Published online Apr 6, 2025. doi: 10.12998/wjcc.v13.i10.97584
Revised: October 23, 2024
Accepted: December 10, 2024
Published online: April 6, 2025
Processing time: 198 Days and 21.8 Hours
Cleidocranial dysplasia (CCD) is an infrequent clinical condition with an auto
We report a rare case of fetal CCD with an unknown family history, confirmed by prenatal ultrasonography and genetic testing at a gestational age of 16 weeks. The genetic reports indicated that the fetus carried pathogenic mutations in the RUNX family transcription factor 2 gene (c.674G>A). After careful consideration, the pregnant woman and her family decided to continue the pregnancy.
Definitive prenatal diagnosis of CCD should include family history, ultrasound diagnosis, and genetic analysis, especially if family history is unknown.
Core Tip: Cleidocranial dysplasia is rare and hidden condition, and many cases are gradually diagnosed after birth. Features include multiple teeth, partial or complete absence of the clavicle, delayed closure of the sagittal suture and fontanel, which have an adverse effect on physical and mental health. We report a rare case of fetal cleidocranial dysplasia with an unknown family history at 16 weeks gestation, confirmed by prenatal ultrasonography and genetic testing. This case can inform future clinicians to aid in timely and correct diagnosis.
- Citation: Wang F, Dai PF, Gao WJ. Prenatal ultrasonography and genetic analysis of fetal cleidocranial dysplasia: A case report. World J Clin Cases 2025; 13(10): 97584
- URL: https://www.wjgnet.com/2307-8960/full/v13/i10/97584.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v13.i10.97584
Cleidocranial dysplasia (CCD) is a rare autosomal dominant systemic bone dysplasia with an incidence of about one in a million people[1]. The disorder is caused by mutations in the RUNX family transcription factor 2 (RUNX2) gene on chromosome 6p21. The RUNX2 gene is essential for differentiation of stem cells into osteoblasts. Therefore, mutations may cause defective bone formation. The disease can occur at any age, and about one-third of the patients have no family history. No significant difference is observed between the sexes. Due to the occult nature of CCD, severe bone growth and developmental abnormalities are gradually observed after birth. There is a need for a multifunctional approach to treat this disease, including tooth extraction and orthodontics, which can have psychological and physical consequences for patients. Therefore, it is important to recognize the disease early, especially in the fetal period. However, early recognition of fetal CCD is difficult when the family history is unknown. Here, we report a case of CCD diagnosed in a pregnant woman at 16 weeks gestation, with unknown family history. Based on a review of previous studies, we explored fetal ultrasonography diagnosis and genetic characteristics of CCD to improve the understanding of obstetricians and imaging physicians.
A 31-year-old woman (gravida 2, para 1), with normal intellectual development, was referred for ultrasound examination after 16 weeks of suspected hypomineralization of the skull bones and absence of the nasal bones.
A routine ultrasound examination was performed at the local hospital at 12 weeks of gestation. The nasal bone was not ossified, but the nuchal translucency thickness was normal. At 16 weeks of gestation, ultrasound examination in a local hospital revealed fetal bone dysplasia. Therefore, she came to our hospital for consultation.
The woman had a regular menstrual cycle, denied a history of exposure to drugs, radiation, and toxins in early pregnancy, and denied having a history of acute and chronic infection, heart disease, hypertension, diabetes, etc.
