Published online Apr 6, 2025. doi: 10.12998/wjcc.v13.i10.101935
Revised: October 31, 2024
Accepted: December 3, 2024
Published online: April 6, 2025
Processing time: 78 Days and 9.6 Hours
Phytosterolemia, also known as sitosterolemia, is a rare autosomal recessive disease characterized by elevated plasma plant sterol levels and xanthomata, which is easily misdiagnosed as familial hypercholesterolemia. Patients with homozygous phytosterolemia often have severe clinical manifestations, with xanthomata in childhood and premature atherosclerosis. Our patient had a milder clinical phenotype.
This report describes a patient with homozygous phytosterolemia who presented with only elevated cholesterol and low-density lipoprotein cholesterol (LDL-C) without xanthomata, arteriosclerosis, or hematological abnormalities. Homo
Phytosterolemia is easily misdiagnosed as familial hypercholesterolaemia and can be treated by dietary modification and cholesterol absorption inhibitors to lower blood lipids.
Core Tip: We have reported a rare homozygous phytosterolemia. We followed up the patient and found that dietary regulation can play a role in lowering blood lipids, and lipid-lowering drugs can be avoided if the diet is well controlled. Second, ezetimibe can reduce plant steroidemia. Most of the parents of homozygous plant steroidemia are close relatives. In this case, the parents of the patient are not close relatives. We will also follow up the patient's family members.
- Citation: Jiang CX, Yang G, Shi LP, Su PY. Homozygous phytosterolemia and a literature review: A case report. World J Clin Cases 2025; 13(10): 101935
- URL: https://www.wjgnet.com/2307-8960/full/v13/i10/101935.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v13.i10.101935
Phytosterolemia, also known as sitosterolemia, is a rare autosomal recessive genetic disease[1], the pathophysiological mechanism of which includes increased intestinal absorption and decreased biliary secretion of plant sterols. Given that its clinical characteristics include extreme elevation of low-density lipoprotein cholesterol (LDL-C), xanthomata, and early onset of coronary artery disease, phytosterolemia is easily misdiagnosed as familial hypercholesterolemia. Some scholars have suggested that phytosterolemia is a recessive inherited form of familial hypercholesterolemia[2]. With wider application of gene detection technology, the rate of diagnosis of phytosterolemia has improved considerably. This report describes a case of homozygous phytosterolemia in a child. The patient had a mild clinical phenotype that seemed to be different from that observed in other patients with homozygous mutations. After treatment of the patient’s blood lipid profile in the outpatient clinic and dietary adjustment, the cholesterol levels have returned to normal.
The patient was an 11-year-old girl who presented to the lipid clinic at our hospital in 2023 with a 6-year history of abnormal lipid metabolism. Her blood lipids had been reviewed at 6-month intervals during the previous 6 years, during which her total cholesterol had ranged from 5.10 to 10.70 mmol/L (normal range, 2.80-5.20) and her LDL-C had ranged from 3.20 to 8.60 mmol/L (normal range, 1.80-3.40). She was treated with statins but the LDL-C remained within 4.0-5.0 mmol/L after treatment. With dietary control and exercise, her LDL-C remained within 3.5-4.0 mmo/L (Table 1). At the time of presentation to our hospital, she had a total cholesterol of 8.60 mmol/L and an LDL-C of 5.70 mmol/L. There were no eyelid or corneal abnormalities. Routine blood investigations showed normal blood cell morphology and number, with no giant platelets or erythrocyte heterogeneity detected in a peripheral blood smear. Echocardiography was normal, and no arteriosclerosis was observed on carotid ultrasound. The thickness of the Achilles tendon was normal on ultrasonography. Total cholesterol was 6.50 mmol/L (normal range, 2.80-5.20) and LDL-C was 4.70 mmol/L (normal range, 1.80-3.40) in the father and 5.88 mmol/L and 4.20 mmol/L, respectively, in the mother. The parents denied a consanguineous union. The father’s ultrasound suggested mild thickening of the carotid intima-media, the mother’s ultrasound suggested normal carotid arteries, and both parents’ cardiac ultrasounds were normal. There was no history of cardiovascular-related disease in the parents. Coronary heart disease was diagnosed in her grandfather at the age of 60 years and in her grandmother at the age of 65 years. Total cholesterol was 6.0 mmol/L (normal range, 2.80-5.20) and LDL-C was 4.10 mmol/L (normal range, 1.80-3.40) in the grandfather and 5.1 mmol/L and 3.70 mmol/L, respectively, in the grandmother. Carotid ultrasound identified plaques in the carotid arteries in both grandparents. The patient was diagnosed with severe hypercholesterolaemia in childhood. Hypercholesterolaemia can be managed with dietary control, and the diagnosis of phytosterolemia or familial hypercholesterolaemia was considered in a review of the literature[1].
