasing thrombosis of left internal jugular vein, sigmoid sinus, and transverse sinus. Likewise, this patient continued to receive anticoagulant treatment.
For young-onset female patients with a family history of thrombosis or recurrent thrombosis events, an inherited predisposition to thrombophilia may significantly contribute to the underlying causes and development of the condition. Inherited thrombophilia can be derived from two different mechanisms: Increased concentrations of procoagulants (gain-of-function disorders) or deficiencies/dysfunctions in the endogenous anticoagulants (loss-of-function disorders) under the circumstance of genotypic milieu[1,8]. Loss-of-function disorders typically have a lower morbidity than gain-of-function disorders, whereas they present more powerful risk factors for thrombosis. In addition to the common hereditary factors, such as thrombophilia - factor V Leiden and prothrombin G20210A, other rare mutations are noteworthy. In this report, we present a novel and infrequent prothrombin Belgrade genetic mutation contributing to recrudescent systemic thrombosis in a young-onset patient.
Prothrombin Belgrade mutation was first proposed in Serbia in 2013, characterized by a G-to-A change at nucleotide position 1787. This alteration results in an arginine-to-glutamine substitution at amino acid position 596 (p.Arg596Gln) within the prothrombin gene[6,9]. Arg596Gln mutation carriers demonstrate decreased fibrinogen clotting activity, impaired procoagulant activity and impaired antithrombin inhibition, shifting the function balance of thrombin towards the procoagulant pathway, which should be the main cause of thrombus embolism in Arg596Gln carriers. A previous study on prothrombin Arg596 related mutations highlighted that the prothrombin Arg596Gln mutation was also an important risk factor for venous thromboembolism in Chinese patients[10].
Furthermore, ATR is one of the most essential hereditary thrombotic mechanisms and ATR caused by prothrombin Belgrade mutation has been reported in Serbia, China, and Japan[6,9-12]. Thrombin-generation assay suggested that the mutant prothrombin functioned to arouse resistance against inhibition by antithrombin, further facilitating blood coagulation[7]. Taking account of the ATR property, selecting an appropriate anticoagulant drug is crucial for the treatment of prothrombin Belgrade mutation. In this case, the patient suffered from recurrent attacks of thrombosis, progressing from cerebral venous sinus thrombosis to systemic thrombosis. A possible reason for recurrence could be drug withdrawal or the therapeutic regimen. It remains to be established whether warfarin is the optimal choice for managing pulmonary thromboembolism due to ATR. Given the high risk of severe thrombosis being fatal, any kind of possibility cannot be neglected. There are many types of anticoagulant drugs with different mechanisms. Despite the fact that the case report fails to draw clear guidelines for the treatment of the disease, it serves as an inspiration and a reminder for selection of anticoagulants. Whether an indirect thrombin inhibitor, such as heparin, or factor Xa inhibitor, such as rivaroxaban, is the best choice for systemic thromboembolism due to ATR remains to be determined. Moreover, examination of ATR assay should be prioritized alongside genetic testing. Furthermore, more stringent and regular follow-up is of great necessity.
In conclusion, our case report displays a novel missense mutation in the prothrombin gene (p.Arg596Gln) in a Chinese patient with recurrent thrombosis. Given that the mechanism and treatment of the prothrombin Belgrade mutation are still not fully elucidated, we provide valuable insights into the hereditary basis of thrombophilia and considerations for targeted therapeutic strategies in managing prothrombin Belgrade mutation.
| 1. | Sachs UJ, Kirsch-Altena A, Müller J. Markers of Hereditary Thrombophilia with Unclear Significance. Hamostaseologie. 2022;42:370-380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 2. | Stevens SM, Woller SC, Bauer KA, Kasthuri R, Cushman M, Streiff M, Lim W, Douketis JD. Guidance for the evaluation and treatment of hereditary and acquired thrombophilia. J Thromb Thrombolysis. 2016;41:154-164. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 161] [Cited by in RCA: 197] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 3. | Miletich JP, Prescott SM, White R, Majerus PW, Bovill EG. Inherited predisposition to thrombosis. Cell. 1993;72:477-480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 105] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 4. | Girolami A, Cosi E, Ferrari S, Girolami B. Heparin, coumarin, protein C, antithrombin, fibrinolysis and other clotting related resistances: old and new concepts in blood coagulation. J Thromb Thrombolysis. 2018;45:135-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 5. | Pastori D, Cormaci VM, Marucci S, Franchino G, Del Sole F, Capozza A, Fallarino A, Corso C, Valeriani E, Menichelli D, Pignatelli P. A Comprehensive Review of Risk Factors for Venous Thromboembolism: From Epidemiology to Pathophysiology. Int J Mol Sci. 2023;24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 177] [Reference Citation Analysis (0)] |
| 6. | Djordjevic V, Kovac M, Miljic P, Murata M, Takagi A, Pruner I, Francuski D, Kojima T, Radojkovic D. A novel prothrombin mutation in two families with prominent thrombophilia--the first cases of antithrombin resistance in a Caucasian population. J Thromb Haemost. 2013;11:1936-1939. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 53] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 7. | Miyawaki Y, Suzuki A, Fujita J, Maki A, Okuyama E, Murata M, Takagi A, Murate T, Kunishima S, Sakai M, Okamoto K, Matsushita T, Naoe T, Saito H, Kojima T. Thrombosis from a prothrombin mutation conveying antithrombin resistance. N Engl J Med. 2012;366:2390-2396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 89] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 8. | Badescu MC, Butnariu LI, Costache AD, Gheorghe L, Seritean Isac PN, Chetran A, Leancă SA, Afrăsânie I, Duca ȘT, Gorduza EV, Costache II, Rezus C. Acute Myocardial Infarction in Patients with Hereditary Thrombophilia-A Focus on Factor V Leiden and Prothrombin G20210A. Life (Basel). 2023;13:1371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 9. | Yoshida R, Seki S, Hasegawa J, Koyama T, Yamazaki K, Takagi A, Kojima T, Yoshimura M. Familial pulmonary thromboembolism with a prothrombin mutation and antithrombin resistance. J Cardiol Cases. 2018;17:197-199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 10. | Wu X, Li L, Ding Q, Wang X, Wu F, Wu W. Screening and functional exploration of prothrombin Arg596 related mutations in Chinese venous thromboembolism patients. J Clin Pathol. 2018;71:614-619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 11. | Kishimoto M, Suzuki N, Murata M, Ogawa M, Kanematsu T, Takagi A, Kiyoi H, Kojima T, Matsushita T. The first case of antithrombin-resistant prothrombin Belgrade mutation in Japanese. Ann Hematol. 2016;95:541-542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 12. | Tsuji A, Miyata T, Sekine A, Neki R, Kokame K, Tomita T, Kashima Y, Asano R, Ueda J, Aoki T, Ogo T. Three Cases of Unprovoked Venous Thromboembolism with Prothrombin p.Arg596Gln Variant and a Literature Review of Antithrombin Resistance. Intern Med. 2023;62:885-888. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/