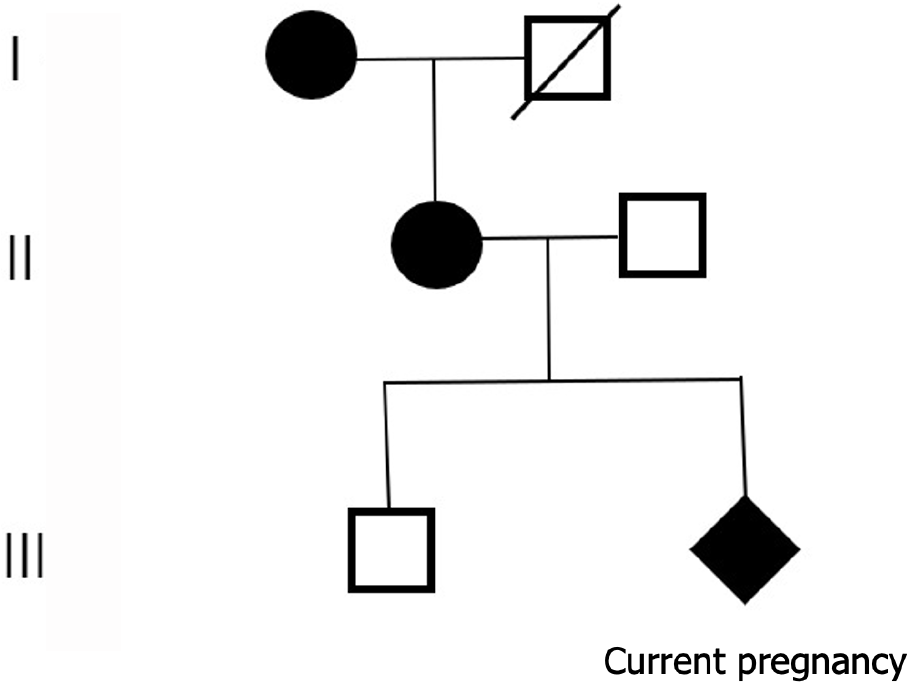
The patient’s family history is unknown, but she self-reported that her first son was normal in appearance and intelligence, but her mother had unclear speech, low intelligence, and a small clavicle and skull.
The physical examination of this pregnant woman revealed short stature, large head and small face, sunken forehead, widening eye distance, low and flat nose, and overcrowding and malocclusion of supernumerary teeth. Besides, the shoulder joint range of motion was large, and the two shoulders could be closed in the chest.
In this genetic test, the whole blood samples of fetal amniotic fluid and both parents of the proband were collected, and whole-exome sequencing was performed to detect the pathogenic genes.
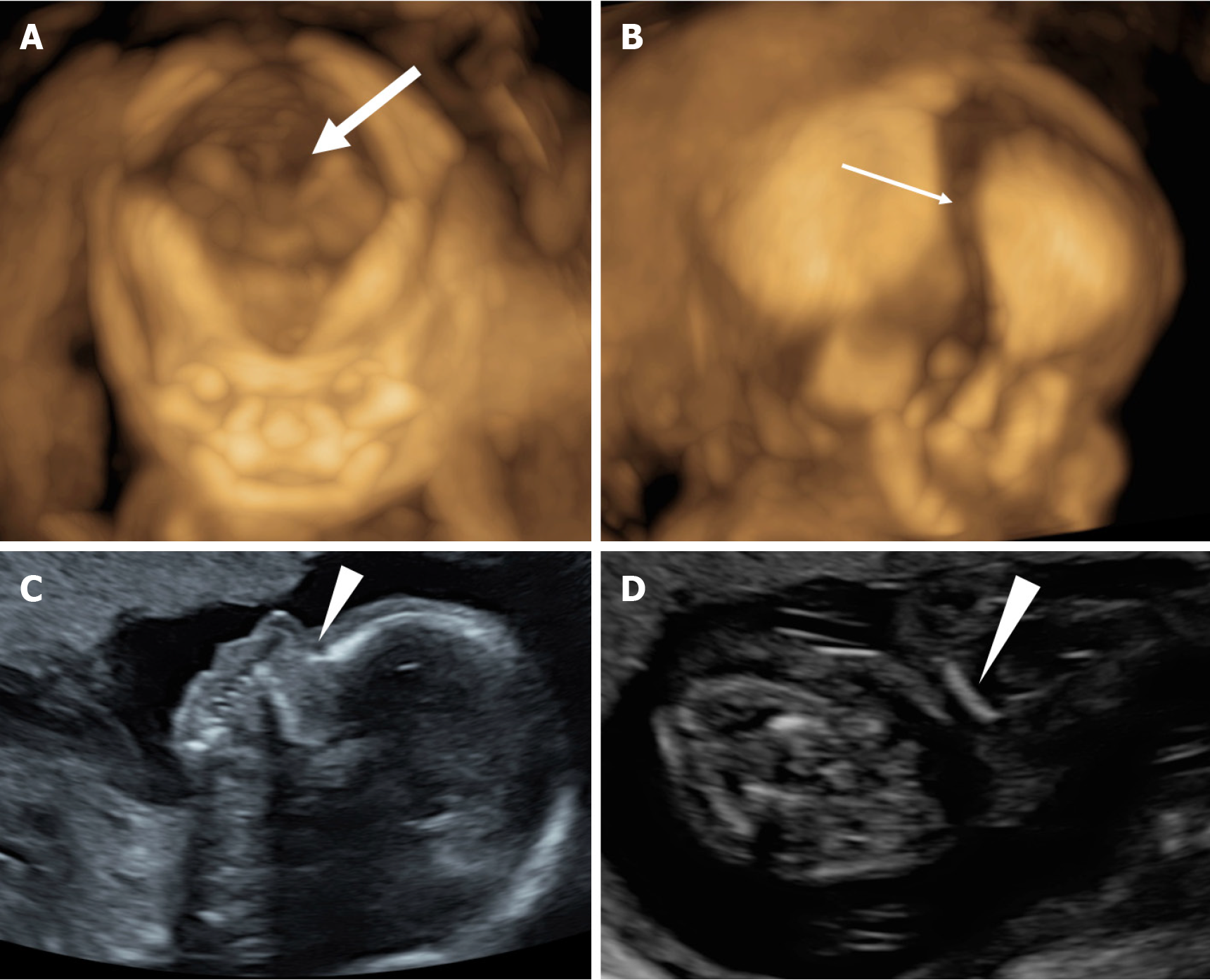
The results of ultrasound examination in our hospital were: Biparietal diameter 3.6 cm, head circumference 13.2 cm, abdominal circumference 10.8 cm, and femur length 1.7 cm. Also, the fetal heart rate was about 145 beats/min. No nasal bone was shown, the cranial suture was widened, ossification of the skull and clavicle (more serious on the right side) was poor, echo decreased, and femur length was less than the 2.5th percentile of corresponding gestational age. The scapula and ilium were also smaller, and no obvious abnormalities were observed in the placenta, amniotic fluid, umbilical cord and spine. The ultrasound measurements were equivalent to 16 weeks and 3 days