| Treatment | Total cholesterol (mmol/L) | Low-density lipoprotein cholesterol (mmol/L) | Triglyceride (mmol/L) | High density lipoprotein cholesterol (mmol/L) |
| Normal lifestyle | 9.5 | 6.3 | 2.3 | 1.1 |
| Exercise and dietary | 6.1 | 4.0 | 1.4 | 1.3 |
| Statin | 6.5 | 4.6 | 0.8 | 1.2 |
| Ezetimibe and dietary | 4.6 | 2.8 | 1.5 | 1.2 |
At the time of presentation to our hospital, she had a total cholesterol of 8.60 mmol/L and an LDL-C of 5.70 mmol/L. There were no eyelid or corneal abnormalities. Routine blood investigations showed normal blood cell morphology and number, with no giant platelets or erythrocyte heterogeneity detected in a peripheral blood smear. Echocardiography was normal, and no arteriosclerosis was observed on carotid ultrasound. The thickness of the Achilles tendon was normal on ultrasonography.
The patient was an 11-year-old girl who presented to the lipid clinic at our hospital in 2023 with a 6-year history of abnormal lipid metabolism. Her blood lipids had been reviewed at 6-month intervals during the previous 6 years, during which her total cholesterol had ranged from 5.10 to 10.70 mmol/L (normal range, 2.80-5.20) and her LDL-C had ranged from 3.20 to 8.60 mmol/L (normal range, 1.80-3.40). She was treated with statins but the LDL-C remained within 4.0-5.0 mmol/L after treatment. With dietary control and exercise, her LDL-C remained within 3.5-4.0 mmo/L.
Total cholesterol was 6.50 mmol/L (normal range, 2.80-5.20) and LDL-C was 4.70 mmol/L (normal range, 1.80-3.40) in the father and 5.88 mmol/L and 4.20 mmol/L, respectively, in the mother. The parents denied a consanguineous union. The father’s ultrasound suggested mild thickening of the carotid intima-media, the mother’s ultrasound suggested normal carotid arteries, and both parents’ cardiac ultrasounds were normal. There was no history of cardiovascular-related disease in the parents. Coronary heart disease was diagnosed in her grandfather at the age of 60 years and in her grandmother at the age of 65 years. Total cholesterol was 6.0 mmol/L (normal range, 2.80-5.20) and LDL-C was 4.10 mmol/L (normal range, 1.80-3.40) in the grandfather and 5.1 mmol/L and 3.70 mmol/L, respectively, in the grandmother. Carotid ultrasound identified plaques in the carotid arteries in both grandparents.
There were no eyelid or corneal abnormalities. Echocardiography was normal, and no arteriosclerosis was observed on carotid ultrasound.
Her LDL-C had ranged from 3.20 to 8.60 mmol/L (normal range, 1.80-3.40). She was treated with statins but the LDL-C remained within 4.0-5.0 mmol/L after treatment. With dietary control and exercise, her LDL-C remained within 3.5-4.0 mmo/L (Table 1). At the time of presentation to our hospital, she had a total cholesterol of 8.60 mmol/L and an LDL-C of 5.70 mmol/L. Routine blood investigations showed normal blood cell morphology and number, with no giant platelets or erythrocyte heterogeneity detected in a peripheral blood smear.
The patient was diagnosed with severe hypercholesterolaemia in childhood, and the patient's severe hypercholesterolemia can be managed with dietary. The physicians at the Lipid Clinic consider phytosterolemia or familial hypercholesterolemia after taking into account the patient's actual condition and reviewing the literature.
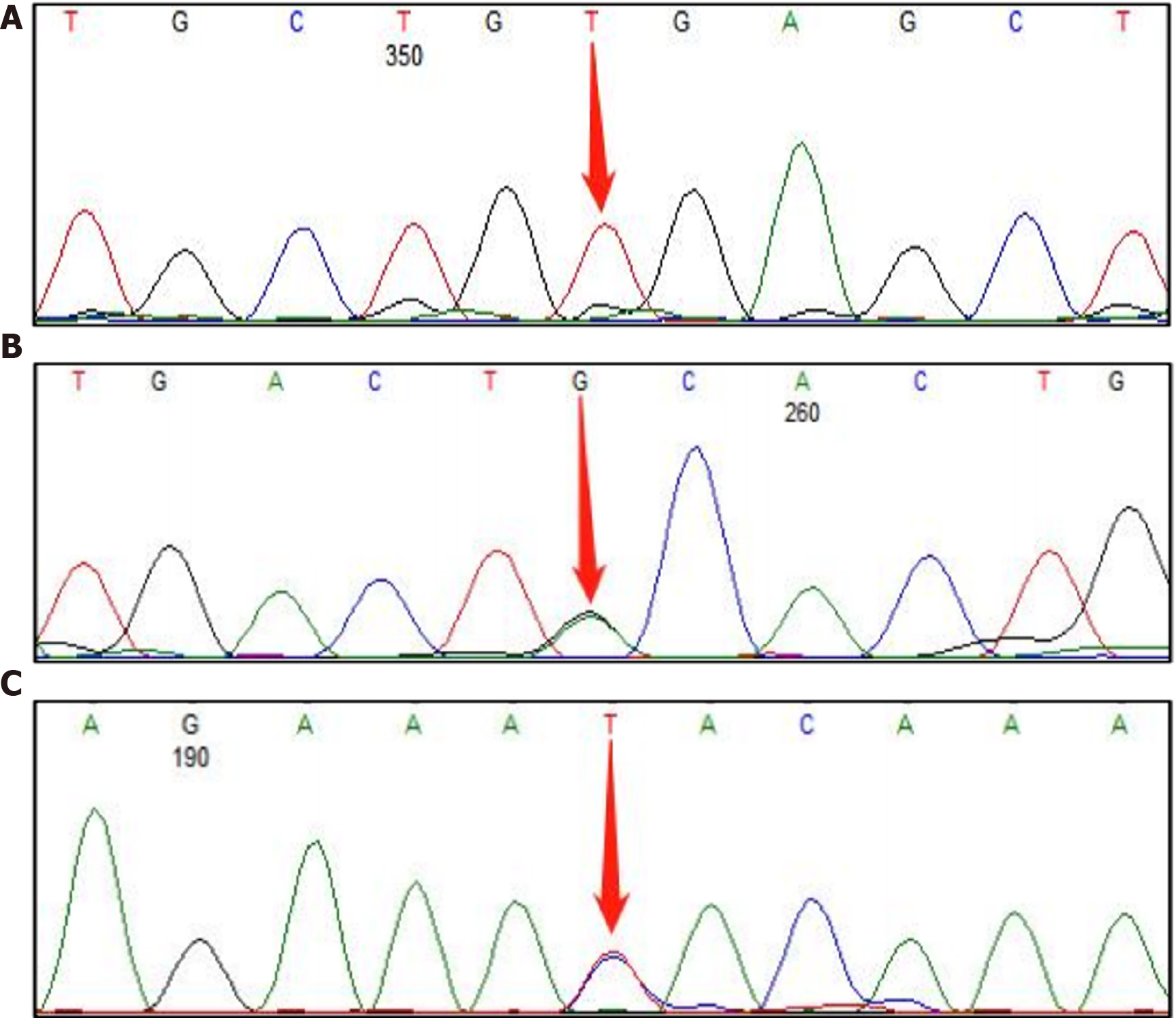
Whole exome sequencing was performed to make the diagnosis in the proband, and three mutations at sites related to lipid metabolism were detected; namely, ABCG5 c.1336C>T (p.Arg446Ter), APOB c.1433G>A (p.Cys478Tyr), and APOB c.11303T>C (p.Ile3768Thr) (Figure 1). ABCG5 c.1336C>T was a homozygous mutation and interpreted as pathogenic, whereas the APOB mutation was interpreted as being of unknown pathogenicity and potentially a benign mutation.