of gestation. Two-dimensional (2D) and 3D ultrasonography of the fetus revealed suspected CCD features, including poor ossification of the skull, large fontanelle, and absence of nasal bones. Furthermore, the clavicle was short and straight, without a typical “S” shape (Figure 1).
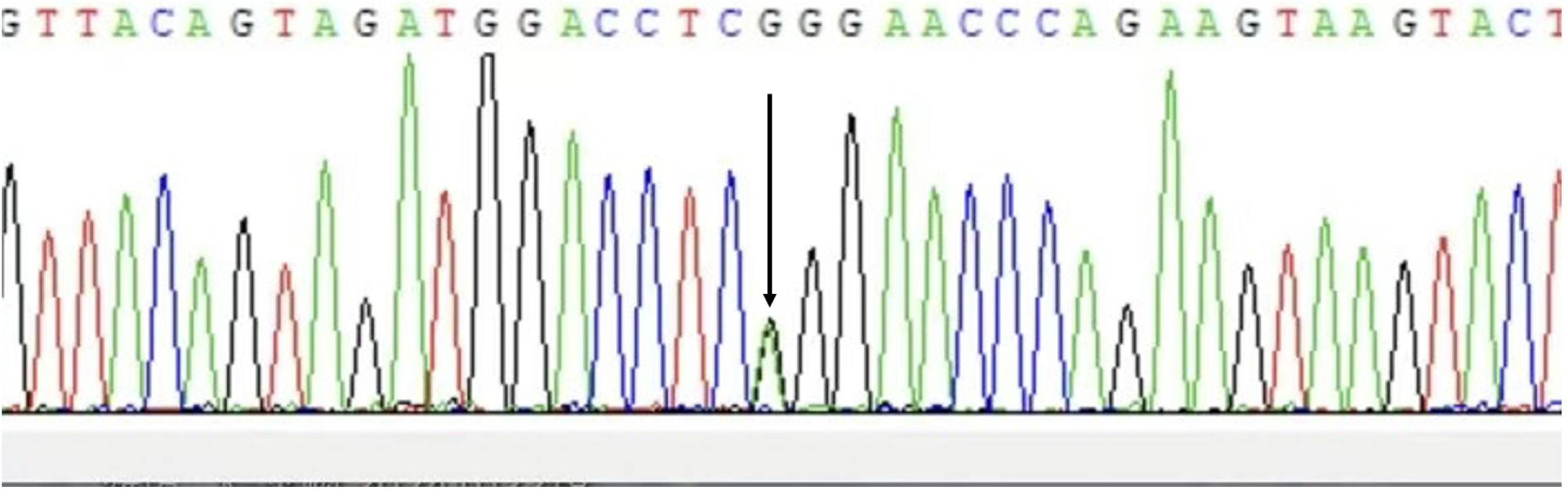
The woman received prenatal counseling due to the suspected CCD on prenatal ultrasound. If a pregnant woman has CCD, the risk of giving birth to a child with CCD per pregnancy is 50%, regardless of the sex of the fetus. Even if similar mutations are found, the severity of the CCD phenotype cannot be predicted. Therefore, fetal amniotic fluid and blood samples from parents were collected for genetic testing. Whole-exome sequencing technology was used to detect the variation related to fetal ultrasound phenotype, RUNX2 gene c.674G>A mutation, which was verified by Sanger sequencing. The results were consistent with the whole exon sequencing results (Figure 2). In addition, it was found that the mother was heterozygous, and the father was wild type. It was suggested that the fetus had clavicular/metaphyseal and maxillary dysplasia with or without short finger (toe) deformity. As the woman was diagnosed with CCD, the whole blood samples of the woman’s first son and her mother were collected for the genetic verification of single-gene genetic disease. It was found that the mother was heterozygous, and her son did not have this mutation (Figure 3).
The obstetrician fully interpreted the ultrasound and genetic test and reported the results to the pregnant woman. They informed the patient about the delivery management measures of CCD and the possible disease characteristics after birth, and respected the woman’s reproductive choice.
After careful consideration by the pregnant woman and her family, she chose to continue the pregnancy until delivery. A male neonate was delivered by cesarean section at 38 + 2 weeks of gestation, with a birth weight of 3250 g, length of 48 cm, and Apgar scores of 9 and 10 at 1 and 5 minutes, respectively. The neonate was discharged home 4 days after delivery, without complications. At 2 years and 7 months follow-up, the boy had well-aligned baby teeth, incomplete closure of the fontanel, short collarbone, and normal intelligence. We will continue to follow up.
CCD triad includes multiple teeth, partial or complete absence of the clavicle, and delayed closure of the sagittal suture and fontanelle[2,3]. CCD can be categorized into three types according to the genetic relationship and different affected bones: (1) Type I is the standard type, with familial inheritance and incomplete ossification of the skull and clavicle; (2) Type II is familial, with familial inheritance, but the skull is not involved; and (3) Type III is sporadic, with no familial inheritance, and the skull and clavicle are involved. This patient had a family history and belonged to type I. As the woman did not know she had CCD, this indicated CCD was insidious, but its heritability became high once it occurred.
Approximately 60%-70% of patients with CCD have a loss of function or haploidy insufficiency mutation of the RUNX2 gene, which is located on chromosome 6p21 and is an important transcription factor in chondrocyte maturation, osteoblast differentiation, and bone formation[4,5]. RUNX2 regulates mesenchymal cell proliferation in sutures and suture closure by inducing the signaling pathways of fibroblast growth factor, parathyroid hormone-like hormone, hedgehog and Wnt genes[6]. At present, fetal CCD with prenatal ultrasound and genetic analysis is rarely reported. Our review of studies published in English revealed only eight cases of fetal CCD diagnosed with prenatal ultrasound, including the present case (Table 1). There are some similarities between this and previous CCD cases, such as poor mineralization of skull and clavicle, and missing nose bone, but the biggest difference is that the family history of this case was unknown, which aggravated the difficulty of diagnosis. Therefore, it is important to carefully ascertain the medical history, family history, and even observe the physical characteristics of the pregnant woman. Finally, we continue with a comprehensive ultrasound and retrospective history, as well as genetic testing to confirm the diagnosis of the disease. CCD mainly affects bones formed by intramembranous ossification, such as the skull and clavicles. Its main characteristics are: (1) Short stature; (2) Hypoplasia of the clavicle, resulting in hypermobility of the shoulder, which can usually be approached forward; (3) Skeletal abnormalities such as conical chest with short ribs, flat feet, genu valgus (genu varus deformity), and scoliosis; (4) Hand abnormalities, such as short fingers and distal phalanges; and (5) Repeated upper respiratory tract and ear infections, hearing loss, early infant respiratory distress, and even language disorders and seizures. The child is in good general health with normal intellectual development[1,7-10]. Although the biological characteristics of the fetus and pregnant woman were consistent with the aforementioned description, the mental health issues of the woman’s mother needed exploration using a large sample.