The patient was diagnosed to have phytosterolemia based on the homozygous ABCG5 mutation (Table 2). Plasma plant sterol levels were measured by liquid chromatography, and dihydrocholesterol, stigmasterol, canola oil-derived sterol, and β-sitosterol levels were found to be significantly increased in the patient (Table 3).
| Gene | Chromosome location | Variation | Homozygous/heterozygous | Pathogenicity |
| ABCG5 | chr2: 44050063 | c.1336C>T (p.Arg446Ter) | Homozygous | Pathogenic |
| APOB | chr2: 21252807 | c.1433G>A (p.Cys478Tyr) | Heterozygote | Unknown |
| APOB | chr2: 21228437 | c.11303T>C (p.Ile3768Thr) | Heterozygote | Unknown |
| Inspection item | Results | Units | Hint | Reference range |
| Squalene | 0.76 | μmol/L | Normal | 0.300-4.00 |
| Cholestanol | 78.37 | μmol/L | 0.01-10.00 | |
| Desmosterol | 5.78 | μmol/L | 0.30-5.00 | |
| Lathosterol | 1.75 | μmol/L | Normal | 0.01-12.50 |
| Campesterol | 319.76 | μmol/L | 0.01-10.00 | |
| Stigmasterol | 32.60 | μmol/L | 0.10-8.50 | |
| Sitosterol | 307.79 | μmol/L | 1.00-15.00 |
Genetic testing of the patient's parents showed that both father and mother carried an ABCG5 c.1336C>T (Table 4). The results confirm that the patient has phytosterolemia, and does not have familial hypercholesterolemia.
| Relationship | Gene | Homozygous/heterozygous | Genotype | Verification result |
| Father | ABCG5 c.1336C>T | Heterozygote | CT | Positive |
| Mother | ABCG5 c.1336C>T | Heterozygote | CT | Positive |
As recommended by the lipid clinic, the patient was started on ezetimibe, and a low phytosterolemia diet with reduced intake of plant sterols, improved dietary composition, and strictly controlled intake of rice, nuts, and vegetables was recommended.
One month later, the patient’s total cholesterol level had decreased significantly to 4.6 mmol/L and her LDL-C level had decreased to 2.8 mmol/L. We will adjust the treatment regimen according to the patient’s lipid profile in future follow-ups.
Phytosterolemia is an autosomal recessive inherited disease caused by mutations in ABCG5 or ABCG8. Under normal circumstances, plant sterols undergo limited absorption in the intestine and excretion in bile, and their concentrations in plasma are very low. However, the ABCG5/ABCG8 mutations can result in excessive intestinal absorption of sterols (including cholesterol and sterols in plants and shellfish) and impaired excretion of sterols in bile[3,4]. Dietary intake of plant sterols can reach approximately 200-500 mg/day, but < 5% is absorbed into the blood[5,6]. Plasma phytosterol levels can reach 30-200 times the normal range in individuals who harbor ABCG5/ABCG8 mutations. Excess phytosterols accumulate in the skin and blood vessels[1,2,7], and elevated free sterols accumulate in the plasma membranes of platelets, resulting in dysregulation of various platelet activation pathways[8], leading to massive thrombocytopenia and hemorrhage[5]. Laboratory tests have detected elevated plasma plant sterol levels, hemolytic anemia, and elevated platelets and transaminases. The estimated incidence of phytosterolemia in the general population is approximately 1 in 160000[9], and only approximately 200 cases have been reported worldwide[10,11]. The clinical manifestations of phytosterolemia include xanthomata, atherosclerosis, and other characteristics that are similar to those seen in familial hypercholesterolemia. Therefore, phytosterolemia is easily misdiagnosed or missed, and genetic testing is needed for a correct diagnosis.
ABCG5 and ABCG8 are major hepatobiliary transporters of dietary cholesterol and endogenous synthetic sterols[12,13]. ABCG5 is located at the p21 position on human chromosome 2, has a total length of 26368 bases, and contains 13 exons. ABCG5 encodes the ATP-binding cassette transporter G5, a member of the ATP-binding cassette subfamily G[4], which transports a variety of molecules through the outer and inner membranes of cells. The ABCG5 protein is located mainly in the apical membrane of intestinal cells and the tubule membrane of the bile duct. ABCG5 regulates the metabolism of sterols (mainly plant-derived) by forming heterodimers with the ABCG8 protein, promoting excretion of cholesterol and plant sterols into bile by hindering the transport of sterols back into the intestinal lumen. The ABCG5 c.1336C>T mutation in our patient is located in exon 10. It introduces a premature stop codon and truncates the encoded protein at amino acid 446, which is predicted to lead to nonsense-mediated mRNA degradation. The pathogenic mechanism is loss of function. Our review of the literature showed that this mutation site is the current hot spot of phytosterolemia mutations in China[9,10,12,14]. Almost all patients with a mutation at this site have severe clinical manifestations, including thrombocytopenia and xanthomata[5,12]. Our patient had no symptoms except elevated plasma plant sterol levels and high LDL-C levels, whereas > 90% of affected patients have varying degrees of xanthomata, thrombocytopenia, and early onset coronary heart disease[15]. We are considering the following possible reasons for this anomaly. First, we became aware through consultations with specialists in genetics that a few mutations are undetectable-for example, large missing fragments and rearrangements. Second, epigenetic inheritance such as low gene expression because of DNA hypermethylation can result in a milder clinical phenotype. These mechanisms need to be studied further. In treating phytosterolemia, lipids are controlled in the following ways. First, review of the patient’s medical history showed that her blood lipids were monitored regularly, and if significantly elevated, she would increase the amount of exercise, which helped to reduce the blood lipid levels (Table 1). Second, the composition of the patient’s diet was changed to include more fats and meat and fewer vegetables, fruits, and other plant sterols, with the aim of reducing the accumulation of plant sterols in the body. We are investigating whether changing the dietary composition decreases the accumulation of plant sterols in the body by observing the patient for other clinical manifestations during follow-up.