| Ref. | Family history | Maternal history | Previous pregnancy | Gestational week at detection | Prenatal ultrasound findings | Genetic diagnosis |
| Hassan et al[9], 1997 | Unknown | G2P1 | Healthy female | 19 weeks | Abnormal skull ossification; short femora; short humeri | No chromosome rearrangement of chromosome 6 |
| Stewart et al[15], 2000 | Positive | G3P2 | CCD male, healthy female | 14 weeks + 4 days | The hypoplastic clavicles and the cranium appeared less well ossified than expected for gestational age | Undetected |
| Paladini et al[11], 2000 | NA | NA | NA | 37 weeks | Late-onset skeletal dysplasia; clear frontoparietal bossing; hypomineralization of the cranial bones; the low nasal bridge; clavicular hypoplasia | Refused genetic testing |
| Winer et al[3], 2003 | Negative | NA | NA | 24 weeks | Clavicular hypoplasia; no ossification of the cranial parietal bone; very poor ossification of the frontal and pubic bones; the long bones growth restriction | An apparently de novo balanced t(2q;6q)(q36;q16) translocation |
| Soto et al[13], 2006 | Positive | G3P1 | Open anterior fontanelle at 5-year-old | 18 weeks + 3 days | 18 weeks + 3 days: Absence of nasal bone, the widened fontanelles; hypomineralized occipital bones; 21 weeks + 3 days: Intrauterine clavicle fracture | No RUNX2 gene mutation |
| Hove et al[14], 2008 | Positive | G2P1 | CCD child | 13 weeks + 6 days | Severely delayed ossification of the vertebral spine; barely visible clavicles; poorly ossified calvarial bones | NA |
| Hermann et al[12], 2009 | Positive | NA | NA | 15 weeks + 4 days | Large fontanelles; lack of nasal bones; clavicles without the typical “S” form; severe delay in calvarial ossification | NA |
| Present case | Unknown | G2P1 | Healthy male | 16 weeks | Lack of nasal bones; large fontanelles; widened cranial suture; the poor ossification of skull and clavicle; short femur | Pathogenic mutations in the RUNX2 gene (c.674G>A) |
The correct diagnosis of CCD involves clinical history, symptoms, signs, imaging findings, and genetic analysis. The main problem in CCD management is early diagnosis. Previous case reports on prenatal ultrasound diagnosis of CCD were based on abnormalities of the clavicle (hypoplasia and hypocalcification) and malcalcification of the skull (large fontanelle and wide cranial suture)[11]. Some researchers found that almost all fetuses with CCD had missing nasal bones, which was consistent with the finding of this study[12,13]. Ultrasonography has found early suggestive features of CCD as early as 14 weeks of gestation, such as severely delayed spinal ossification, clavicular hypoplasia, and skull ossification below gestational age. However, few studies have reported the prospective diagnosis of CCD in patients with a negative family history[14,15]. In addition, the ultrasonic diagnostic criteria of fetal fontanel and cranial suture width should be further studied to avoid missed diagnosis due to subjective factors. Natural delivery may cause potential damage to the fetal brain due to the delayed nature of CCD skull ossification, and a planned cesarean section is recommended in these cases. Therefore, the prenatal identification of CCD is of clinical importance.
Ultrasound may be the only prenatal diagnostic technique that can detect CCD when family history is negative and DNA testing is not available during pregnancy. 3D ultrasound is a better alternative compared with 2D ultrasound when the craniofacial disease is suspected. The ultrasound findings of clavicular hypoplasia are an important marker of CCD in high-risk families. However, clavicular hypoplasia is also seen in other disorders, including skeletal dysplasia syndrome and chromosomal abnormalities. For example, osteogenesis imperfecta congenita, an autosomal dominant genetic disorder, has prenatal ultrasound findings that include generalized hypomineralization of the long bones and calvarium, multiple bone fractures and severe shortening and bowing of the long bones. Although nasal bone deletions are most common in chromosomal disorders (e.g., trisomy 21 and 18), fetuses with trisomy 21 often have a thickening of the nuchal pellucidum, nasal bone dysplasia, abnormal venous blood flow, heart defects, and excessive amniotic fluid. Other bones should be carefully examined in fetuses with nasal bone deletions but normal karyotypes.