Phytosterolemia is usually treated by pharmacological therapy and improvement of dietary composition. Although phytosterolemia and familial hypercholesterolemia both present with extremely elevated LDL-C levels, familial hypercholesterolemia is characterized mainly by mutations in LDLR, PCSK9, APOB, and LDLRAP1[16], whereas phytosterolemia is characterized by mutations in ABCG5/ABCG8 and increased intestinal absorption of plant sterols, resulting in increased LDL-C and total cholesterol levels. Thus, the mechanism of the increase in cholesterol levels is different in the two conditions. Phytosterolemia may be more dependent on dietary sterol intake[3]. Therefore, the LDL-C level may decrease in response to an appropriate diet in patients with phytosterolemia but not in those with familial hypercholesterolemia. Phytosterolemia is treated with ezetimibe, a cholesterol absorption inhibitor, or cholestyramine, a bile acid-chelating agent, whereas familial hypercholesterolemia is treated using statins, which lower cholesterol by inhibiting the liver enzyme HMG-CoA reductase[2]. In view of the gastrointestinal adverse reactions associated with cholestyramine[15], we chose ezetimibe for treatment in this case. Ezetimibe acts via glucuronidation in the gut and liver and blocks the absorption of dietary- and bile-derived cholesterol. Both the parent compound and glucuronic acid are located at the brush border in the small intestine, where they block the absorption of dietary- and bile-derived cholesterol without affecting the absorption of triglycerides[12]. Although dietary fiber and plant sterols are usually beneficial for reducing intestinal absorption of cholesterol, dietary intake of phytosterols and shellfish sterols should be avoided in patients with phytosterolemia and ABCG5 and/or ABCG8 variants. Such patients should be advised to continue to limit their intake of plant sterols[17], to reduce grains, vegetable oils, fruits and vegetables, nuts, and chocolate, and to avoid other grains such as buckwheat, rice, and beans[18]. After taking ezetimibe for one month, our patient’s plasma total cholesterol was 4.60 mmol/L and her LDL-C was 2.8 mmol/L. Routine blood and liver function tests were normal.
Phytosterolemia is an extremely rare genetic disease that has a severe impact on the blood lipid profile. Given that plasma plant sterol levels are not routinely measured, it is easy for patients with phytosterolemia to be misdiagnosed as having familial hypercholesterolemia. Genetic counseling and treatment for phytosterolemia is different from those for familial hypercholesterolemia. Considering that phytosterolemia is characterized mainly by elevated plant sterol levels, genetic testing and determination of plasma plant sterol levels in offspring are essential for early intervention and treatment of the disease. The clinical phenotype of homozygous phytosterolemia is severe, whereas the clinical phenotype in our patient is mild. We plan to further explore the mechanisms underlying the light clinical phenotype. We are continuing to monitor this patient for clinical manifestations of hematological and cardiovascular abnormalities.
| 1. | Tada H, Kojima N, Takamura M, Kawashiri MA. Sitosterolemia. Adv Clin Chem. 2022;110:145-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 2. | McGowan MP, Hosseini Dehkordi SH, Moriarty PM, Duell PB. Diagnosis and Treatment of Heterozygous Familial Hypercholesterolemia. J Am Heart Assoc. 2019;8:e013225. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 156] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 3. | Yang C, Yu L, Li W, Xu F, Cohen JC, Hobbs HH. Disruption of cholesterol homeostasis by plant sterols. J Clin Invest. 2004;114:813-822. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 116] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 4. | Yu L, Hammer RE, Li-Hawkins J, Von Bergmann K, Lutjohann D, Cohen JC, Hobbs HH. Disruption of Abcg5 and Abcg8 in mice reveals their crucial role in biliary cholesterol secretion. Proc Natl Acad Sci U S A. 2002;99:16237-16242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 558] [Cited by in RCA: 555] [Article Influence: 23.1] [Reference Citation Analysis (0)] |
| 5. | Bastida JM, Benito R, Janusz K, Díez-Campelo M, Hernández-Sánchez JM, Marcellini S, Girós M, Rivera J, Lozano ML, Hortal A, Hernández-Rivas JM, González-Porras JR. Two novel variants of the ABCG5 gene cause xanthelasmas and macrothrombocytopenia: a brief review of hematologic abnormalities of sitosterolemia. J Thromb Haemost. 2017;15:1859-1866. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 38] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 6. | Buonuomo PS, Iughetti L, Pisciotta L, Rabacchi C, Papadia F, Bruzzi P, Tummolo A, Bartuli A, Cortese C, Bertolini S, Calandra S. Timely diagnosis of sitosterolemia by next generation sequencing in two children with severe hypercholesterolemia. Atherosclerosis. 2017;262:71-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 24] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 7. | Nomura A, Emdin CA, Won HH, Peloso GM, Natarajan P, Ardissino D, Danesh J, Schunkert H, Correa A, Bown MJ, Samani NJ, Erdmann J, McPherson R, Watkins H, Saleheen D, Elosua R, Kawashiri MA, Tada H, Gupta N, Shah SH, Rader DJ, Gabriel S, Khera AV, Kathiresan S. Heterozygous ABCG5 Gene Deficiency and Risk of Coronary Artery Disease. Circ Genom Precis Med. 2020;13:417-423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 51] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 8. | Kanaji T, Kanaji S, Montgomery RR, Patel SB, Newman PJ. Platelet hyperreactivity explains the bleeding abnormality and macrothrombocytopenia in a murine model of sitosterolemia. Blood. 2013;122:2732-2742. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 54] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 9. | Xia Y, Duan Y, Zheng W, Liang L, Zhang H, Luo X, Gu X, Sun Y, Xiao B, Qiu W. Clinical, genetic profile and therapy evaluation of 55 children and 5 adults with sitosterolemia. J Clin Lipidol. 2022;16:40-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 19] [Reference Citation Analysis (0)] |
| 10. | Abifadel M, Boileau C. Genetic and molecular architecture of familial hypercholesterolemia. J Intern Med. 2023;293:144-165. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 71] [Article Influence: 23.7] [Reference Citation Analysis (0)] |
| 11. | Su SQ, Xiong DS, Ding XM, Kuang JA, Lin YC. Pediatric patients with familially inherited sitosterolemia: Two case reports. Front Cardiovasc Med. 2022;9:927267. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 12. | Qin M, Luo P, Wen X, Li J. Misdiagnosis of sitosterolemia in a patient as Evans syndrome and familial hypercholesterolemia. J Clin Lipidol. 2022;16:33-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 13. | Salen G, von Bergmann K, Lütjohann D, Kwiterovich P, Kane J, Patel SB, Musliner T, Stein P, Musser B; Multicenter Sitosterolemia Study Group. Ezetimibe effectively reduces plasma plant sterols in patients with sitosterolemia. Circulation. 2004;109:966-971. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 248] [Cited by in RCA: 216] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 14. | Su X, Shao Y, Lin Y, Zhao X, Zhang W, Jiang M, Huang Y, Zeng C, Liu L, Li X. Clinical features, molecular characteristics, and treatments of a Chinese girl with sitosterolemia: A case report and literature review. J Clin Lipidol. 2019;13:246-250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 15. | Bastida JM, Girós ML, Benito R, Janusz K, Hernández-Rivas JM, González-Porras JR. Sitosterolemia: Diagnosis, Metabolic and Hematological Abnormalities, Cardiovascular Disease and Management. Curr Med Chem. 2019;26:6766-6775. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 30] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 16. | Sustar U, Kordonouri O, Mlinaric M, Kovac J, Arens S, Sedej K, Jenko Bizjan B, Trebusak Podkrajsek K, Danne T, Battelino T, Groselj U. Universal screening for familial hypercholesterolemia in 2 populations. Genet Med. 2022;24:2103-2111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 24] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 17. | Lee MH, Lu K, Hazard S, Yu H, Shulenin S, Hidaka H, Kojima H, Allikmets R, Sakuma N, Pegoraro R, Srivastava AK, Salen G, Dean M, Patel SB. Identification of a gene, ABCG5, important in the regulation of dietary cholesterol absorption. Nat Genet. 2001;27:79-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 438] [Cited by in RCA: 464] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 18. | Ghosh S, Devereaux MW, Anderson AL, Gehrke S, Reisz JA, D'Alessandro A, Orlicky DJ, Lovell M, El Kasmi KC, Shearn CT, Sokol RJ. NF-κB Regulation of LRH-1 and ABCG5/8 Potentiates Phytosterol Role in the Pathogenesis of Parenteral Nutrition-Associated Cholestasis. Hepatology. 2021;74:3284-3300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 26] [Article Influence: 5.2] [Reference Citation Analysis (1)] |