The prenatal diagnosis of CCD requires confirmation by ultrasound and genetic testing. Other bones should be carefully examined, especially for fetuses with nasal bone loss of unknown family history. 3D ultrasonography is more likely than 2D ultrasonography to show an enlarged fontanelle and a low mineralized skull. Understanding the family history, clinical features, ultrasound findings, and genetic characteristics of CCD can help in the early diagnosis of fetal CCD so that clinicians can decide on an appropriate delivery mode and postnatal bone monitoring so as to improve function, prognosis, and aesthetics.
We thank the patients for consenting us to share the details. We also appreciate the support of the hospital and the ultrasound department.
| 1. | Cooper SC, Flaitz CM, Johnston DA, Lee B, Hecht JT. A natural history of cleidocranial dysplasia. Am J Med Genet. 2001;104:1-6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 108] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 2. | Farrow E, Nicot R, Wiss A, Laborde A, Ferri J. Cleidocranial Dysplasia: A Review of Clinical, Radiological, Genetic Implications and a Guidelines Proposal. J Craniofac Surg. 2018;29:382-389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 44] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 3. | Winer N, Le Caignec C, Quere MP, David A, Boceno M, Aubron F, Joubert M, Boog G, Philippe HJ, Rival JM. Prenatal diagnosis of a cleidocranial dysplasia-like phenotype associated with a de novo balanced t(2q;6q)(q36;q16) translocation. Ultrasound Obstet Gynecol. 2003;22:648-651. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 4. | Morrison NA, Stephens AA, Osato M, Polly P, Tan TC, Yamashita N, Doecke JD, Pasco J, Fozzard N, Jones G, Ralston SH, Sambrook PN, Prince RL, Nicholson GC. Glutamine repeat variants in human RUNX2 associated with decreased femoral neck BMD, broadband ultrasound attenuation and target gene transactivation. PLoS One. 2012;7:e42617. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 5. | Wu LZ, Su WQ, Liu YF, Ge X, Zhang Y, Wang XJ. Role of the RUNX2 p.R225Q mutation in cleidocranial dysplasia: a rare presentation and an analysis of the RUNX2 protein structure. Genet Mol Res. 2014;13:1187-1194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 6. | Motaei J, Salmaninejad A, Jamali E, Khorsand I, Ahmadvand M, Shabani S, Karimi F, Nazari MS, Ketabchi G, Naqipour F. Molecular Genetics of Cleidocranial Dysplasia. Fetal Pediatr Pathol. 2021;40:442-454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 7. | Jirapinyo C, Deraje V, Huang G, Gue S, Anderson PJ, Moore MH. Cleidocranial Dysplasia: Management of the Multiple Craniofacial and Skeletal Anomalies. J Craniofac Surg. 2020;31:908-911. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 8. | Ma Y, Zhao F, Yu D. Cleidocranial dysplasia syndrome with epilepsy: a case report. BMC Pediatr. 2019;19:97. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 9. | Hassan J, Sepulveda W, Teixeira J, Garrett C, Fisk NM. Prenatal sonographic diagnosis of cleidocranial dysostosis. Prenat Diagn. 1997;17:770-772. [PubMed] |
| 10. | Biswas A, Babu GS, Rao K, Sakthivel S, Castelino R. Application of 3-D Imaging in a Familial Case of Cleidocranial Dysplasia. Cumhuriyet Dent J. 2020;23:142-148. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 11. | Paladini D, Lamberti A, Agangi A, Martinelli P. Cleidocranial dysostosis. Prenatal ultrasound diagnosis of a late onset form. Ultrasound Obstet Gynecol. 2000;16:100-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 12. | Hermann NV, Hove HD, Jørgensen C, Larsen P, Darvann TA, Kreiborg S, Sundberg K. Prenatal 3D ultrasound diagnostics in cleidocranial dysplasia. Fetal Diagn Ther. 2009;25:36-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 13. | Soto E, Richani K, Gonçalves LF, Devers P, Espinoza J, Lee W, Treadwell MC, Romero R. Three-dimensional ultrasound in the prenatal diagnosis of cleidocranial dysplasia associated with B-cell immunodeficiency. Ultrasound Obstet Gynecol. 2006;27:574-579. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 14. | Hove HD, Hermann NV, Jørgensen C, Kreiborg S, Sundberg K. An echo-poor spine at 13 weeks: an early sign of cleidocranial dysplasia. Fetal Diagn Ther. 2008;24:103-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 15. | Stewart PA, Wallerstein R, Moran E, Lee MJ. Early prenatal ultrasound diagnosis of cleidocranial dysplasia. Ultrasound Obstet Gynecol. 2000;15:154-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 18] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/